J Pharm Pharmaceut Sci (www.cspscanada.org) 10(1):9-16, 2007
EFFECT OF AGE AND GENDER ON THE PHARMACOKINETICS OF R667, A NOVEL AGENT FOR THE TREATMENT OF EMPHYSEMA, IN HEALTHY VOLUNTEERS
B Brennan, Y Chiu, L Berthelon, S Kolis, B Davies
Hoffmann-La Roche, Nutley, USA. Institut de Pharmacologie Clinique Roche, Strasbourg, France
Received January 2, 2007. Reviewed January 11, 2007. Revised February 16, 2007. Accepted February 16, 2007. Published February 19, 2007.
Corresponding Author: Barbara Brennan, PharmD 340 Kingsland St., Building 721 Nutley, NJ 07110 Telephone: (973) 235-7503 Fax: (973) 235-5635 Email: Barbara.Brennan@roche.com
ABSTRACT -- The purpose of the present study was to assess the impact of age and gender on the pharmacokinetics (PK) of R667. Thirty six healthy male and female volunteers (12 m 18-45 years; 12 m and 12 f ≥ 65 years) received a single 1 mg oral dose of R667. Serial blood samples were collected for determination of plasma R667 and metabolite concentrations. The PK parameters of R667 were similar between elderly males and young males (Cmax= 9.8 vs 9.8 ng/mL; AUC0-last=50.9 vs 47.3 ng.h/mL, respectively). Exposure of R667 increased in elderly females compared to elderly males (Cmax= 13.1 vs 9.8 ng/mL; AUC0-last= 60.8 vs 47.3 ng.h/mL, respectively). When the CL/F was corrected for BSA and V/F corrected for weight these differences were no longer evident. In conclusion these exposure differences are not considered clinically relevant, and no dose adjustment of R667 is required based on gender or age.
Introduction
Chronic obstructive pulmonary disease (COPD) is currently the fourth leading cause of death worldwide and the only leading cause of death with a continued increase in incidence[1]. Its prevalence is estimated to be approximately 14% in smokers, 7% in ex-smokers, and 3% in never-smokers[2]. COPD is characterized by the development of a progressive, irreversible airflow limitation associated with an abnormal inflammatory response in the lungs. The diagnosis of COPD includes two pathophysiologic conditions, chronic bronchitis and emphysema. The majority of patients have signs and symptoms of both conditions with one condition predominating.
The American Thoracic Society defines emphysema as the presence of permanent enlargement of the airspaces distal to the terminal bronchioles, accompanied by destruction of their walls and without obvious fibrosis[3]. The two major causes of emphysema are tobacco-induced inflammation and the disease alpha-1-antitrypsin deficiency (AATD) which causes a chronic proteinase-antiproteinase imbalance in the lung. The chronic lung injury associated with either of the above risk factors, results in the loss of lung parenchyma which reduces the alveolar surface area available for gas exchange, impairing the oxygen transfer function of the lung. Furthermore, the loss of lung matrix reduces lung elastic recoil pressure and results in diminished driving pressure of expiration, leading to airflow limitation and chronic pulmonary hyperinflation. These progressive changes have been considered irreversible and therapy has been focused on smoking cessation, symptomatic relief, and reduction of complications. Commonly used pharmacotherapy include bronchodilators and corticosteroids which only treat symptoms with limited success. There are currently no agents available that reduce the progression of the disease.
New treatments for COPD are therefore urgently needed, especially agents that are able to target the underlying destructive inflammatory processes. R667 (Figure I) is a novel pharmacologic agent currently in clinical development that has the potential to promote alveolar repair.
Potential clinical outcomes of alveolar repair in patients with emphysema include slowing of the progression of the disease and partial restoration of lung mechanical and gas exchange functions. Improvement in lung physiological functions in emphysema may be accompanied by a reduction of symptoms of dyspnea and an improvement in the quality of life. R667 is predominantly metabolized by CYP3A4 to four major oxidative metabolites (M2, M3, M4a, and M4b) all with significantly less pharmacologic activity than R667[4].

Figure 1. Molecular Structure of R667
Because it is possible to have differences in enzyme activity between different age or gender groups, the effect of age and gender on the pharmacokinetics of R667 and its metabolites was investigated in healthy subjects. Initial pilot studies indicated that there was little difference in the pharmacokinetics of R667 and its metabolites between male and female subjects or between young and elderly subjects. This study was designed to verify these data using fully validated analytical methodology for the metabolites. The primary objective of this study was to compare the pharmacokinetic profile of a single 1 mg oral dose of R667 between healthy elderly males and healthy younger males and between healthy elderly females and healthy elderly males. The 1 mg dose of R667 was selected because it was shown to be safe in preclinical and clinical studies and it is within the anticipated clinical dose range. Healthy young females were not studied because of the teratogenic potential of R667. Additionally, the effect of hormone replacement therapy (HRT), on the pharmacokinetics of R667 was evaluated in the elderly female cohort.
Materials and METHODS
Subjects
Thirty six healthy male and female volunteers were enrolled in the study, having first given written informed consent, providing they met all of the inclusion and none of the exclusion criteria. To be included in the study, subjects had to be a male between the ages of 18 to 45 years or a male or female aged 65 or older with a body mass index between 18 and 30 kg/m2. Exclusion criteria included clinically significant signs of infectious or any major illness within 4 weeks of the first dosing day, history of recurrent infections, history of clinically relevant cardiovascular, gastrointestinal, biliary, hepatic, renal, endocrine, autoimmune, hematological, pulmonary, neurological, psychiatric, allergic, or skin disorders, clinically significant asthma and/or atopic disease, clinically relevant history of alcohol and/or substance abuse, human immunodeficiency virus, hepatitis B or C virus, use of any medication within 7 days prior to study drug administration or any medication taken within 6 times the elimination half-life of the medication prior to study drug intake, whichever was longer (with the exception of HRT and acetaminophen), regular smokers (> 10 cigarettes or > 3 pipefulls or > 3 cigars per day), blood loss or donation of more than 400 mL in the previous 3 months, participation in a clinical study with an investigational drug in previous 3 months, history of drug sensitivity, triglycerides, amylase or lipase outside upper limit of normal, or semi-supine systolic blood pressure greater than 160 mm Hg or diastolic blood pressure greater than 95 mm Hg. Male volunteers had to agree to use barrier contraception during the study and for 90 days after discontinuation.
Study design
This was an open-label, parallel-group, non-randomized, single dose study that was conducted at the Institut de Pharmacologie Clinique Roche (Strasbourg, France) and was approved by an independent ethics committee (Comite Consultatif de Protection des Personnes dans la Recherche Biomedicale d’Alsace No. 1 Strasbourg). A screening examination was performed between 40 and 2 days before study drug administration. Screening included medical history, physical examination, clinical laboratory tests, vital signs, and electrocardiogram (ECG) recording. On study day 1, all subjects received a single 1 mg oral dose of R667 following a safety assessment which included ECG and vital signs measurement. Pharmacokinetic samples were taken predose and up to 72 hours after R667 administration. The subjects remained in-house from the evening of day -1 until day 2 after the 36-hour blood sample was obtained. The subjects returned to the study site on days 3 and 4 for collection of the 48- and 72-hour blood samples. The subjects also returned to the study site for a follow-up assessment between study days 9 and 16. The follow-up assessment included a physical examination, vital signs, body weight and ECG measurement. Adverse events were monitored throughout the study.
Sample Collection
Serial blood samples (7 mL) were collected in lithium heparin Vacutainers for the measurement of R667, M2, M3, M4a, and M4b concentrations predose and 1, 2, 3, 4, 6, 8, 10, 12, 16, 24, 36, 48, and 72 hours after study drug administration. The plasma was separated by centrifugation within 30 minutes of collection and decanted into plastic storage tubes. Samples were immediately frozen and stored at approximately -20 ºC for 4 to 6 hours and then at -70 ºC until assayed.
Analytical Technique for R667 and Metabolites
R667, M2, M3, M4a and M4b concentrations were determined in human plasma samples using a validated liquid chromatography turbo ion spray mass spectrometry LC/MS/MS procedure[5]. Stable labeled isotopic internal standards (IS) were used. The isotopic internal standards were: [13C 2H4]R667, [13C 15N2 2H5]M2, [13C15N22H5]M3, [13C 15N2 2H4]M4a and [13C 15N2 2H4]M4b. All analytes were isolated from human plasma samples (500 mL) by a solid-phase extraction (SPE) procedure. Sample extracts were evaporated to dryness and reconstituted in acetonitrile:methanol:water. The extract concentrates were analyzed by turbo ion spray liquid chromatography/tandem mass spectrometry (LC/MS/MS) in the positive ion mode. R667, M4a and M4b were separated using a a Chiralcel OJ-RH column with a Betabasic C18 guard column . M2 and M3 were separated using a BDS hypersil column (C18), with a Betabasic CN guard column. The lower limit of quantification (LLQ) was 0.01 ng/mL for all analytes. The calibration curve ranges were linear from 0.01 ng/mL to 2 ng/mL for all analytes.
Inter-assay precision and accuracy were assessed using quality control (QC) samples that were prepared by spiking known concentrations of the analytes into blank plasma. The precision was assessed by the coefficient of variation (CV), determined by dividing the standard deviation by the mean. The accuracy was assessed by determining the relative error (RE), determined by the formula: 100*[(mean QC concentration - nominal concentration)/nominal concentration]. The inter-assay precision of QC samples (QC1, QC2, and QC3) ranged from to 5.8 to 9.4% for R667, from 5.2 to 7.6% for M2, from 4.1 to 8.5% for M3, from 4.6 to 5.1% for M4a, and from 3.2 to 8.5% for M4b. The inter-assay accuracy ranged from 0.2 to 6.9% for R667, from -1.4 to 3.2% for M2, from -2.7 to 4.9% M3, from -5.8 to -0.5% for M4a, and from -5.6 to 1.9% for M4b.
Pharmacokinetic Methods
Pharmacokinetic parameters for R667, M2, M3, M4a, and M4b were estimated by standard noncompartmental methods using WinNonlin 4.1a (Pharsight Corporation, Mountainview, Ca.). The terminal rate constant (lz) was estimated by linear regression of the last 4 to 5 log-transformed data points. The terminal half-life was calculated as ln(2)/lz. AUC0-last was the area under the concentration time curve from time zero to the last measurable concentration calculated by the linear trapezoidal method. AUC0-∞ was calculated by the addition of Clast/lz to AUC0-last. Cmax was defined as the maximum observed concentration and Tmax was defined as the time at which Cmax occurred. The apparent oral clearance (CL/F) was calculated by dose/AUC0-∞ and the apparent volume of distribution (V/F) was calculated as (CL/F)/lz.
Statistical Methods
The primary parameters for comparability testing were the ratio of the least squares means of AUC0-last, and Cmax of R667 when administered to elderly males relative to young males and elderly females relative to elderly males. AUC0-last was used as the primary parameter rather than AUC0-∞ because the degree of extrapolation to infinity from the last measurable time point was very small (~5%). Therefore AUC0-last provided a good estimate of the extent of exposure following a single dose of R667. Other pharmacokinetic parameters of R667 and its four metabolites were considered secondary and will be presented by descriptive summary statistics. To assess the effect of age and gender on the pharmacokinetics of R667, the natural log transformed values of AUC0-last and Cmax were compared using the bioequivalence wizard in WinNonlin 4.1a for a parallel design. From this analysis, the least squares means for each treatment group, estimated treatment group differences, and associated 90% confidence intervals (CIs) were calculated. Absence of an effect was declared if the 90% CIs for the ratio of the adjusted least squares means fell within the defined limits of 80-125%.
RESULTS
Thirty-six healthy subjects met the entry criteria and were enrolled in the study. The mean age of the young male group was 31 years (range, 21 to 40), compared with a mean age of 67 years (range, 65-74) for the elderly male and female group. The mean weight was higher in the young (70.1 kg) and elderly male (76.3 kg) groups then in the elderly female (62.0 kg) group. All 36 subjects completed the study and were included in the pharmacokinetic, statistical, and safety analysis.
Pharmacokinetic and Statistical Analysis
Age Effect
The mean (± SD) pharmacokinetic parameters R667 in young and elderly males are provided in Table I. The pharmacokinetic parameters (Cmax, AUC0-last, Tmax, t1/2, CL/F, V/F) were very similar between the age groups. The mean R667 plasma concentration versus time curves in young and elderly male subjects are displayed in Figure I. The curves appear very similar between the two cohorts. Tmax was approximately 3 hours and the terminal half life of R667 was estimated to be about 10 hours, consistent with the data reported in the previous R667 studies[6] (approximately 7 to 10 hours). The intersubject variability for the AUC0-last and Cmax was about 17% to 25%.
A statistical comparison of Cmax and AUC0-last for R667 between elderly and young males is presented in Table II. The least squares means of Cmax and AUC0-last were very similar between the two groups and the 90% confidence intervals for the ratio of the adjusted means were within the range of 80% to 125%. Based on these results, the age of the subjects did not appear to have any significant impact on the pharmacokinetics of R667.
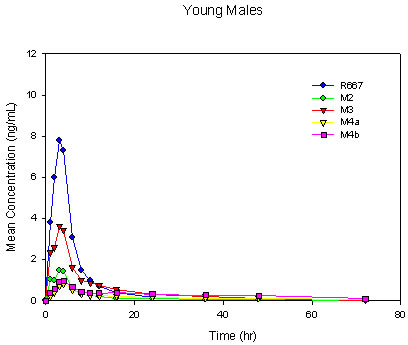
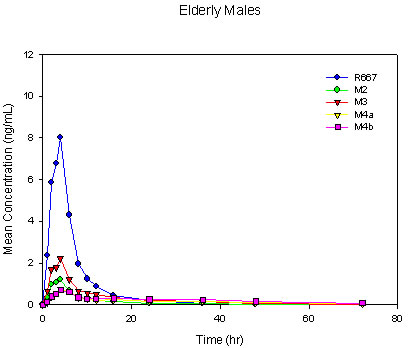
Four major metabolites, M2, M3, M4a, and M4b, were observed following a single-dose oral administration of 1 mg of R667. The rank order for Cmax was R667 > M3 > M2 > M4b > M4a in young males. The rank order for Cmax was similar in elderly males with a reverse in the order of the M4 metabolites as R667 > M3 > M2 > M4a > M4b. The rank order for AUC0-last was R667 > M3 > M4b > M4a > M2 in both the young and elderly male groups. This was slightly different from what has been seen in previous studies, in which the AUC0-last of M2 was slightly higher than that of M4a[7],[8]. However, in accordance with the previous studies, M3 remained the primary metabolite. Mean R667 and metabolite plasma concentration-time profiles for young and elderly males are shown in Figure II. Overall, the plasma concentration versus time profiles were similar for men greater than 65 years of age and men between the ages of 18 to 45 years with the exception of M3 and M4b (formed directly from M3). These metabolites were somewhat decreased in the elderly group as compared with the young group.
Table I: Single Dose R667 Pharmacokinetic Parameters
Parameter (Units) |
Study Group |
Mean ± SD |
CV (%) |
Cmax |
Young males |
9.78 ± 1.91 |
19.5 |
(ng/mL) |
Elderly males |
9.78 ± 2.48 |
25.4 |
|
Elderly females |
13.1 ± 2.95 |
22.5 |
|
|
|
|
Tmax |
Young males |
2.92 ± 1.17 |
40.0 |
(h) |
Elderly males |
3.59 ± 1.45 |
40.3 |
|
Elderly females |
2.91 ± 1.56 |
53.6 |
|
|
|
|
AUC0-last |
Young males |
47.3 ± 8.86 |
18.7 |
(ng×h/mL) |
Elderly males |
50.9 ± 9.02 |
17.7 |
|
Elderly females |
60.8 ± 10.7 |
17.6 |
|
|
|
|
t1/2 |
Young males |
11.9 ± 2.70 |
22.7 |
(h) |
Elderly males |
10.0 ± 2.57 |
25.7 |
|
Elderly females |
9.26 ± 1.93 |
20.9 |
|
|
|
|
CL/F |
Young males |
21.7 ± 4.11 |
18.9 |
(L/h) |
Elderly males |
20.3 ± 4.42 |
21.8 |
|
Elderly females |
17.0 ± 3.64 |
13.1 |
|
|
|
|
V/F |
Young males |
377 ± 146 |
38.8 |
(L) |
Elderly males |
287 ± 82.0 |
28.6 |
|
Elderly females |
221 ± 39 |
17.6 |
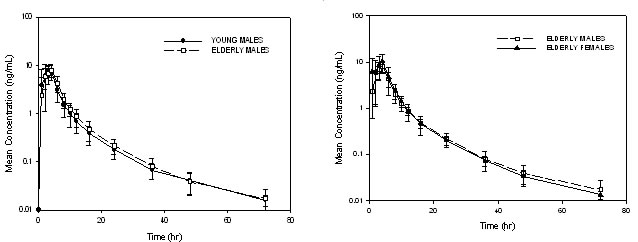
Figure I. Mean Single Dose R667 Plasma Concentrations in Elderly Males as Compared to Young Males and in Elderly Females as Compared to Elderly Males
Table II. Results of the Statistical Analysis of R667 AUC0-last and Cmax by Age and Gender
PK parameter |
Study Group |
Least Squares Means |
|
90% Confidence Intervals (%) |
Conclusion |
Cmax (ng/mL) |
Young males |
9.6 |
-- |
-- |
Equivalence Non-equivalence |
AUC0-last (ng×h/mL) |
Young males |
46.5 |
-- |
-- |
Equivalence Non-equivalence |
Gender Effect
The mean (± SD) pharmacokinetic parameters R667 in elderly males and females are provided in Table I and the mean R667 plasma concentration-time curves in elderly male and female subjects are provided in Figure I. R667 exposure was somewhat higher in elderly female subjects as compared to elderly male subjects. Cmax and AUC0-last were approximately 36% and 20% higher, respectively, in the elderly females than in the elderly males. This appeared to have resulted from a decrease in the CL/F and V/F in the elderly female subjects. Tmax occurred slightly earlier in the elderly female group (approximately 2.5 hours) than in the elderly male group (approximately 3.3 hours).
The terminal half life of R667 was estimated to be about 10 hours, a value consistent with that reported in the previous R667 studies [6] (approximately 7 to 10 hours). The intersubject variability for the AUC0-last and Cmax was about 18% to 25%.In accordance with the young and elderly males, four major metabolites were observed following a single-dose oral administration of 1 mg of R667 in healthy elderly female subjects. The metabolite profile was similar between elderly females and elderly males with the same rank order for both Cmax and AUC0-last of each metabolite. Mean R667 and metabolite concentration-time profiles for elderly females and elderly males can be found in Figure II.
..
...
..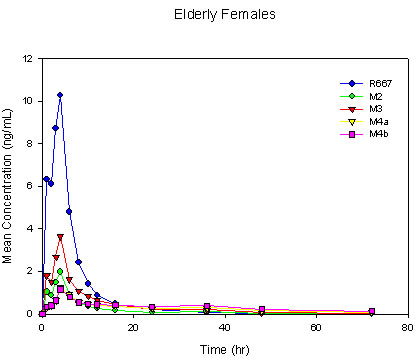
Figure II. Mean Single-Dose Plasma Concentrations of R667 and Metabolites in Young Males, Elderly Males and Elderly Females
The plasma exposure of R667 and its metabolites was higher in elderly females as compared to elderly males. A statistical comparison of Cmax and AUC0-last for R667 in elderly females and elderly males is presented in Table II. The least squares means of Cmax and AUC0-last were slightly higher in elderly females than in elderly males. Consequently, the upper limit of the 90% confidence interval fell outside the 80% to 125% bioequivalence range for both exposure parameters. This appeared to have resulted from a decrease in the CL/F and V/F values by approximately 16% and 23%, respectively in the elderly females compared with the elderly males. However, when the CL/F values were corrected for the body surface area and the V/F values were corrected for the body weight, these differences were no longer evident (Table III).
Effect of Hormone Replacement Therapy
Five of the elderly females included in the current study were receiving HRT (including estradiol, progesterone, and/or norethindrone) throughout the duration of the study. Many of these hormones are metabolized through the same pathway (CYP3A4) as R667. Table IV provides a descriptive listing of pharmacokinetic parameters for R667 and its metabolites in elderly females who received R667 in the presence and absence of concomitant HRT. Although the number of subjects was small, the exposure of R667 and its metabolites did not appear to be affected by HRT.
Table III. Normalized R667 Pharmacokinetic Parameters of Elderly Females as Compared with Elderly Males
Study Group |
(CL/F)/BSA (L/h/m2) |
(V/F)/BW (L/kg) |
|
|
|
|
|
|
|
||
Table IV. Pharmacokinetic Parameters of R667 and Metabolites in Elderly Females with and without Hormone Replacement Therapy
Analyte |
PK Parameter (Units) |
HRT Administration |
Mean ± SD |
CV (%) |
R667 |
Cmax (ng/mL) |
Alone |
13.1 ± 2.4 |
18.4 |
|
|
With HRT |
13.2 ± 3.9 |
30.0 |
|
AUC0-last (ng.h/mL) |
Alone |
63.4± 8.3 |
13.0 |
|
|
With HRT |
57.2 ± 13.5 |
23.7 |
|
|
|
|
|
M2 |
Cmax (ng/mL) |
Alone |
2.4 ± 0.9 |
39.1 |
|
|
With HRT |
2.7 ± 1.5 |
54.6 |
|
AUC0-last (ng.h/mL) |
Alone |
14.7± 5.3 |
35.7 |
|
|
With HRT |
14.7 ± 6.2 |
42.2 |
|
|
|
|
|
M3 |
Cmax (ng/mL) |
Alone |
4.5 ± 1.7 |
37.6 |
|
|
With HRT |
5.1 ± 2.9 |
58.0 |
|
AUC0-last (ng.h/mL) |
Alone |
28.7± 9.2 |
32.2 |
|
|
With HRT |
29.6 ± 13.2 |
44.6 |
|
|
|
|
|
M4a |
Cmax (ng/mL) |
Alone |
1.4 ± 0.8 |
54.5 |
|
|
With HRT |
1.4 ± 0.7 |
51.4 |
|
AUC0-last (ng.h/mL) |
Alone |
18.8 ± 5.9 |
31.3 |
|
|
With HRT |
22.3 ± 9.9 |
44.2 |
|
|
|
|
|
M4b |
Cmax (ng/mL) |
Alone |
1.2 ± 0.6 |
49.6 |
|
|
With HRT |
1.4 ± 0.7 |
52.6 |
|
AUC0-last (ng.h/mL) |
Alone |
22.6 ± 6.1 |
27.2 |
|
|
With HRT |
26.2 ± 10.8 |
41.1 |
HRT = hormone replacement therapy. |
||||
Safety
Three (25.0%), one (8.3%), and four (33.3%) subjects in the young male, elderly male, and elderly female groups, respectively experienced a total of 9 adverse events. The reported adverse events included toothache, headache, herpes zoster, back pain, myalgia, cystitis, and skeletal injury. The adverse event term “skeletal injury”, which was considered unrelated to study treatment, was used for one patient in the elderly female group. This patient fell on her buttocks and injured her coccyx on study day 7. Headache was the most frequent adverse event, which occurred in 3 subjects (2 young males and one elderly female). All these adverse events were mild or moderate with 7 of the 9 events considered remotely or possibly related to study treatment.
Discussion
In the present study, the pharmacokinetic parameters of R667 and its metabolites were very similar in the elderly and young male subjects with the exception of two metabolites, M3 and M4b, which were somewhat decreased in the elderly males as compared with the young males. However, this decrease was not considered to be clinically relevant, considering the absolute size of the difference, the relative contribution of the two metabolites to the overall plasma concentration of drug related material, and the difference in activity of the two metabolites in comparison to R667 (approximately 7% for M3 and approximately 12% for M4b).
The exposure (Cmax and AUC) of R667 and its metabolites was higher in the elderly females as compared with the elderly males. This appeared to result from a difference in the apparent total body clearance and apparent volume of distribution between the two groups. Both parameters decreased in the elderly females with apparent volume of distribution decreasing to a greater extent than the apparent total body clearance. This resulted in a slight decrease in half-life in healthy elderly females. However, when volume was normalized to body weight and clearance was normalized to body surface area, the differences were no longer evident.
The metabolite to parent AUC0-last ratios were relatively similar for both the elderly male and female subjects, indicating that much of the differences in metabolite exposures in the elderly females were accounted for by the increase in R667 exposure (data not shown). Two of the metabolites, M4a and M4b, had an increase in the AUC0-last ratio of approximately 29% each. However, due to the considerable variability in the AUC0-last ratio and the position of these metabolites at the end of the metabolic pathway, it was difficult to determine whether or not these increases represented true differences in the metabolism in the healthy elderly females compared with the healthy elderly male subjects.
Despite the fact that the pharmacokinetic differences in R667 exposure between the healthy elderly males and females were statistically significant, these differences were not considered clinically important and appeared to have resulted from differences in the body size between the elderly male and elderly female subjects.
Many of the female sex hormones included in HRT regimens are metabolized by CYP3A4. R667 is also metabolized by CYP3A4. Because five subjects in the elderly female group were receiving HRT throughout the duration of the study, this provided an opportunity to evaluate the potential for a drug-drug interaction between R667 and HRT. Although the subject numbers were small, the pharmacokinetic parameters of R667 and its metabolites appeared unchanged in the presence and absence of concomitant HRT. This result was not unexpected based on the lack of drug interaction with another CYP3A4 substrate, midazolam[9] and provides supportive evidence of the lack of drug interaction between R667 and other CYP3A4 substrates.
Conclusion
There were no clinically relevant differences in R667 pharmacokinetics between the young males and elderly males and between the elderly males and elderly females. The difference in exposure between the elderly male and elderly female subjects was a result of differences in body size between the two groups and is not considered to have clinical consequence. Therefore, no dosing adjustments are recommended for subjects based on age or gender. There were no differences in exposure of R667 or its metabolites in the presence of HRT. This provides additional evidence for the lack of a drug interaction between R667 and other CYP3A4 substrates. A single dose of 1 mg of R667 was well tolerated in all three groups, and no unexpected safety concerns were observed in any groups.