J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 7(1):84-87 2004
Stability of sulfadiazine oral liquids prepared from tablets and powder.
Umma Pathmanathan, David Halgrain, Fouad Chiadmi, Joël Schlatter1, Norbert Vermerie
Pharmacy Department, University Hospital of Jean Verdier, Bondy, France.Received 04 February 2004, Revised 02 April 2004, Accepted 02 April 2004, Published 21 April 2004
PDF Version
Abstract
PURPOSE: To assess the stability of sulfadiazine (SDZ) oral liquids prepared from tablets and powder at two temperatures. METHODS: Solutions of SDZ 200 mg/mL were prepared from commercially available 500 mg tablets and powder in sterile water for irrigation. They were stored in amber glass bottles at 4°C and 23°C. The concentrations of SDZ were determined in duplicate by high-performance liquid chromatography at 0, 1, 3, 7 and 14 days. The initial and final pH of solutions was compared. The recovery of SDZ from tablets was determined. A loss exceeding 10% of the initial concentration of SDZ was considered excessive degradation. RESULTS: The recovery of SDZ from tablets was 100 ± 3%. The initial pH values were significantly different between solutions prepared from tablets and powder, 6.9 and 9.8 respectively. No significant difference was found between initial and final pH values for the two all formulations. Detectable change in odor was observed for the solutions stored at 23°C. The solution prepared from powder was stable 3 days stored at 4°C. Other formulations lost over 10% of the initial SDZ concentration within 2 days. CONCLUSIONS: SDZ 200 mg/mL oral solution prepared from powder could be used to facilitate drug administration to very young children by nurses but by taking account of its fast degradation.
Introduction
Toxoplasmosis is caused by the protozoan parasite Toxoplasma gondii. Congenital infection with this germ may result in spontaneous abortion, fetal death or severe disease (1, 2). The sequelae in live-born infants with signs of infection are generally severe and include a potentially fatal syndrome in which hydrocephalus, mental retardation and chorioretinitis may occur (3-7). The recommended dose of sulfadiazine in children is to 25 to 50 mg/kg four times daily (8). Sulfadiazine, a 2-sulfanilamidopyrimidine (SDZ), is a sulfonamide anti-infective agent available in compressed tablets only. SDZ is a white or yellow crystalline powder insoluble in water and very slightly soluble in ethanol. It is slowly darkening on exposure to light with decomposition (9). A liquid dosage form would be highly desirable for pediatric patients and allows the dose to be easily adjusted. Because of the lack of published data on the stability of SDZ in oral liquid dosage form, we designed a study to determine the stability of the drug in liquid prepared from commercially available tablets and powder at two controlled temperatures.
Materials
Materials
Sulfadiazine powder1 was of analytical grade. Sulfadizaine 500-mg tablets2 were obtained commercially. They were consisted of potato starch 17.5 mg, magnesium stearate 1.15 mg, corn starch 135 mg and talc 6 mg. Ranitidine 50 mg/2 ml for injection3 (internal standard) and sterile water for irrigation4 were obtained commercially. Methanol5 and diethylamine6 were HPLC grade.
Formulations
A suspension of SDZ 200 mg/ml was prepared by crushing 100 500-mg tablets using a glass mortar and pistil and diluting with sterile water for irrigation to make a paste. Sterile water for irrigation was then added and mixed to a 250 ml final volume. The suspension was transferred in twelve amber glass prescription bottles. The recovery of SDZ obtained from tablets was also studied from five suspensions prepared independently. Another oral liquid of SDZ 200 mg/ml was prepared with SDZ powder. A sufficient quantity was weighed and mixed with sterile water for irrigation. This solution was transferred in twelve amber glass prescription bottles. Duplicate 1 mL samples were removed from each bottle and analyzed immediately.
Storage of solutions
Six bottles from each formulation were stored at room temperature (22 ± 3°C) with direct exposition at the sunlight and at 4 ± 2°C. Five bottles of SDZ suspension prepared independently were stored at +40 ± 3°C for forced degradation. The room temperature was measured each day by a pH meter (Model pH 302) with a temperature probe.
Sampling
From each bottle, 1 ml sample was taken, diluted in 2 ml of NaOH 1N and shacked. After 5 minutes, 50 mL of this solution were diluted in 50 mL of sodium bicarbonate 4.2%. The diluted sample was assayed in duplicate by high-performance liquid chromatography (HPLC), immediately after preparation, 1, 3, 7 and 14 days. The appearance and color were assessed by observing the samples against black and white backgrounds under normal light. The pH was measured with a pH meter (Model pH302, Hanna Instruments, Tanneries, France) in triplicate initially and at 14 days after preparation.
HPLC Analysis
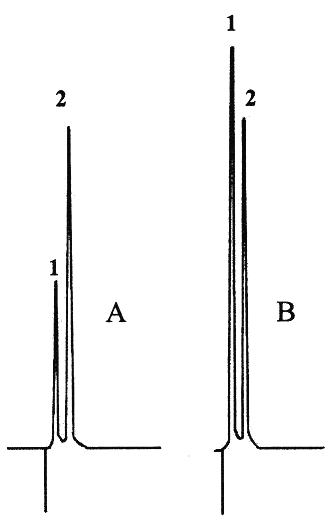
SDZ was quantified by using a modified HPLC method (10). The HPLC system consisted of a pump7, a 20 mL manual injector8, and a C18 column9. The mobile phase, consisting of sterile water for irrigation, methanol and diethylamine (60:39:1 v/v/v). A flow rate of 1 mL/min was used throughout the run. The ultraviolet variable-wavelength detector10 was set at 270 nm. Stock standard solutions of SDZ (2 g/L) and of ranitidine (5 g/L) were prepared in sterile water for irrigation and stored at 4°C. Six calibration standards were prepared by diluting SDZ stock standard solution with sterile water for irrigation to concentrations of 5, 10, 25, 30, 40 and 50 mg/L with 200 mg/mL, each containing of internal standard 200 mg/mL. The standard curve was constructed by plotting the peak-height ratio of SDZ to ranitidine against the SDZ concentration and was used for calculating the drug concentrations of the samples. The straight line of linear regression of SDZ HPLC assay was y = 0.047x + 0.226 ± 0.021. The standard curve was linear with a coefficient of correlation of 0.999 ± 0.002. The limits of detection and quantification of SDZ were 1.3 and 3.9 mg/L, respectively. The intraday coefficients of variation were 3.5% (n=10) and 1.4% (n=10) for respectively concentrations of 5 and 40 mg/L. The interday coefficients of variation were 4.5% (n=10) and 1.0% (n=10) for respectively concentrations of 5 and 40 mg/L. The retention times for the SDZ and internal standard were 0.8 and 2.4 minutes, respectively (Figure 1). In order to establish the stability-indicating nature of the assay, SDZ solutions obtained from powder and tablets were stored at 40°C until the chromatographic peak was not detected. Any degradation peak appeared during the study period.
Figure 1: Chromatograms of sulfadiazine solutions partially degraded (A) and not degraded (B). Peak 1: Sulfadiazine (retention time = 0.8 min), peak 2: internal standard (retention time = 2.4 min).
Data analysis
The initial concentration of SDZ was defined as 100%, and sample concentrations were expressed as a percentage of the initial concentration remaining as the Anaizi et al. Method (11). A loss exceeding 10% of the initial concentration of SDZ was considered excessive degradation. The pH values were expressed as mean ± standard deviation (S.D.). Difference of initial and final pH values was evaluated by a student's t test (á=0.05).
Results and Discussion
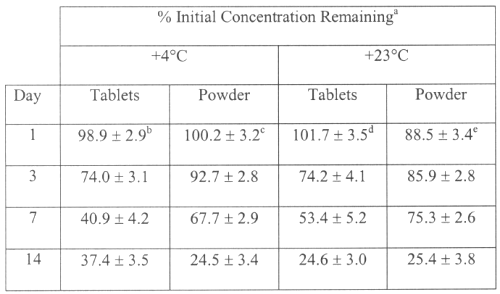
The recovery of SDZ solution from compressed tablets was of 100 ± 3% (n=5). The initial pH values were significantly different between liquids prepared from tablets and those prepared from the powder, 6.9 ± 0.1 and 9.8 ± 0.07 respectively. No significant difference was found between initial and final pH values for all formulations. Detectable change in odour was observed at the end of the study for the solutions stored at room temperature. The solution prepared from powder was stable 3 days stored at 4° C. The formulation prepared using the tablet lost over 10% of the initial SDZ concentration within 2 days (Table 1). SDZ can be prepared extemporaneously in liquid formulations from tablets or powder. However, the SDZ oral liquids are not stable for long time and cannot be prepared by pharmacists. Excipients and pH of suspensions prepared from tablets could explain the difference in stability with solutions prepared from powder. SDZ is not available in a liquid dosage form for the management of paediatric patients. SDZ 200 mg/mL oral solution prepared from powder was stable 3 days at 4° C and could be used to facilitate drug administration to very young hospitalized children by taking account of her fast degradation. However, the formulations are not suitable for preparation in large batches.
Table 1: Stability of SDZ 200 mg/mL oral liquids prepared from tablets and powder at two temperatures.
a Reported as mean ± S.D. of duplicate determinations for six samples.
b The actual mean ± S.D. initial concentration was 210.1 ± 6.3 g/mL.
c The actual mean ± S.D. initial concentration was 213.2 ± 8.5 g/mL.
d The actual mean ± S.D. initial concentration was 196.4 ± 6.9 g/mL.
e The actual mean ± S.D. initial concentration was 201.3 ± 9.0 g/mL
References
Pratlong, F., Boulot, P., Issert, E., Miska, M., Dupont, F., Bachelard, B., Sarda, P., Viala, J.L., Jarry, D., Fetal diagnosis of toxoplasmosis in 190 women infected during pregnancy. Prenat Diagn, 14:191-198, 1994.
Qublan, H.S., Jumaian, N., Abu-Salem, A., Hamadelil, F.Y., Mashagbeh, M., Abdel-Ghani, F., Toxoplasmosis and habitual abortion. J Obstet Gynaecol, 22:296-298, 2002.
Berrebi, A., Kobuch, W.E., Bessieres, M.H., Bloom, M.C., Rolland, M., Sarramon, M.F., Termination of pregnancy for maternal toxoplasmosis. Lancet, 344:36-39, 1994.
Kutova, K., Peicheva, Z., Popova, A., Markova, V., Mincheva, N., Todorov, T., Congenital toxoplasmosis: eye manifestations in infants and children. Ann Trop Paediatr, 22:213-218, 2002.
Foulon, W., Villena, I., Stray-Padersen, B., Decoster, A., Lappalaiven, M., Pinon, J.M., Treatment of toxoplasmosis during pregnancy: a multicenter study of impact on fetal transmission and children’s sequelae at age 1 year. Am J Obstet Gynecol, 180:410-415, 1999.
Wilson, C.B., Remington, J.S., Stagno, S., Reynolds, D.W., Development of adverse sequelae in children born with subclinical congenital toxoplasma infection. Pediatrics, 66:767-774, 1980.
Mombro, M., Perathoner, C., Leone, A., Nicocia, M., Moiraghi-Rugenini, A., Zotti, C., Congenital toxoplasmosis: 10 year follow-up. Eur J Pediatr, 154:635-639, 1995.
Kieffer, F., Thulliez, P., Brezin, A., Nobre, R., Romand, S., Yi-Gallimard, E., Voyer, M., Magny, J.F., Treatment of subclinical congenital toxoplasmosis by sulfadiazine and pyrimethamine continuously during 1 year: apropos of 46 cases. Arch Pediatr, 9:7-13, 2002.
Reynolds, J.E.F., Sulphadiazine. Martindale, The Extra Pharmacopoeia, The Pharmaceutical Press, London, pp. 206-207, 1993.
Kishida, K., Furusawa, N., Matrix solid-phase dispersion extraction and high-performance liquid chromatographic determination of residual sulfonamides in chicken. J Chromatogr A, 937:49-55, 2001.
Anaizi, H., Swenson, C., Instability of aqueous captopril solutions. Am J Hosp Pharm, 50:486-488, 1993.
Appendices
1 Sulfadiazine powder, Sigma, St Quentin, France, lot 91K1044
2 Adiazine®, Bouchara, Levallois-Perret, France, lot 8647
3 Raniplex® for injection, Fournier, Dijon, France
4 Sterile water for irrigation, Fresenius, Sèvres, France, lot 0146/36
5 Methanol, Chromanorm“, Prolabo, Paris, France
6 Diethylamine, Chromanorm“, Prolabo, Paris, France
7 LC-6A, Shimadzu Corporation, Duisbourg, Germany
8 Rheodyne“, Bensheim, Germany
9 Lichrospher® 25 cm, 100 RP-C18, 5 mm, Merck, Darmstadt, Germany
10 SPD-6A, Shimadzu Corporation, Duisbourg, Germany
Corresponding Author: Schlatter Joël, avenue du 14 juillet, 93140 Bondy, France. joel.schlatter@jvr.ap-hop-paris.frPublished by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps