J Pharm Pharmaceut Sci (www.cspscanada.org) 10(1):61-70, 2007
Enhanced nuclear delivery and cytotoxic activity of hydro -xycamptothecin using o/w emulsions
Yongxing Zhaoa,b; Jianqing Gaoa; Xiaoyi Suna; Hailiang Chena; Limao Wu a; Wenquan Lianga
aCollege of Pharmaceutical Sciences, Zhejiang University, Hangzhou, Zhejiang 310058, PR ,China bCollege of Pharmaceutical Sciences, Zhengzhou University, Zhengzhou, Henan 450052, PR, China
Received, January 4, 2007, Revised, April 1, 2007, Accepted April 5, 2007, Published, April 5, 2007
Corresponding Author: W Liang, College of Pharmaceutical Sciences, Zhejiang University Zijin’gang Campus, Hangzhou, Zhejiang Province China Tel/Fax: +86-571-8820 8436 Email: wqliang@zju.edu.cn
ABSTRACT - PURPOSE. Hydroxycamptothecin -Emulsions (HCPT-E), being a promising delivery system for intravenous applications, were prepared. Here we evaluated the relation of cytotoxicity and intracellular drug amounts. FuThe mechanism of the enhanced antiproliferative activity was also investigated. METHODS. In vitro cytotoxic potential were assessed by the methyl thiazol tetrazolium (MTT) assay,. The capability of emulsions to preserve lactone form of HCPT and the time course of subcellular (cytoplasm and nuclei) drug distribution in vitro of HCPT-E were quantified by a reversed-phase HPLC assay. RESULTS. MTT results showed that the antiproliferative activity of HCPT-E was stronger than that of HCPT-Injections (HCPT-I) which is presently used in clinic. IC50 values of HCPT-E was much lower, by 4 and 7 times than those of HCPT-I. HeLa cells treated with HCPT-E for 4 h exhibited a considerable morphology change and displayed a hint of apoptotic cell death at 72 h. The lactone is the key position in HCTP molecule which is sensitive to pH. Lactone ring of HCPT in HCPT-E was stable for longer time than that of HCPT-I in the presence of human plasma or culture medium. Both the nuclear and cytoplasmic level of HCPT in HeLa cells exposed to HCPT-E at each time were higher by 18 to 33 times than those treated with HCPT-I. Drug amounts of nuclei was higher than that of cytoplasm in HeLa cells incubated with HCPT-E while it was reverse in HeLa cells exposed to HCPT-I. CONCLUSIONS. HCPT-E improved stability of HCPT, enhanced uptake, changed its intracellular distribution in favor of accumulating in the intracellular targets.
Introduction
Hydroxycamptothecin (HCPT) is a promising derivative of camptothecins (CPT) which possesses the ability to inhibit the growth of a wide range of human tumors. HCPT acts on topoisomerase I, inhibit DNA replication and RNA transcription by stabilizing the cleavable complexes formed between topoisomerase I and DNA (1, 2). Therefore, HCPT and other camptothecin analogues have recently undergone extensively clinical evaluation worldwide (3-5). The preservation of α-hydroxy-δ-lactone ring of HCPT is crucial for its anti-tumor activity (6). However, the delivery of the lactone form is quite challenging, since the lactone exists in a pH-dependent equilibrium with an open carboxylate (Figure 1), and the lactone form with the closed E-ring has poor solubility. Furthermore, human serum albumin is shown to bind preferentially to the carboxylate form resulting in the more rapid opening of the lactone ring in blood (7).
At present, CPT injection is formulated in sodium salt of the carboxylate which possess only 10% of the cytotoxic activity of CPT-lactone form (6) and several side-effects have been found in phase I clinical trials (8).Due to its low aqueous solubility and activity in vivo, with respect to drug hydrolysis and blood-protein interactions (9) the pharmaceutical development and clinical utilization of HCPT have been limited. Therefore, the development of a safer and more potent intravenous formulation is timely. Recently, specifically designed dosage forms and techniques for camptothecins, water- and lipid-soluble prodrugs (10), cyclodextrines inclusion compounds (11), polymer-bound derivatives (12, 13), nanoparticles (14), microspheres (15), and liposomes (11, 16) have been evaluated to overcome the hydrophobic and unstable characters of camptothecins. However, the solubility and stability of camptothecins in these formulations are also in question. Emulsion systems offer potential advantages for the delivery of poorly-soluble drugs because of the high volume fraction of the oil phase that can be used (17) and submicron lipid emulsion for parenteral used is relatively easy to produce on a large scale. In our work, HCPT is dissolved in oil phase existing as an active lactone form. Moreover, emulsions may be a potent candidate in sustained-release and targeted delivery systems (18).
Many studies demonstrated that the intracellular accumulation of anti-cancer agents strongly influenced the efficiency of chemotherapy for cancer (19-21). Moreover, the subcellular distribution of anti-cancer agents might play a significant role in their cytotoxic potency and contributes to their pharmacological features (10, 16). Some studies of in vitro cytotoxicity and intracellular drug uptake indicated that increasing intracellular anticancer drug level likely to lead to an enhanced antiproliferative activity (17, 22-25). But relationship between subcellular (especially nuclei) drug distribution and antiproliferative activity is not studied. The subcellular localization of anti-cancer agents is crucial as individual drugs are designed to exert their activities against specific subcellular targets (26). For example, topoisomerase I in nuclei is the target organelle of HCPT.
In the present work we prepared HCPT-Emulsions (HCPT-E) and determined the time course of subcellular (cytoplasm and nuclei) drug distribution of HCPT-E compared with that of injection in HeLa cells. The relationship between cytotoxicity and intracellular drug amount of two preparations was also evaluated.
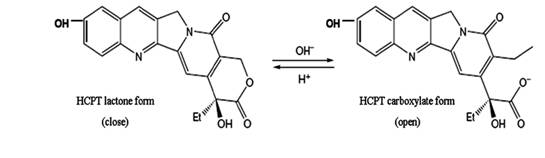
Figure 1. The structural equilibrium of HCPT in aqueous solution. HCPT undergoes reversible pH-sensitive hydrolysis with opening of the lactone ring to form the carboxylate isoform.
Materials and METHODS
Materials
HCPT (purity >98.6%) was provided by China Aroma Chemical Co. Ltd (Hangzhou, Zhejiang, China). HCPT-I were purchased from Harbin Sanctity Pharmaceutical Co. Ltd (Harbin, Heilongjiang, China). Acetonitrile and methanol of HPLC grade were obtained from Siyou Chemical Reagent Co. Ltd (Tianjin, China). Dulbecc’s Modified Eagle Medium (DMEM) and trypsin were purchased from Genom BioMed Technology Inc (Hangzhou, Zhejiang, China). Fetal bovine serum was purchased from Beijing Yuan Heng Sheng Ma BioMed Technology Inc (Beijing, China). Cholesterol was purchased from Pharmacia Biotech (Piscataway, NJ, USA), and Lecithin, Soya phosphatidylcholine from Lipoid GmbH (Ludwigshafen, Germany). Soybean oil and olive oil were purchased from Sinopharm Chemical Reagent Co. Ltd (Shanghai, China). Ploxamer188 was purchased from BASF (Germany). All other chemicals were of analytical grade.
HCPT-E preparation
Oil-in-water emulsions containing HCPT (0.05%, w/v), soybean oil (5%, w/v) and olive oil (5%, w/v) as oily phase and lecithin as emulsifier (2.0%, w/v) were prepared as reported earlier (27) after some modifications.
Firstly, we dissolved HCPT and lipids (Lecithin and cholesterol, 3:1, w/w) in a mixed organic solvent of methanol and chloroform (1:2, v/v) which was then removed by the rotary evaporator under vacuum with a water bath maintained at 35℃. The lipid film formed on the flask was hydrated with pH 6.0 phosphate-buffered saline (PBS) by agitation, assisted with sonication in order to obtain a colloidal suspension. Poloxamer 188 (0.5%, w/v) and glycerol (22.5%, w/v) were then added to the colloidal suspension. Lecithin was then dissolved in the oil phase composed of soybean and olive oil at 70℃. Finally, the oily phase was added to the aqueous solution phase previously heated at 70℃ and emulsified by a homogenizer (FJ-200, Shanghai Specimen and Model Factory, Shanghai, China) at 12000 rpm for 5 min. The pH of the crude emulsion was adjusted to 5.6 by citric acid (0.1 M). The emulsion was then passed though a high-pressure homogenizer (EmulsiFlex-C5, AVESTIN, Canada) at 150000 PSI for 5 discrete volume cycles to produce a fine emulsion. The HCPT content of the HCPT-E was measured using a HPLC method.
Characteristics of HCPT-E
The droplet size and zeta potential were measured using a Malvern Zetasizer Nano-ZS90 (Malvern instruments, UK). A pH meter (PHS-3C Shanghai REX Instrument Factory, China) was used for the determination of the pH value of emulsions at room temperature (21℃ ± 2). In order to determine the physical stability of the emulsions, the emulsions were heated at 100°C for 30 min. Then, the particle size, zeta potential and pH value were measured again.
Cell culture experiment
HeLa, human cervical cancer cell line, was obtained from Medical school of Zhejiang University (Hangzhou, Zhejiang, China) and grown in DMEM supplemented with 10% (v/v) fetal bovine serum. Cells were incubated in a humidified atmosphere at 37℃ gassed with 5% CO2 in air, and subcultured every 2 days with 0.25% trypsin.
For determining the intracellular drug distribution, HeLa cells were seeded 1×107 cells/bottle and allowed for attach for 12 h. The cells were incubated with HCPT-E or HCPT-I at 13.7 μM for 0.5, 1, 2, 4, 7 and 12 h, respectively. The incubation was stopped by washing the cells thrice with ice-cold PBS (pH 7.4). Nuclei were isolated by modifying a method that employed isotonic conditions and detergent lysis (28, 29). HeLa cells were collected by centrifugation at 3000 rpm for 5 min in a centrifuge (Hermle Z323K Germany). The resulting cell pellet was washed thrice with 3 ml ice-cold PBS. Resuspended the pellet in 300µl TM-2 buffer (0.01M Tris-HCl, pH7.4, 0.002M MgCl2, 0.0005M phenylmethyl sulfonyl fluoride) and incubated at room temperature for 1 min. The tube containing cell in TM-2 buffer was incubated in ice water for 5 min. Triton X-100 was added to the suspension to a final concentration of 0.5% (v/v) and the suspension was incubated in ice water for an additional 5 min. The nuclei were isolated from the cytosol by centrifugation at 800rpm at 4℃ for 10 min. The supernatant was collected for determining the drug amount in cytoplasm. The pellet of the isolated nuclei was re-suspended in 1 ml of TM-2 buffer for determining the level of drug in nuclei.
The in vitro Cytotoxic effects of the preparations was measured by a proliferation assay utilizing tetrazolium dye, MTT (30). In brief, cells seeded into a 96-well plate at a density of 1×105 cells/well, were incubated with HCPT-I and HCPT-E at varied concentrations (10, 5, 2.5, 1.25, 0.27, 0.137, 0.069 and 0.034 μM) for different time (48-72 h). The medium was replaced by fresh medium and the cells were incubated for 4 h with 5 mg·ml-1 MTT, and then the media were replaced with 150 μl DMSO. The optical densities at 492 nm were determined using a microplate reader (SUNRISE TECAN, Austria). Cell survival was expressed as a percent of control.
Assay of HCPT
To elucidate protective effects of emulsion on the lactone ring of HCPT against its hydrolysis, fifty microliters HCPT-E or HCPT-I were incubated with 10 ml human plasma or culture medium
supplemented with 10% fetal bovine serum at 37℃ under gentle agitation at 50 rpm. Four hundred microliters aliquot was withdrawn at time interval (0, 2, 5, 10, 15, 20, 30, 60, 90, 120, 240, 300 and 360 min), dissolved in a cold organic mixture (acetonitrile: methanol =1:1) and vortexed for 5 min then centrifuged for 15 min at 4℃ at 12000 rpm. The supernatant was immediately injected into reverse-phase HPLC system for simultaneous determination of the lactone and carboxylate form ratio of HCPT as reported by Shenderova (31). Reverse-phase HPLC was carried out using an Agilent 1100 modules. The chromatographic condition was set up as follows: an Agilent Zorbax SB-C18 guard column(4.6 × 12.5mm, 5μm); Analytical column (4.6 mm × 150 mm, DiamonsilTM C18 , 5 μm); mobile phase, a 75:25 (v/v) mixture of 0.1% triethylamine-phosphoric acid buffer (pH 3.0) and acetonitrile, flow-rate, 1.0 ml/min; Diode-array detector (DAD), 384 nm; injection volume, 20 μl; temperature 350oC. The limit of quantification of the method was 100 ng/ml. The intra- and inter day variation was <14 %.
The HCPT concentration in nuclei and cytoplasm were quantified by a reversed-phase HPLC assay described previously (16). Fifty-microliter phosphoric acid was added to the isolated nuclear or cytoplasmic solutions described in ‘Cell culture experiment’, and vortexed for 3 min. The mixture was extracted with 5.0 ml of ethyl acetate, vortexed for 5 min then centrifuged for 15 min at 3500 rpm. The upper organic layer was collected and evaporated to dryness with N2 at 35℃. The residue was reconstituted in 100µl methanol, vortexed for 3 min and centrifuged for 10 min at 3500 rpm before analysis. The chromatographic condition was the same as in the determination of protective effect of emulsion on HCPT except for using fluorescence detection instead of DAD at excitation and emission wavelengths of 382 and of 528 nm, respectively.
The limit of quantification of the method was 2 ng/ml. The intra- and inter day variation was <10 %.
Microscopic studies
Morphological change of HeLa cells was observed using an optical microscope HeLa cells were grown on cover-slips to 80% confluence and incubated with HCPT-E and HCPT-I (13.7 μM HCPT) diluted in culture medium at 37°C for 4 h. Cells were then washed with PBS and incubated with drug-free fresh media for further 72 h. Morphological changes was observed and photographed at 0, 12, 24, 48 and 72h.
Statistical Analysis
The statistical analysis of experimental data was carried out using the Student’s t-test at p<0.05. Data are presented as mean ± S.D.
RESULTS
The size analysis, zeta potential measurements, polydispersity, pH values and physical appearance of HCPT-E are listed in Table 1. The results showed that the characterizations of emulsion were not significantly affected by heat process (100oC, 30min). The drug loading of emulsions was 656 µM and no crystal was subsequently detected by optical microscope at this loading level.
In previous studies, drug-free emulsion did not show any cytotoxicity in the concentration range applied (data not shown). Thecytotoxicity assay measured for HCPT using MTT proved that both the injection and the emulsion were cytotoxic against HeLa cells. HCPT-E showed higher antiproliferative activity than HCPT-I (Figure 2a). IC50 values of HCPT-E were lower than HCPT-I to about 4 and 7 times when incubation time is 48 and 72h, respectively (Figure 2b). Enhanced cytotoxicity was observed as the incubation time prolonged. Even though the incubation time for emulsion group was the two thirds of the control, IC50 of HCPT-E was lower.
Table 1. Effects of heat sterilization on physicochemical properties of sub-micro emulsion
|
Before heating |
After heating (100℃, 30min) |
Particle size (nm) |
267±3.6 |
280±4.3 |
Polydispersity |
0.0768 |
0.0545 |
Zeta potential (mV) |
-27.8±5.2 |
-26.2±7.2 |
pH |
5.61±0.04 |
5.53±0.03 |
physical appearance |
Homogenous; No creaming, no crystal observed |
|
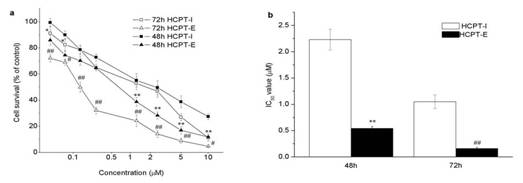
Figure 2. Antiproliferative activity of HCPT-I and HCPT-E in HeLa cells. Dose-and time-dependent cytotoxicity of HCPT (a), IC50 values ( µM ) of HCPT preparations (b). Data as mean ± S.D., n=4. *P#0.05, **P#0.01 vs. HCPT-I for 48h; # P#0.05, ## P#0.01 vs. HCPT-I for 72h.
The morphological change of cells treated with HCPT-I and HCPT-E were observed as shown in Fig.3. Cells treated with HCPT-I and HCPT-E gradually changed from the typical rhomboid–tetrahedral (Figure 3. a1 and b1) to an elongated, fibroblast-like shape (Figure 3. a5 and b4). The dramatic morphological change was observed after treated with HCPT-E and HCPT-I for 48h and 72 h, respectively. HCPT-E induced cells became round-shape and shrunk, with shrinking of the nuclear membrane, displayed morphological clue of apoptotic cell death (Figure 3. b5 ) .
The results on the protective effect of emulsions on lactone ring showed that the chemical structure was stable in oil droplets for a long time, whereas the lactone ring opening was observed only within 2 min in the presence of human plasma or culture medium in HCPT-I (Figure 4). Emulsions preserved 21.8% and 20.98 % original form in human plasma and culture medium respectively after 6 h. Whereas the results were 11.7% and 5.92% in HCPT-I. This indicates that the incorporation of HCPT into emulsions is advantageous for preserving active lactone form at a high concentration for a long period. The concentration of drug in nuclei and cytoplasm at each incubation time were shown in Figure 5a. HeLa cells exposed to HCPT-I, HCPT tends to accumulate in cytoplasm as the level were significantly higher than that in the nuclei at all prefixed times. The profiles showed an initial rapid increase of drug level both in the nuclei and cytoplasm followed by a slight increase of the drug amounts when the incubation time prolonged. Compared with HCPT-I, the drug level of nuclei and cytoplasm were obviously higher in HeLa cells treated with HCPT-E. The drug concentrations both in nuclei and cytoplasm showed a initial rapid increase, followed by a drop and then reached a platform after 4h. Surprisingly, HCPT present in nuclei was more than that in cytoplasm at all time points when HeLa cells were exposed emulsion. Significant differences were found except for 2 and 7 h. The results demonstrated that the HCPT-E may has the capability to change the distribution of HCPT in cell, and tend to accumulate in nuclei. Figure 5b is the percent of nuclear drug concentration in the total HCPT uptaken by cells. In the emulsion group, all the values exceed 50% compared with injection group below 50%.

Figure 3. Morphological changes of HeLa cells at 0, 12, 24, 48 and 72h after treating with HCPT-I (a1, a2, a3, a4 and a5) or HCPT-E (b1, b2, b3, b4 and b5) for 4h (magnification ´ 200).
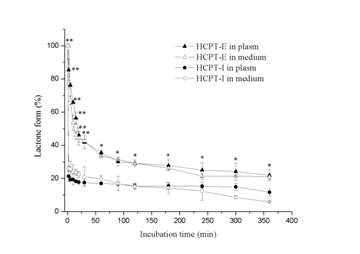
Figure 4. Preservation of lactonic ring by HCTP-E in the presence of human plasma and culture medium supplemented with 10% fetal bovine serum. Data as mean ± S.D., n=3. *P<0.05, **P<0.01 vs. HCPT-I.
Discussion
The submicro emulsion is semihomogeneous dispersion of two immiscible liquids which can be administrated intravenously for anticancer therapy. HCPT is a typically hydrophobic and unstable in water, The HCPT-E containing water insoluble HCPT in oil droplets was successfully prepared to overcome the lack of sufficient aqueous solubility and instability. The HCPT-E had small particle size, low polydispersity value, relatively high zeta potential, and appeared stable.
As shown in Fig.1, HCPT can exist as an E-ring closed lactone or an E-ring opening carboxylate form in aqueous solution depending on pH. Structure-activity studies have shown that successful inhibition of DNA topoisomeraseⅠby HCPT requires an intact lactone E-ring. Oil droplets of submicron lipid emulsion can avoid drug directly contacting with aqueous solution, which may cause the opening of the E-ring. Thus, the emulsion exerted as a reservoir of HCTP. Attributed to higher levels of lactone HCPT present in emulsion in the presence of human plasma or culture medium, stronger antiproliferative activity was achieved.
The cytotoxicity of HCPT in emulsion against the HeLa cell was provided greater than the injection. HeLa cells treated with HCPT-E for 4 h exhibited a considerable morphology change and displayed a hint of apoptotic cell death at 72 h. The enhanced cytotoxicity might be not only due to the preservation of closed E-ring in emulsion. The emulsion-based delivery systems may influence the intracellular distribution of drug (18). MTT results and morphological changes of HeLa cells indirectly indicated that the enhanced cytotoxicity might be due to large amount of HCPT with the active lactone form released from oil droplets in cells, especially in nuclei. The subcellular localization of anti-cancer agents is crucial as individual drugs are designed to exert their activities against specific subcellular targets (26).
We further determine the intracellular concentrations of HCPT. Previously, we have developed a simple, rapid, sensitive HPLC method to determine the accumulation of HCPT in HeLa cells after incubation with HCPT-liposomes and HCPT-I (16). Surprisingly, the subcellular distributions of emulsion and solution were quite different. The HCPT-E most likely to accumulate in nucleus where HCPT could exert its pharmacological activity. The drug amounts in nuclei and cytoplasm of HeLa cells exposed to HCPT-E exceeded those exposed to HCPT-I, about 18 to 33 times. The detail mechanism research is under way. The increased HCPT intracellular accumulation and preferential nuclear distribution partially attributed to the nature of emulsion (32). Many studies demonstrated that emulsion as a gene carrier may enhance gene sustained expression in nuclei (33-35). However, it has not been reported whether a variety of chemotherapeutic agents may enhance their nuclei distribution in tumor cells delivered by emulsion.
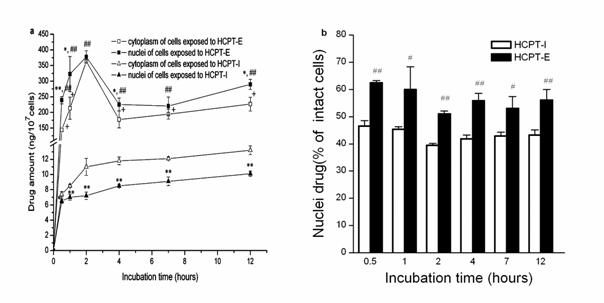
Figure 5. Intracellular distribution of HCTP-E and HCTP-I. HCTP levels in cytoplasm and nuclei (a).The percentage of nuclei drug in total uptaken drug amounts (b). Data as mean ± S.D., n=3. *P<0.05, **P<0.01 vs. cytoplasm; # P<0.05, ## P<0.01 vs. nuclei of HeLa cells incubated with HCPT-I; + P<0.01 vs. cytoplasm of HeLa cells incubated with HCPT-I.
Our results suggested that emulsion may enhance HCPT delivery to nuclei. The detail mechanism and intracellular kinetics of HCPT-Emulsions is under way.
Conclusion
The emulsion can protect the lactone ring of the HCPT and protect its loss of activity by hydrolytic degradation in medium and plasma. The HCPT-E could enhance the intracellular HCPT accumulation and change its intracellular distribution in favor of a targeting effect towards the nuclei. This would suggest that the o/w emulsion could be suitable formulation for intravenous administration of HCPT.
ACKNOWLEDGEMENTS
This study was supported in part by the Specialized Research Fund for the Doctoral Program of Higher Education of China (SRFDP No.20050335044) and the National Natural Science Foundation of China (NSFC No.30672553)