J Pharm Pharmaceut Sci (www.cspscanada.org) 10(1):17-25, 2007
In Vitro Percutaneous Permeation of the Repellent DEET and the Sunscreen Oxybenzone across Human Skin
Tao Wang, Xiaochen Gu
Faculty of Pharmacy, University of Manitoba, Winnipeg, MB, Canada R3T 2N2
Received December 7, 2006; Revised February 13, 2007, Accepted February 25, 2007; Published March 6, 2007.
Corresponding Author: Dr. Xiaochen Gu, Faculty of Pharmacy, University of Manitoba, 50 Sifton Road, Winnipeg, MB, Canada, PH: +1(204)474-6903, FX: +1(204)474-7617 email: xgu@cc.umanitoba.ca
ABSTRACT - PURPOSE. DEET and oxybenzone are two essential active ingredients in repellent and sunscreen products. The percutaneous permeation of the two compounds across human skin from five commercially available repellent and sunscreen products was investigated in vitro. METHODS. Diffusion studies were carried out at 37 °C, using Franz-style diffusion cells and human epidermis (380 µm in thickness). The test products were evaluated either individually or in various combinations for up to 6 hours. Concentrations of both compounds permeated through the skin were measured using an HPLC assay. Permeability and permeation percentage of DEET and oxybenzone from different application approaches were calculated and statistically compared. RESULTS. The accumulated transdermal permeation was 0.5-25.7% for DEET and 0.3-1.6% for oxybenzone, respectively. Repellent lotion produced an 18-fold increase in transdermal permeation in comparison to that of repellent spray, while using repellent spray prior to sunscreen lotion resulted in the highest penetration of DEET among the study groups. Premixing sunscreen lotion with repellent spray at different ratios also produced significantly higher permeation of oxybenzone across the skin than the control, but other application approaches did not differentiate from the single sunscreen lotion. CONCLUSION. It was concluded from this study that human skin was less permeable to DEET and oxybenzone than artificial membranes, but was comparable to pig skin in permeability. DEET permeated transdermally more across human skin than oxybenzone, and both compounds acted as permeation enhancers when used simultaneously. Premixing repellent and sunscreen enhanced the overall penetration of both DEET and oxybenzone. Using different application sequences and amounts resulted in variable percutaneous permeation of DEET and oxybenzone through the skin.
Introduction
Insect repellents and sunscreens are two types of over-the-counter, consumer healthcare products that have been extensively used by the general public for the protection of vector-borne diseases and sunburns. DEET (N,N-diethyl-m-toluamide) is one of the most effective active ingredients in over 90% of all commercially available insect repellents (1,2). Oxybenzone is a primary UVA/UVB sun-blocking agent that is also present in many commercial sunscreen preparations (3,4). Concurrent application of both repellent and sunscreen products for outdoor activities has gradually become prevalent in recent years around the world, especially in mosquito-infested regions and areas, due mainly to health threats from mosquitoes including West Nile fever and malaria and better promotion of sunscreen use for skin cancer prevention.
Designed as topical preparations, repellents and sunscreens should exert minimal transdermal and systemic absorption under ideal application conditions. Percutaneous characterization of DEET and oxybenzone has been individually studied and reported (5-8). Both compounds are able to permeate across the skin and reach the systemic circulation. Recently, we have reported a synergistic percutaneous permeation of DEET and oxybenzone when both repellents and sunscreens were simultaneously applied (9-12). In addition, synergistic permeation was found between oxybenzone and some other compounds (13,14); co-application of repellents and sunscreens also reduced sun protection efficiency of the sunscreens (15,16). These characteristics of transdermal absorption are certainly considered undesirable as it could compromise the safety and efficiency of either repellents or sunscreens.
The use of in vitro diffusion experimentation for drug research and development has been well established and documented over the years (17-20). The method is able to provide insight into characterization of percutaneous drug permeation and systemic absorption before necessary in vivo testing in living subjects actually takes place. In order to achieve reliable and predicable results that are clinically correlative, human skin is generally regarded as the best testing membrane for diffusion experiments. Biological membranes from other mammalian animals and synthetic membranes are sometimes also used as substitutes when ample human skin specimens are difficult to obtain. Differences between human skin and other biological or artificial membranes have been studied and reported (18,21,22).
In this study, we investigated the transdermal permeation of DEET and oxybenzone across human skin when five commercially available repellent and sunscreen products were applied separately or in combination. The permeability and overall permeation percentages of DEET and oxybenzone across human skin were also statistically compared with those obtained from in vitro diffusion studies using pig skin and numerous artificial membrane models, with the hope of determining the similarity or difference in permeation rate and extent among various membrane models.
Materials and METHODS
Materials
Pure chemical standards DEET and oxybenzone were purchased from Fluka Chemika GmbH (Buchs, Switzerland) and Riedel-de Haën GmbH (Seelze, Germany) respectively. Polyoxyethylene 20-oleyl ether (Brij® 98) was obtained from Sigma Chemical Co. (St. Louis, MO, USA). Acetonitril (HPLC grade), glacial acetic acid, potassium phosphate monobasic and sodium hydroxide were purchased from Fisher Chemicals (Fair Lawn, NJ, USA). All chemicals used were of AC-Grade unless specified otherwise. HPLC-Grade deionized water was obtained from a Milli-Q® Pure Water System (Millipore, Nepean, ON, Canada) in the laboratory.
Table 1. Experimental design of the in vitro diffusion study
|
Study Code |
|||||
Study Design |
1* |
2* |
3* |
4 |
5 |
6 |
Repellent (g) |
1.0(A) |
1.0(B) |
0 |
0.5(A) |
0.5(A) |
0.5(A) |
Sunscreen (g) |
0 |
0 |
1.0(C) |
0.5(C) |
0.5(C) |
0.5(C) |
Application Method |
Direct application |
Direct application |
Direct application |
A: top |
A: bottom |
Prior mixing |
Study Design |
7 |
8 |
9 |
10 |
11 |
|
Repellent (g) |
0.5(A) |
0.25(A) |
0.5(B) |
0.5(D) |
0.5(E) |
|
Sunscreen (g) |
0.25(C) |
0.5(C) |
0.5(C) |
|
||
Application Method |
Prior mixing |
Prior mixing |
Prior mixing |
Direct application |
Direct application |
|
*: Control studies; A: OFF Spray; B: OFF Lotion; C: Coppertone Lotion; D: OFF
Repellent/Sunscreen Lotion; E: Muscol Repellent/Sunscreen Lotion
The five commercially available insect repellent and sunscreen preparations tested in the study were purchased from a local pharmacy, and used as obtained without further manipulation. They were OFF® Skintastic Spray (Product A, 7.0% DEET), OFF® Skintastic Lotion (Product B, 7.5% DEET), OFF® Skintastic Repellent/Sunscreen Lotion (Product D, 7.5% DEET and 5.0% oxybenzone, SPF 15) (S.C. Johnson and Son Ltd., Brantford, ON, Canada), Coppertone® Sunblock Lotion (Product C, 5.0% oxybenzone, SPF 30) (Schering-Plough Healthcare Products, Mississauga, ON, Canada), and Muskol® Repellent/Sunblock Lotion (Product E, 10.0% DEET and 4.0% oxybenzone, SPF 15) (Schering-Plough Healthcare Products, Mississauga, ON, Canada). Table 1 lists the study designs of the in vitro diffusion experiments where these five preparations were tested either individually or in combination.
Skin Preparation
The study was approved by the Research Ethics Boards at both the University of Manitoba and St. Boniface General Hospital. The full human skin was obtained from the Department of Surgery, St. Boniface General Hospital of Winnipeg. The skin specimens were kept at –20 °C after receiving from the Hospital and thawed at 4 °C overnight prior to the diffusion experiment.
On the morning of the study day, the freshly-thawed human skin was rinsed with deionized water to remove trace of biological fluids, and then dried by blotting both surfaces with paper towels. The skin was fully spread on the table and dermatomed to a thickness of 380 µm with an electric dermatome (Padgette Instruments, Kansas City, MO, USA). The dermatomed epidermis was further soaked in saline solution to prevent the membrane from dehydrating and shrinking. The integrity of the skin specimens were carefully examined; only undamaged section with even membrane thickness was selected for the diffusion studies.
Diffusion Study
The diffusion experiment was conducted in an automatic transdermal diffusion system (Logan Instruments Corporation, Somerset, NJ, USA), which was composed of six vertical Franz-style diffusion cells, a thermally-controlled circulating water bath, a magnetic stir console and an automatic sampling collector. Before the skin membrane was mounted onto the cell, a very thin layer of vacuum grease was applied to the connection surface of the receptor and donor cells to prevent the testing materials from leaking. The skin was mounted with the stratum corneum facing the donor cell. Each diffusion cell consisted of a 1 mL donor compartment and a 7 mL receptor compartment, with a diffusion surface area of 0.64 cm2. The test sample was accurately weighed and carefully placed in the donor cell so that a complete and intimate contact between the test material and stratum corneum was maintained throughout the experiment. The amount of test samples that was in direct contact with the skin surface and adjacent effective diffusion area was precisely measured to be 0.1 g, which was also used for the calculation of the overall permeation percentage of DEET and oxybenzone.
The receptor fluid was a pH 7.4 phosphate buffer solution that also contained 4% Brij® 98 (w/v). The use of a surfactant in the receptor medium was to solublize the highly lipophilic DEET and oxybenzone, thus achieving required sink conditions in the diffusion experiments. The preheated receptor fluid was left in the receptor cell for 30 minutes under continuous agitation at 300 rpm to reach temperature equilibrium (37 ± 0.05 °C) before the test sample was added to the donor cell. Both temperature and agitation were maintained constant as described for the duration of the complete diffusion experiment. An aliquot of receptor medium (100 µL) was collected hourly from the receptor cell for six hours, followed by the replenishment of the same volume of fresh, preheated receptor medium after each sampling interval. Four replicates were performed for each diffusion experiment. Concentrations of DEET and oxybenzone in the collected samples were directly injected into an HPLC system for drug analysis.
HPLC Assay
The HPLC assay for the simultaneous determination of DEET and oxybenzone was developed and validated in our laboratory; it had been previously used for various studies (9-12). Briefly, the HPLC system was composed of a Waters® Alliance 2690 Solvent Delivery Module, a 996 Photodiode Array Detector, and a Nova-Pak® C18 column (3.9 mm ´ 150 mm, 4 µm) (Milford, MA, USA). The mobile phase was composed of acetonitrile, methanol and water (pH 3.0, acidic acid) at the ratio of 65:20:15 (v/v/v), and delivered at a flow rate of 1 mL/min. DEET and oxybenzone were detected at the wavelength of 254 nm and 287 nm, with a detection limit of 5 ng and 20 ng respectively. The calibration linearity (r2 ³ 0.99, CV £ 1.0%) ranged 50–2000 ng for DEET and 8–500 ng for oxybenzone. All diffusion samples were analyzed directly without pretreatment; no interference was observed from excipients or additives present in the commercially available preparations.
Data Analysis
The overall permeation percentages of DEET and oxybenzone across the human skin were calculated based on the ratio of accumulated permeation amount to the actual application amount of the test products in the donor cell. The steady-state permeability coefficients (Kp) of DEET and oxybenzone were calculated using the following empirical diffusion equation derived from the Fick’s First Diffusion Law (23),
Js = KpCs Eq. 1
Where Js is the steady-state diffusion flux and Cs is the saturated drug concentration. Permeability coefficient is often used to compare permeation profiles for solutes examined under different conditions and related to the rate of diffusion of a solute within a membrane adjusted for differences in membrane thickness and solute concentration.
Statistical analysis was performed using two-way ANOVA followed by the Tukey’s test (PC-SAS® Version 8.02, SAS Institute Inc., Cary, NC, USA). The following statistical analyses of the data were conducted: (a) the overall permeation percentages and permeability of DEET and oxybenzone among various application designs; (b) the overall permeation percentages of DEET and oxybenzone among human skin, pig skin (10) and artificial membranes (12). Differences were considered statistically significant at p £ 0.05.
RESULTS
Permeation of Repellent DEET
Percutaneous permeation of DEET across human skin was detected in all diffusion experiments in vitro. Figure 1 shows the overall permeation percentages of DEET after 6 hour diffusion from different study designs. The lowest permeation was found to be 0.5% in Control Study 1, where the repellent spray was applied individually, while the highest permeation was found to be 25.7% in Study 5, in which the repellent spray was completely covered by the sunscreen lotion on the top without prior mixing. DEET in the single repellent lotion (Control Study 2) permeated at a significantly higher amount than that in the single repellent spray; an increment of 18 folds in DEET permeation was found between the two control studies.
Concurrent use of the repellent spray and the sunscreen lotion with different application approaches all increased the percutaneous permeation of DEET through the human skin. Placing the repellent spray on the top of the sunscreen lotion (Study 4) and below the sunscreen lotion (Study 5) without mixing resulted in a significant 10-fold and 47-fold increment in DEET permeation, respectively. Premixing the repellent spray and the sunscreen lotion at different use ratios also enhanced DEET permeation, with significant increase of 13 folds (Study 7) and 11 folds (Study 8) respectively. However, premixing the repellent spray and the sunscreen lotion at same amount did not yield significant change in DEET permeation. In comparison to the repellent lotion, only Study 5 exhibited significant permeation of DEET at an increment of 173%.
Mixing the repellent lotion with the sunscreen lotion prior to application (Study 9) resulted in significant increase of DEET permeation (630%) compared to the single repellent spray. However, this permeation value was significantly lower than that of the single repellent lotion. While permeation percentages of DEET from the two commercial combination lotions (Study 10 and Study 11) were all higher than that from the repellent spray, the values were all significantly lower in comparison with that from the repellent lotion.
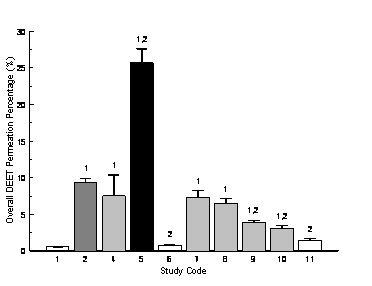
Figure 1. Overall permeation percentage of DEET across human skin after 6 hours; n = 4, mean ± SEM; 1: significant difference from Study 1, 2: significant difference from Study 2, p £ 0.05; bars with the same color indicate no significant difference among the studies; for study codes see Table 1.
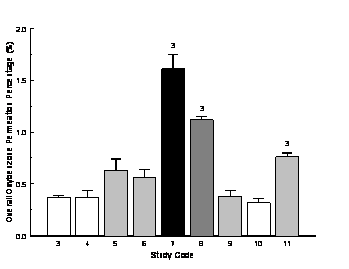
Figure 2. Overall permeation percentage of oxybenzone across human skin after 6 hours; n = 4, mean ± SEM; 3: significant difference from Study 3, p £ 0.05; bars with the same color indicate no significant difference among the studies; for study codes see Table 1.
Permeation of Sunscreen Oxybenzone
Synergistic percutaneous permeation of sunscreen oxybenzone across human skin was also observed in 7 different in vitro diffusion experiments. Figure 2 shows the overall permeation percentages of oxybenzone after 6 hour diffusion studies. The lowest permeation percentage of oxybenzone was observed with the Control (Study 3) and OFF repellent/sunscreen lotion (Study 10) at 0.4% and 0.3% respectively, while the highest permeation percentage of oxybenzone was found in Study 7 at 1.6%, in which 2 volumes of the repellent spray and 1 volume of the sunscreen lotion were premixed. In general, percutaneous permeation of oxybenzone was significantly lower than percutaneous permeation of DEET across human skin membrane.
Concurrent application of the repellent spray with the sunscreen lotion facilitated percutaneous permeation of oxybenzone in different manners. Premixing the repellent spray and the sunscreen lotion at 2:1 and 1:2 ratios significantly increased oxybenzone permeation across the skin; the increment was 335% for Study 7 and 202% for Study 8, respectively. While placing the repellent spray on the top of the sunscreen lotion without physical mixing (Study 4) did not affect the diffusion characteristics of oxybenzone from the lotion preparation, placing the sunscreen lotion on the top of the repellent spray without premixing (Study 5) did generate a 70% increase in percutaneous permeation of oxybenzone across the membrane, indicating the diffusion ability of the compound through the repellent spray layer. Premixing both the repellent spray and the sunscreen lotion prior to application (Study 6) also enhanced the permeation of oxybenzone across the skin membrane (53%).
Premixing the repellent lotion with the sunscreen lotion (Study 9) did not influence percutaneous permeation of oxybenzone in comparison to the control. For the two commercial combination repellent/sunscreen lotions, significant permeation of oxybenzone was observed with only Muskol® brand (Study 11), at an increment of 105%; OFF® brand product (Study 10) did not exhibit any change in oxybenzone permeation compared to the control sunscreen lotion.
Discussion
In vitro diffusion experimentation is the most feasible and cost-saving method available to characterize percutaneous permeation and absorption of various chemical compounds across the human skin. In order to realistically correlate the in vitro diffusion results to in vivo performance of a drug delivery system in humans, skin specimens from human subjects are considered the best testing model for diffusion studies in terms of clinical relevance. Obtaining ample amount of human skin samples for a reasonably well designed diffusion experiment might not be always possible. Biological membranes from various animals such as pigs, rats and mice, as well as certain synthetic membranes including polyethylene and cellulose derivatives, have been used as substitute membrane models for in vitro diffusion studies. It is important to establish satisfactory correlation between the human skin and other surrogate membranes. Some success has been observed and documented with both animal skin specimens and artificial membranes (22,24,25). Artificial membranes are appropriate for assessing drug release characteristics from a formulation vehicle or a delivery system.
Similar to what has been observed in previous studies (9,10,12), concurrent use of repellent and sunscreen preparations resulted in synergistic percutaneous permeation of both DEET and oxybenzone across the human skin. The rate and extent of percutaneous permeation were also dependent on various application approaches, including formulation type, application sequence and application ratio. Repellent lotion produced significantly higher transdermal permeation of DEET than repellent spray, due mainly to its low viscosity and intimate contact with the stratum corneum. In addition, the presence of other excipients and additives in the lotion preparation such as emulsifiers and surfactants might have facilitated the diffusion and permeation of DEET through the formulation as well as across the skin layers. This significant increase of DEET permeation from the repellent lotion had also been observed in all previous studies with both biological and artificial membranes. Mixing repellent spray with sunscreen lotion promoted DEET permeation in comparison to single repellent spray; the overall permeation percentages of DEET were however smaller than that from single repellent lotion. Premixing repellent lotion and sunscreen lotion resulted in lower permeation of DEET in human skin, which was contrary to what was found in pig skin and artificial membranes (10,12). For sunscreen oxybenzone, the overall permeation percentage was enhanced when repellent products were simultaneously applied, which was also identical in patterns compared to previous studies with both biological and synthetic membranes. Applying repellent spray on the top of sunscreen lotion without premixing did not prevent DEET from diffusing across various skin layers and reaching the receptor medium (Fig. 1, Study 4). This phenomenon was not observed in previous diffusion studies, indicating the differences in percutaneous absorption between human skin and other surrogate membrane models. Covering the repellent spray with the sunscreen lotion should definitely be avoided according to the result from this study, because this application approach would produce an occlusive condition on the skin surface, preventing repellent liquid from evaporating and subsequently promoting more permeation of DEET across the skin layers. The general application recommendation is therefore always to apply the sunscreens first to seal the skin surface and then to use the repellents at appropriate dose on the top. This will also generate the most optimal protection efficacy for both repellents and sunscreens (10,11). For oxybenzone, its transdermal permeation was not significantly affected regardless of application sequence. Nevertheless, premixing the sunscreen lotion with the repellent spray at 2:1 and 1:2 proportions did significantly enhanced the permeation percentage of oxybenzone across the human skin. This permeation pattern was identical to what was reported in other diffusion experimentation.
Commercial products that combine both repellent and sunscreen ingredients in one single preparation have been discontinued in the Canadian market by Health Canada. The primary reason for this withdrawal of such products was based on the fact that these products served neither protection purposes when utilized. Used as a repellent at sparse dose, no sufficient sun protection efficiency could be achieved. When used as sunscreens, overexposure to repellent component could take place (26). In addition, studies have indicated that Sun Protection Factor (SPF) of a sunscreen product was reduced by 33% when a repellent was applied simultaneously (15). In our previous study with pig skin, commercially available combined preparations resulted synergistic permeation of both DEET and oxybenzone, which was significantly higher than the control studies (10). In particular, physical mixing of the repellent lotion and the sunscreen lotion immediately before the application yielded higher overall permeation extent than the two commercial compound products. It was hypothesized that chemical interactions between DEET and oxybenzone could take place when the two products were physically mixed, which subsequently resulted in enhanced percutaneous permeation of the two compounds. In this study, synergistic permeation enhancement of DEET was also found from Studies 9, 10 and 11. However, the overall permeation extent was higher than the control repellent spray, but not the control repellent lotion. For oxybenzone, only Study 11 generated significantly higher percutaneous permeation than the control sunscreen lotion. The discrepancy of these permeation properties from combined products might be attributed to differences between human skin and piglet skin in terms of percutaneous drug absorption.
Table 2 contains the steady-state permeability coefficients of DEET and oxybenzone across the human skin. Compared to similar diffusion studies with piglet skin and artificial membranes as the testing models, permeability of DEET and oxybenzone in human skin was found to be comparable to that of pig skin. Human skin has been proven to be generally less permeable than other animal skin models in drug permeation and absorption (22). This trend was found in most experimental designs with both DEET and oxybenzone in this study. Significant difference in permeability of DEET and oxybenzone had been previously found between piglet skin and three other artificial membranes; therefore there was significant difference of permeability between human skin and synthetic membrane models. The overall permeation percentage of DEET through human skin was higher than that with low-density polyethylene (LDPE) and low fouling composite (LFC) membranes, while the overall permeation percentage of oxybenzone through the human skin was significantly lower than that in LDPE, LFC and mixed cellulose esters (MCE) membranes. This discrepancy in permeability of DEET and oxybenzone among various testing membrane models was attributed to the solubility of compounds in the preparations and in the membranes, the partition between the preparations and the membranes, as well as the lipophilicity of the compounds. While using surrogate membranes other than human skin specimens may provide insightful data to evaluate skin permeation and systemic absorption of topically administered medications, data generated using human skin offers more clinically relevant and reliable results as compared with those from in vitro diffusion studies. The former, therefore, should be considered as the first choice where sufficient human skin samples are available.
Table 2. Steady-state permeability coefficient (´10-4, cm/h) of DEET and oxybenzone
|
Study Code |
|||||
Study Design |
1 |
2 |
3 |
4 |
5 |
6 |
DEET |
1.0±0.2 |
26.8±5.0 |
--- |
21.1±8.1 |
65.8±13.1 |
1.3±0.3 |
Oxybenzone |
--- |
--- |
1.0±0.2 |
1.8±0.4 |
2.3±0.5 |
1.6±0.3 |
|
|
|
|
|
|
|
Study Design |
7 |
8 |
9 |
10 |
11 |
|
DEET |
17.9±3.4 |
18.2±3.7 |
10.2±1.9 |
6.8±1.3 |
3.7±0.8 |
|
Oxybenzone |
21.5±12.4 |
3.6±0.7 |
1.0±0.1 |
1.0±0.2 |
1.0±0.2 |
|
* n = 4, mean ± SEM
Variations of experimental results from using human skin were larger than those from either pig skin (10) or synthetic membranes (9,12), as human skin specimens were collected from different subjects whose skin conditions and ages were variable. In addition, the four replicates in each diffusion experiment might have come from different skin samples with different size of the skin specimens available and consistency in skin preparations. This was not the case with pig skin and artificial membranes; ample membrane samples from the same animal or batch were available for the experiments, subsequently resulting in more uniform and tighter result variations. All these factors should therefore be taken into consideration in order to carry out a reliable and accurate in vitro diffusion study for transdermal drug development and assessment.
In conclusion, results from this in vitro study indicated a synergistic percutaneous permeation of the insect repellent DEET and the sunscreen oxybenzone when various commercially available products were tested. The enhanced transdermal absorption was dependent upon the test formulations and the application approaches. Human skin was less permeable to DEET and oxybenzone than some artificial membranes; permeability of oxybenzone was lower than that of DEET. Even though the study was conducted under in vitro conditions, percutaneous patterns of this kind would be considered undesirable in vivo. Insect repellents and sunscreens are extensively used by the general public for summer outdoor activities, and concurrent application of both products is prevalent in many areas and regions. As a result, further investigations should be carried out to ensure the application safety and protection efficacy from concurrent use of DEET-based insect repellent and sunscreen products.
ACKNOWLEDGEMENT
We thank Dr. K. A. Murray and his staff at the St. Boniface General Hospital of Winnipeg for coordinating the Human Ethics Protocol and the collection of human skin specimens. We also acknowledge research support from Canadian Institutes of Health Research (CIHR), Manitoba Health Research Council (MHRC) and Canada Foundation for Innovation (CFI). Tao Wang received a Graduate Studentship from the University of Manitoba.