J Pharm Pharmaceut Sci (www.cspscanada.org) 10(3):358-367, 2007
Polypropylene Imine Dendrimer Mediated Solubility Enhancement: Effect of pH and Functional Groups of Hydrophobes
Umesh Gupta, Hrushikesh Bharat Agashe and Narendra Kumar Jain
Pharmaceutics Research Laboratory, Department of Pharmaceutical Sciences, Dr. Hari Singh Gour University, Sagar 470 003, INDIA.
Received, December 16, 2006, Revised, July 11, 2007, Accepted, August 8, 2007, Published August 10, 2007.
Corresponding Author: Dr. Narendra Kumar Jain Professor of Pharmaceutics Research Laboratory, University of Sagar, Email: jnarendr@yahoo.co.in
ABSTRACT - Dendrimers today are known for their three dimensional, monodispersed, highly branched, macromolecular nano-scopic architecture with number of reactive end groups. Dendrimers have been reported to act as solubilizing agents to host both hydrophilic and hydrophobic drugs. The present study was performed to investigate the effect of pH on poly(propylene) imine dendrimers (5.0G) mediated solubility enhancement of hydrophobes differing in functional groups (pKa). Weakly basic, (famotidine, -NH2 functional group; pKa 7.1), weakly acidic (indomethacin, -COOH functional group; pKa 4.5) and amphoteric (amphotericin B, -COOH and -NH2 functional groups; pKa 5.7 and 10.0) hydrophobes were selected for the study. The experiment was carried out at pH 4.0, 7.4 and 10.0. The solubility of all the drugs was enhanced at pH 7.4 and 10.0 but not at pH 4.0. The drug-dendrimer complexes followed 1:1 stoichiometry (AL type of curve) and were characterized for stability of complex, complexation efficiency and thermodynamic properties. Thermodynamic properties were utilized to elucidate the mechanism behind dendrimer mediated solubility enhancement. The data suggested that hydrophobic and electrostatic interactions were responsible for solubility enhancement. Conclusively, PPI dendrimers were found useful in solubility enhancement of not only acidic and basic but also amphoteric drugs, their solubilization ability was clearly regulated by pH and chemical nature of drug.
INTRODUCTION
Dendrimers are novel three dimensional, hyperbranched, monodisperse nanometric macromolecules obtained by reiterative sequence of reactions (1). Dendrimer’s applicability is well established in drug delivery (2-8), gene delivery (9-11) and as MRI agents (12-14). Newkome et al. in 1991 observed that dendrimers have “container” properties in the solution. Dendrimer’s amphiphillic nature i.e. hydrophilic exterior and hydrophobic interior makes them analogous to unimolecular micelles. Dendrimers are considered as static unimolecular micelles and their micellar structure remains stable at even higher concentrations of solvents (15-18). Micelle-like behaviour of dendrimers resulted into their application to solubilize hydrophobic drugs. A recent study of dendrimer mediated solubilization has been found to be superior to cyclodextrin mediated solubilization (19). Dendrimers enhance the solubility of hydrophobes probably due to hydrophobic interactions, hydrogen bonding and electrostatic interaction between terminal functional groups of the dendrimers and hydrophobes (20-22). Several hydrophobes such as nifedipine, niclosamide, ibuprofen, methotrexate, 5-fluorouracil, indomethacin, propranolol, flurbiprofen etc. have been successfully solubilized in dendrimers (2, 4, 19, 23-26). Literature suggests that PAMAM dendrimers are the most investigated dendrimers in solubilization. Poly (propylene)imine dendrimers (PPI) constitute an equally important family of dendrimers reported first by Brabander and Meijer (27) . PPI dendrimers are commercially available as AstramolTM by DSM. These dendrimers closely resemble PAMAM dendrimers (except repeating units). This indicates that PPI dendrimers also hold promise as solubilizing agents but surprisingly very few studies are available exploring PPI dendrimer’s ability in this field (28-30). Difference in repeating units in PAMAM and PPI dendrimers creates an internal microenvironment with different polarity. Alkyl chains in PPI dendrimers make the internal microenvironment less polar compared to PAMAM dendrimers, which have alkyl chains with amido groups as repeating units (31). Anticipated potential of PPI dendrimers as solubilizing agent, their greater hydrophobic microenvironment compared to PAMAM dendrimers and paucity of reports proving PPI dendrimer mediated solubilization prompted us to design a comprehensive study on PPI dendrimer mediated solubilization. It has been reported that solubilization potential of dendrimers increases with generation number. Therefore it was designed to utilize fifth generation of poly(propylene) imine dendrimers (PPI-5.0G). Three hydrophobic drugs indomethacin (weakly acidic), famotidine (weakly basic), and amphotericin B (amphoteric) were selected to investigate the effect of nature of hydrophobes on dendrimer-drug interaction (Fig.1). The mechanism behind dendrimer mediated solubilization was evaluated on the basis of thermodynamic data. Though various studies on solubility enhancement of weakly acidic and basic drugs using dendrimers are available, to the best of our knowledge, this is the first study investigating solubility enhancement of an amphoteric drug, as well.
METHODS AND MATERIALS
Ethylene diamine (EDA), acrylonirile was purchased from CDH, India. Raney Nickel was purchased from Merck, India. Amphotericin B (AmB) was obtained as generous gift from Bharat Serums and Vaccines Ltd. Mumbai, India. Indomethacin (IND) was purchased from Sigma, USA. Famotidine (FMD) was obtained as generous gift from Torrent Pharmaceuticals Ltd. Ahmedabad, India. Nylon membrane filter (0.45 mm) was obtained from Pall Gelman Sciences, USA. All other reagents and chemicals were of analytical grade and used as received. Acid phthalate buffer (pH 4.0), Borate alkaline buffer (pH 10.0) and Phosphate buffer saline (pH 7.4) were prepared according to Indian Pharmacopoeia 1996 (32).
Synthesis of PPI-5.0G Dendrimers
Poly (propylene imine) dendrimers were synthesized using repetition of double Michael addition of acrylonitrile to primary amines, followed by heterogeneously catalyzed hydrogenation of the nitriles, resulting in a doubling of the number of primary amines. In this sequence, ethylenediamine (EDA) was used as dendrimer core, but a variety of molecules with primary or secondary amine groups such as 1,4-diaminobutane etc. can also be used. The divergent approach followed in synthesis was according to a study reported elsewhere (27).

Indomethacin

Famotidine

Amphotericin B
Figure 1. Structures of the drugs.
Half Generation synthesis
Acrylonitrile (8.35 mol, 443 g), which was about 2.5 molar times per terminal NH2 group of core amine moiety, was added to a solution of ethylenediamine (1.67 mol, 100.34 g) in 1.176 kg water. The exothermic reaction caused the temperature to rise to 38oC. After this exothermic effect the reaction mixture was heated at 80oC for 1 h to complete the addition reaction. The excess of acrylonitrile was then removed as a water azeotrope by vacuum distillation (16 mbar, bath temperature 40oC). Obtained crystalline solid was 0.5 G PPI dendrimer. General formula of half generation can be represented as (EDA-dendr-(CN)4n) where n is generation of reaction or reaction cycle.
.........
Full Generation Synthesis
Hydrogenation, which is an important step during conversion of half to full generation dendrimers, was carried out in a laboratory scale catalytic hydogenator (Superfit, India). To the hydrogenation vessel, filled with Raney Nickel catalyst pretreated with hydroxide (900g) and water was added, EDA-dendr-(CN)4 dissolved in methanol. Subsequently this mixture was hydrogenated at 70oC and 40 atm hydrogen pressure for 1hr. The cooled reaction mixture was filtered and solvents were evaporated at reduced pressure. The residue contained was 1.0 G PPI dendrimer [EDA-dendr-(NH2)4]. PPI dendrimers up to 5.0 G (MW 7140.01; with 62 tertiary amine groups and 64 terminal primary amines) were prepared by repetition of all the above steps consecutively, with increasing quantity of acrylonitrile (Scheme 1). General formula of half generation can be represented as [EDA-dendr-(NH2)4n] where n is generation of reaction or reaction cycle (27).
Characterization of PPI dendrimers
Spectroscopic methods were used to characterize synthesized dendrimers. IR spectroscopy of dendrimers was carried out using KBr pellet method after adsorption of smaller amount of dendrimer on KBr pellets in Perkin-Elmer IR spectroscope. NMR spectroscopy of dendrimer samples were carried out at 300 MHz, after dissolving in D2O (Bruker DRX 300 MHz USA).
Phase Solubility Studies
Phase solubility analysis was carried out according to the method described by Higuchi and Connors (33). Excess of drugs was added separately into screw-capped vials containing different concentrations (0.3 to 3 mM) of PPI-5.0G dendrimer in buffers of pH 4.0, 7.4 and 10.0. Vials were shaken for 48 hours in metabolic shaker (Indian Equipment Corporation, Mumbai, India) at 25oC and allowed to stand for 24 hours to attain equilibrium. Solutions were filtered using nylon membrane filter of pore size 0.45 mm. The pH of the filtered solutions were determined to confirm that it remained unchanged. The samples were diluted appropriately with respective buffer solutions and analyzed in a UV Visible spectrophotometer (Shimadzu 1601 UV-Vis spectrophotometer, Japan) at 320 nm (IND), and 286 nm, (FMD) 405 nm (AmB).
Mathematical Treatment of Data:
Stability constants (Ks) for the complexes were calculated by the formula
![]()
Where, S0 is the intrinsic solubility of drug; slope was obtained from phase solubility curves. Different thermodynamic parameters were calculated using following equation (34-35).
S. No. |
Parameters |
Equation |
1. |
DG (Free energy) |
-2.303 RT log Ks |
2. |
DH (Enthalpy) |
4.606 RT log Ks |
3. |
DS (Entropy) |
DS = DH-DG /T |
Results
Synthesis and Characterization of PPI dendrimer
PPI dendrimers of 5.0 G were synthesized using ethylene diamine as core. In the initial stage synthesis of 0.5 G PPI dendrimer was confirmed through IR spectroscopy which clearly reveals the nitrile peak at 2248.2 cm-1. Upon hydrogenation of 0.5 G PPI dendrimers all the nitrile terminals got converted into EDA-(NH2)4 i.e. 1.0 G. The conversion of 0.5 G to 1.0 G was further confirmed by IR spectrum of 1.0 G PPI that gave major peaks at 3373.9 cm-1of primary amine (N-H stretch). The sequence was continued upto 5.0 G PPI dendrimers. Synthesis of PPI 5.0 G was confirmed by IR peaks for C-N stretching of CH2-NH2 (1118.3 cm-1); C-H stretching (2957.5 cm-1, 2824.2 cm-1); N-H bending vibrations of amine (1597 cm-1) and most importantly N-H stretching of primary amine (3396.5 cm-1), which confirms that most of the terminal nitrile groups of dendrimer were converted to primary amine terminals. 1H-NMR spectral peaks and shifts also further confirmed the synthesis. Peaks of alkane were obtained between 0.6- 1.25 ppm and that of secondary alkane was obtained between 1.3-1.9 ppm. Alkyl amines exhibited peaks between 2.3 and 2.9 ppm. Peak at 7.933 ppm confirmed the presence of primary amines. Results were similar to the synthesis of PPI dendrimers reported by De Brabander van den berg and Meijer (27).
Phase solubility studies
Phase solubility studies were planned and carried out at three different pH conditions i.e. at pH 4.0, 7.4 and 10.0. The pH of the solutions was observed to be same before and after the addition of dendrimers. Three hydrophobic molecules, namely indomethacin (pKa 5.7, active functional group -COOH), famotidine (pKa 7.1, active functional group –NH2) and amphotericin B (pKa 5.7 and 10.0 with both –NH2 and-COOH groups) were selected for the study. These hydrophobic drugs differ on the front of molecular weight as well. All these properties of the hydrophobic drugs selected are summarized in Table 2. All the drugs produced Higuchi’s AL type of phase solubility curve at different pH values (except at pH 4.0) (Fig. 2) and hence considered to be having 1:1 stoichiometry (33). Solution behavior of these three drugs is discussed separately as follows.
Indomethacin (IND)
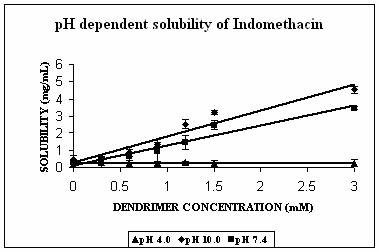
The maximum increment in solubility in case of IND was found at pH 7.4 (12 folds), followed at pH 10.0 (9 folds) and 2 folds at pH 4.0 over its corresponding intrinsic solubility (Fig. 3A). Increment was significant at pH 4.0 (p=0.0103) and extremely significant at pH 7.4 (p< 0.0001) and at pH 10.0 (p=0.0003). At pH 7.4 IND exists in almost 99% ionized form (Table 2). The protonation status of the dendrimers is reported to be a pH dependent phenomenon. At pH 10.0, PPI 5.0 G dendrimer exists in deprotonated state while at pH 4.0 it is completely protonated (Table 1). At pH 7.4 however PPI dendrimer exhibits a characteristic protonation profile (36). At this pH all primary amines and 2/3rd of tertiary amines are protonated. This could attribute an electrostatic interaction between cationic dendrimers and COO- groups of indomethacin. However, this electrostatic interaction becomes impossible at pH 10 as the dendrimer is completely deprotonated at this pH. Still at this pH 9 folds increment in solubility was observed. We propose that this could be due to hydrophobic encapsulation of indole ring of IND (Fig.1) in hydrophobic micro cavities of PPI dendrimers with COOH groups orienting away from dendrimers in aqueous environment. Stability constant of IND -PPI dendrimer complex (Table 2) at pH 7.4 was higher than that at pH 10.0 which is an indication of the highest strength of complex formed at pH 7.4. Slight increment in solubility of IND at pH 4 could be attributed to electrostatic interaction between charged –COOH and –NH2 groups of drug and dendrimers, respectively. However the extent of this interaction was limited due to the presence of only 24% of ionized IND at this pH (Table 2).
Famotidine (FMD)
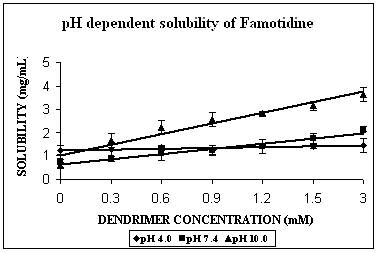
FMD remains almost completely unionized at pH 10.0 (Table 2). The solubility enhancement was maximum at this pH. It was found to be 6 folds at pH 10.0, 3 folds at pH 7.4 and 1.5 folds at pH 4.0 (Fig. 2C). In case of FMD also increment was significant at pH 4.0 (p=0.0035), and extremely significant in case of remaining two pH conditions (p< 0.0001 at both pH 7.4 and 10.0). The maximum increment in solubility of FMD at pH 10.0 could be due to both hydrophobic interactions as well as hydrogen bonding. There is a possibility of unionized FMD partitioning into favorable apolar microenvironment of PPI-5.0G dendrimers. This microenvironment however becomes considerably polar at pH 7.4 and completely polar at pH 4.0 and could be the reason behind reduced effectiveness of dendrimers in solubility enhancement at these pH values. The solubility enhancement at pH 7.4 could again be ascribed to both hydrophobic interaction and hydrogen bonding between deprotonated/unionized fractions of dendrimer/FMD. Observations of the present study are in agreement with previously reported results of the study on solubility enhancement of nifedipine in presence of PAMAM dendrimers (22). Phase solubility curve indicated 1:1 stoichiometry for FMD-PPI dendrimer complex at pH 10.0 and 7.4. A comparison of stability constants suggested that complex exhibits highest stability at pH 10.0 (Table 2).
Amphotericin B (AmB)
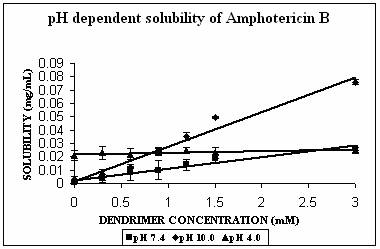
AmB has both –COOH and –NH2 groups in its structure. Its behavior was very interesting. AmB demonstrated comparable solubility enhancement at pH 7.4 and 10.0 (Fig. 3B). This increment was statistically extremely significant (p<0.0001 in both cases). However the mechanism involved could be different at different pH conditions. At pH 10.0 where increment in solubility was 25 folds, hydrophobic encapsulation of drug and hydrogen bonding between OH and NH2 groups of drug and dendrimer, respectively were thought to be responsible for solubility enhancement. At pH 7.4 solubility enhancement was attributed to electrostatic interaction between COOH groups of drug and primary amines of dendrimer. At pH 4.0, however, a relatively lesser yet statistically significant solubility enhancement was observed (p= 0.0045). This may be due to the ionization/protonation status of the interacting species, which reduces the interaction between them as compared to two other pH conditions (Table 2).
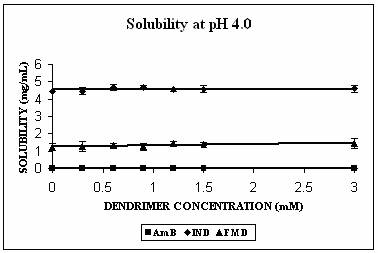
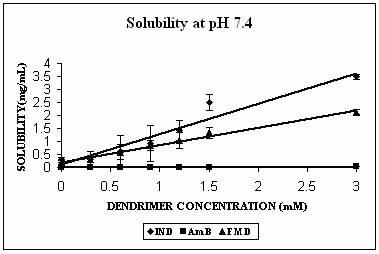
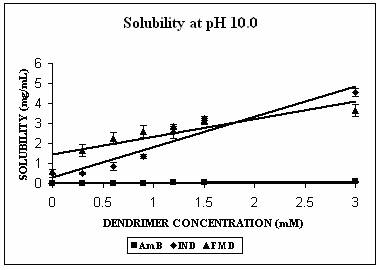
Figure 2. Solubility profiles of Indomethacin (IND), Amphotericin B (AmB) and Famotidine (FMD) at different pH.
 (A)
(A)
 (B)
(B)
 (C)
(C)
Figure 3. Solubility profiles of (A) Indomethacin (IND), (B) Amphotericin B (AmB) and (C) Famotidine (FMD) at different pH with increasing concentration of dendrimer.
Thermodynamics of Solubilization
At this stage it became apparent that either hydrophobic interaction and/or hydrogen bonding and/or electrostatic interactions are the possible mechanisms involved in solubility enhancement of selected drugs. The thermodynamic parameters of complexation could be used to ascertain the mechanism involved in solubilization (34-35). It was decided to use thermodynamic parameters as a tool for confirmation of underlying mechanism of solubility enhancement. Thermodynamic parameters calculated for different drugs at different pH conditions at 250C are tabulated in Tables 3, 4 and 5. Negative DG values for all the complexes are an indication of their stability (Table 3). Values of DG increase with increasing pH. This indicates that the system becomes more stable thermodynamically with increasing pH. With the increasing pH dendrimer becomes more deprotonated. So it can be concluded that dendrimers in deprotonated form are able to form more stable complexes with hydrophobic drugs resulting in higher solubility enhancement. A comparison of DH and TDS indicates that solubilization is an entropy driven process (Table 5). Positive values of DS (Table 4) indicated that hydrogen bonding could not be the underlying mechanism (34-35). We had earlier hypothesized that at pH 10.0 (and in case of FMD, pH 7.4), either hydrogen bonding or hydrophobic interactions were involved. Positive DS values ruled out the involvement of hydrogen bonding. This was further confirmed by spectroscopic evaluation, i.e. lmax values of drugs, which remained unchanged, even after complexation with dendrimers (21). Also, positive DS values support our inference of involvement of electrostatic interaction at pH 7.4 (34-35). Higher DS values also indicate increasing disorderliness or randomness. Any of these kinds of interactions (hydrophobic or electrostatic) are possible only after breaking ordered cluster sheets of water present around both, drug and dendrimer. This might be the origin of disorderliness, and, in turn, high DS values. A positive value also supports involvement of hydrophobic forces as hydrophobic interactions are normally characterized by a positive DH and a large DS (37-38). A positive value for DH indicated endothermic reaction (Table 5). This absorbed heat might have been used to produce randomness in ordered sheets of water.
Table 1. Protonation status of PPI-5.0 G dendrimer at different pH.
pH |
Primary amines |
Tertiary amines |
4.0 |
P |
P |
7.4 |
P |
(2/3) P |
10.0 |
DP |
DP |
P-protonated, DP- deprotonated
DISCUSSION
Dendrimers have found many diverse applications in the field of pharmaceutical sciences, solubility enhancement being one of them. PAMAM obviously remains the most investigated family of dendrimers. PPI dendrimers might also prove useful in solubility enhancement like PAMAM dendrimers. Through many reports demonstrating ability of PAMAM dendrimers as solubilizing agents are available, studies exploring similar potential of PPI dendrimers are rare. Against this backdrop it was decided to undertake thorough investigation on utility of PPI dendrimers in solubility enhancement. So, it was decided to focus on variables such as dendrimers concentration, environmental pH and nature of hydrophobic drugs. Polypropylene imine dendrimers were synthesized up to fifth generation. Dendrimers were characterized through IR, NMR spectroscopic techniques. Synthesis was in well accordance with a
Table 2. Percentage ionization of Famotidine, Amphotericin B and Indomethacin at pH 4.0, 7.4 and 10.0*
pH |
Famotidine |
Amphotericin B |
Indomethacin |
||||||
%I |
%UI |
Ks (M-1) |
%I |
%UI |
Ks (M-1) |
%I |
%UI |
Ks (M-1) |
|
4.0 |
99.92 |
0.08 |
20.44 |
1.96 (acid group) |
98.04(acid group) |
55.06 |
24.02 |
75.98 |
20.44 |
7.4 |
33.38 |
66.62 |
403.09 |
98.04(acid group) |
1.956(acid group) |
8572.86 |
99.87 |
0.13 |
403.09 |
10.0 |
0.13 |
99.87 |
4917.90 |
99.99(acid group) |
0.01(acid group) |
8341.89 |
99.99 |
0.01 |
4917.99 |
%I- percentage of ionized drug, %UI- percentage of unionized drug, Ks- stability constants (M-1) *Values are according to Henderson-Hasselbach equation
Table 3. Stanadard free energy (DG, kcal/mole) change in drug dendrimer complexation at different pH.
pH |
Indomethacin |
Famotidine |
Amphotericin B |
4.0 |
-2.04 |
-1.78 |
-2.4 |
7.4 |
-5.34 |
-3.5 |
-5.30 |
10.0 |
-5.87 |
-5.03 |
-5.34 |
Table 4. Entropy (DS, cal/mole x K) change in drug dendrimer complexation at different pH.
pH |
Indomethacin |
Famotidine |
Amphotericin B |
4.0 |
20.52 |
17.99 |
23.28 |
7.4 |
53.76 |
35.63 |
53.43 |
10.0 |
59.09 |
50.68 |
53.83 |
Table 5. Comparison of enthalpy (DH kcal/mole) and TDS (cal/mole) at different pH.
pH |
Indomethacin |
Famotidine |
Amphotericin B |
|||
DH |
TDS |
DH |
TDS |
DH |
TDS |
|
4.0 |
4.12 |
6114.96 |
3.57 |
5361.02 |
4.72 |
6937.44 |
7.4 |
10.68 |
16020.48 |
7.08 |
10617.14 |
10.62 |
15922.14 |
10.0 |
11.74 |
17608.82 |
10.10 |
15102.64 |
10.70 |
16041.34 |
similar reported method (27). Phase solubility studies were performed at three different pH conditions i.e. at pH 4.0, 7.4 and 10.0. Three hydrophobic molecules indomethacin, famotidine and amphotericin B were selected for the study because of their different chemical behavior. It was observed that solubility enhancement was governed by both pH and functional groups present in case of all the hydrophobic drugs. In case, of indomethacin the maximum increment in solubility was at pH 7.4 and electrostatic interaction was predominant mechanism of solubility enhancement. Famotidine, being a weakly basic drug, did follow a trend different from indomethacin. The maximum solubility increment in case of famotidine was found to be at pH 10.0. Hydrophobic interactions were found to be the possible mechanisms of the solubility enhancement as proved by the thermodynamic which further helped in the elucidation of the underlying mechanism of solubility enhancement. In case of amphotericin B the solubility enhancement was found to be comparable at pH 7.4 and 10.0. The underlying mechanisms were found to be possibly due to electrostatic interactions and hydrophobic encapsulations. Results clearly confirm that solubility enhancement was due to presence of dendrimers at different pH compared to their corresponding aqueous solubility’s at different pH.
CONCLUSIONS
Utility of dendrimers in solubility enhancement is well established. However, very few reports establishing direct evidence of PPI dendrimer mediated solubility enhancement are available. Present study was aimed at comprehensively establishing utility of PPI dendrimers in solubility enhancement of hydrophobes of different chemical nature. It can be concluded from the data that PPI dendrimers are useful for solubilization of not only weakly acidic and weakly basic drugs (IND and FMD, respectively in present study) but also drugs like AmB, which is amphoteric in nature. It was observed that, in general, electrostatic and hydrophobic interactions played a major role in solubility enhancement. It can also be concluded that mechanism of solubilization largely depends on protonated/deprotonated state of dendrimer. This was suggested by observation that electrostatic interactions were never involved at pH 10.0 and hydrophobic interactions appeared a rare possibility at pH 7.4. In case of all three hydrophobes solubility enhancement at pH 4.0 was negligible. At this pH dendrimer is predominantly protonated. It seems that protonated PPI dendrimers will be able to enhance solubility of only those drugs, which remain predominantly in ionized form at pH 4.0.