J Pharm Pharmaceut Sci (www.cspscanada.org) 10(3):278-287, 2007
Evaluation of skin permeation and accumulation profiles of ketorolac fatty esters
Krishna Hari Bhandari 1, Madhuri Newa 1, Sung Il Yoon 2, Jung Sun Kim 2, Dae-Duk Kim 3, Jung Ae Kim 1, Bong Kyo Yoo 1, Jong-Soo Woo 1, Won Seok Lyoo 4 , Jun Young Choi 1, Hyun Tae Lim 1, Jae H. Lee 5 , Han Gon Choi 1**, Chul Soon Yong1*
1College of Pharmacy, Yeungnam University, 214-1, Dae-Dong, Gyongsan 712-749, South Korea 2Department of Biotechnology, Dongseo University, Busan 617-716, South Korea 3College of Pharmacy, Seoul National University, San 56-1, Shinlim-Dong, Kwanak-Ku, Seoul 151-742, South Korea 4 School of Textiles, Yeungnam University, 214-1, Dae-Dong, Gyongsan, 712-749, South Korea 5 Division of Pharmaceutical Sciences, Chung-Ang University, 221, Heuksuk-Dong, Dongjak-Gu, Seoul 156-756, South Korea
Received, January 1, 2007; Revised, April 30, 2007; Accepted, May 7, 2007; Published, May 18 2007.
Corresponding Author: Dr. Chul Soon Yong, College of Pharmacy, Yeungnam University, 214-1, Dae-Dong, Gyongsan 712-749, South Korea; E-mail: csyong@ynu.ac.kr
ABSTRACT - Purpose: Classic penetration enhancement/retardation methods for improved dermal drug delivery primarily focus on co-applied chemicals aided alterations in skin accumulation/permeation profile, and in many cases, this has been achieved by compromising the systemic absorption/toxicities of penetrant/enhancer/retarder. In this study, higher dermal accumulation without systemic absorption of ketorolac and its fatty esters (esters) will be achieved by synthesizing lipophilic fatty ester soft prodrugs of ketorolac. Methods: Ketorolac decenoate (C10:1), dodecenoate (C12:1) and palmitoleate (C16:1) were synthesized and evaluated for their lipophilicity, enzymatic hydrolysis, chemical stabilities, and skin permeation and accumulation profiles using the combination of common permeation enhancing techniques such as the use of lipophilic receptor solution, enhancer pretreatment of skins, removal of stratum corneum and delipidization of skins etc. Results: Esters were highly lipophilic, chemically stable, enzymatically unstable in hairless mouse skin/liver homogenates and impermeable into the receptor solution. Conclusion: Higher dermal accumulation, absence of skin permeation, relative enzymatic stability in whole skins during permeation study and the pharmaceutical stability of esters could delineate a preliminary possibility for designing safer dermal agents with minimum potential for systemic absorption without the co-application of permeation enhancers or retarders.
Introduction
One of the major objectives of the dermal drug delivery is to attain higher dermal accumulation without significant systemic washout of the topically applied drugs. Unfortunately, most of the skin disorders requiring topical application of such protective/therapeutic medications are localized in the areas of high permeation flux, and in most cases, stratum corneum (SC) barrier resistance is decreased allowing their easier permeation to attain plasma levels that could sometimes be sufficient to elicit adverse reactions (1). On the other hand, many potential dermal drugs lack the ability to overcome SC barrier to attain higher dermal concentration. There have been many attempts to minimize the systemic absorption by the co-application of chemicals- the penetration retardants (2). At the same time, a lot of studies have been carried out to increase the dermal accumulation using chemical enhancers. However, their success rate and pharmaceutical suitability have been reported not to be very encouraging and in many cases, retardation/enhancement has been achieved by compromising systemic/local toxicities of such enhancers/retarders (3). Therefore, it would be an advantage if the systemic permeation could be minimized, or high dermal accumulation could be achieved without co-applying exogenous chemical retarders or enhancers.
It is a well known fact that the permeability barrier properties of skin are mediated by a series of lipid multilayers segregated within SC interstices, and their hydrophobic nature and tortuous, extracellular localization restrict the transport of most compounds across the SC (4). When this barrier is acutely perturbed by removing lipids with organic solvents, detergents, or tape stripping; a sequence of biological responses is initiated including accelerated epidermal lipid synthesis (5-7) that replenishes the skin lipid content which contribute to rapidly restore the skin barrier function (8). The specific requirement for epidermal lipids to create the barrier has also been delineated using the specific inhibitors of the rate-limiting enzymes of lipid synthesis (9-12), and such selective inhibition of the synthesis of one or more of SC lipids increased SC permeability to epicutaneously applied drugs (13). Topical application of 5-(tetradecyloxy)-2-furancarboxylic acid (TOFA)- the rate limiting enzymes of fatty acid synthesis- immediately after barrier disruption inhibited the early stages of barrier recovery due to deletion of the target lipid from the extracellular membranes. But, the barrier recovery was normalized, and abnormalities in lamellar body and SC membrane structure were corrected by the co-application of palmitate (C16: 0 fatty ester) with TOFA, indicating that the delay was due to the deficiency in bulk lipids (9-12). In addition, previous studies about the role of SC lipids as the determinant of the permeability barrier to the drug penetration reported an inverse correlation between the solute penetration and the total SC lipid weight (neither the number of cell layers nor the thickness of the SC correlated with the observed differences in permeability) (14-15), and showed that the enhanced epidermal lipid synthesis in SC or providing exogenous lipids resulted into a more efficient barrier function (9-13).
Although many fatty alcohols / acids / esters have been shown to act as penetration enhancers for a variety of drugs including ketorolac, their enhancing efficiency has been found to have a parabolic relationship to the degree of carbon chain saturation and/or length (16-22), which when increased beyond certain values (depending upon the nature of a penetrant) their permeation enhancing efficiency was decreased because of their decreased mobility within the skin layers due to the combined effect of correspondingly increased molecular weight, lipophilicity etc (21). Also, the permeability of highly lipophilic molecules has been reported to be low due to their accumulation in SC because of their low aqueous solubility (23-24). However, such compounds may sometime be ineffective retardants for other molecules or their self permeation could eventually increase because of their enzymatic degradation into parent drug and free enhancer molecule that may increase the permeation of the liberated parent drug and/or the original ester. Hence, in case of fatty esters (that are enzymatically hydrolysed into free fatty alcohols or acids enhancers) in addition to their lipophilicity, their enzymatic stability in skin would be an important factor for their permeation retardant effect.
Not all of the previously synthesized ketorolac ester prodrugs were efficient to enhance the permeation of ketorolac e.g. faster enzymatic hydrolysis of ketorolac [(N, N-dimethylamino) carbonyl] methyl ester than that of ketorolac ethyl ester into parent ketorolac was the reason behind the increased permeation flux of ketorolac from methyl ester (25). In our earlier studies on ketorolac alkyl ester prodrugs and amide prodrugs (26-27), it was found that their lipophilicities were proportional to their carbon chain length and good linear relationships between log P and capacity factor were observed. Similarly, parabolic relationships were found between skin permeation rate and lipophilicity indicating a possibility for designing suitably lipophilic ketorolac esters without systemic washout. However, they were enzymatically unstable. Since the lipophilicity and the stability have been reported to be improved by synthesizing fatty ester derivatives (28), which have also been shown to have high dermal accumulation (29), if suitably lipophilic and esterase stable fatty ester derivatives of a model drug (ketorolac) could be synthesized, their skin accumulation might be high with lower potential for skin permeation because of their lipophilicity and low aqueous solubility, and the application of exogenous bulk lipids in the form of fatty esters might result into a more efficient barrier function (9-12). Although a lot of earlier works have clearly shown as to how the skin permeation of drugs can be increased using free fatty alcohols/acids or esters, less attentions have been paid to synthesize and evaluate lipophilic fatty ester soft prodrugs. Soft prodrugs are metabolically stable in whole skin and might be especially useful for targeting skin with topical delivery and localized exposure (30).
Materials and METHODS
Materials
Ketorolac was purchased from Fluka (Sleeze, Germany) and its fatty esters (Fig 1) were provided by Medicinal Chemistry Lab, Dongseo University, Busan, 617-716, South Korea. They were received as light yellow oil. Their molecular weights are presented in Table 1.
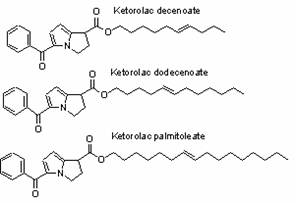
Figure 1 Structures of esters.
A specific purity study was not done because standard samples were not available. However, the compounds were purified by column chromatography and no impurities were detected by Thin layer chromatography (TLC) and High-performance liquid chromatography (HPLC). Moreover, the Nuclear magnetic resonance (NMR) spectra of the compounds did not reveal any additional peaks indicating that no significant impurities were present (data not shown- to be published in the near future). All other reagents were of analytical grade and used without further purification.
Methods
Determination of capacity factors
Capacity factors were determined as per our earlier methods (26-27) using methanol: distilled water (90:10 v/v) as the mobile phase. 90 %v/v methanol was used because of the relatively longer retention time of esters compared to ketorolac when the methanol concentration of mobile phase was less.
Determination of chemical stability
Chemical stability of the esters was determined in PG-PBpH 3, 5, 7, 9 or 11 mixtures (85:15 v/v, final pH readjusted to 3,5,7,9 or 11 using 20 % phosphoric acid or 5 %w/v sodium hydroxide solution in water). Small amount of esters (in PG) were sprinkled into the stability media, well mixed and kept in a water bath at 37 °C. At predetermined time intervals, small amounts were sampled, diluted with respective stability media, filtered and analyzed by HPLC for remaining esters and any liberated parent ketorolac. Considering the initial concentration of esters as 100 %, the remaining percentage of esters in each solution was determined as the function of time.
Enzymatic hydrolysis studies
Animals care and procedures were conducted according to the ''Guide to the Care and Use of Experimental Animal Care" (Canadian Council on Animal Care guidelines, 1984) and the study protocol was approved by the Animal Care and Use Committee, College of Pharmacy, Yeungnam University. Skin/liver homogenates were prepared by homogenizing freshly collected hairless mouse skin/liver with isotonic phosphate buffer (IPB, pH = 7.4) to obtain 25 % w/v tissue suspension for 20 minutes in an ice bath. The supernatants obtained after centrifugation for 20 minutes at 10000 X g were mixed with ester solutions of appropriate concentration in PG in 80:20 volume ratios and shaken at 37 °C. At predetermined time intervals, 0.45 mL of samples were mixed with 0.05 mL internal standard solution (flufenamic acid 1mg/mL) and 0.5 mL tetrahydrofurone (THF):acetonitrile (ACN):dimethylsulphoxide (DMSO) -30:65:5 v/v (for maximum extraction and proteins precipitation). The clear supernatants obtained after immediate mixing and centrifugation for 2 minutes at 10000 X g were analyzed by HPLC. Considering the initial concentration of esters as 100 % and that of ketorolac as 0 %, their remaining percentage in each solution were determined as a function of time. PG was used as the cosolvent and 0.01 % w/v gentamycin was used in all stability media to prevent the bacterial degradation of esters (and homogenates).
Preparation of SC free and delipidized skins
SC free skins were obtained by adhesive tape stripping of intact skins for 30 times (31-32). Delipidized skins were prepared according to the method described by Kuo et al., 1989 (32). 0.01 %w/v gentamycin was added in the buffer solution, and the extracting solution was changed every 12 hours and the extraction was performed for 48 hours to achieve more favorable lipid extraction.
Table 1 Molecular weight, capacity factor and accumulation profiles of esters and parent ketorolac released. Data are expressed as mean ± SD (n=6)
Ketorolac |
M.W. |
Capacity factor |
Accumulation of esters |
Accumulation of released ketorolac |
||||
Intact |
SC stripped |
Delipidized |
Intact |
SC stripped |
Delipidized |
|||
Ketorolac |
255.3 |
0.307 |
0.0198 |
0.0238 |
0.0365 |
0.02 |
0.0238 |
0.0365 |
Decenoate |
393.5 |
2.26 |
40.31 |
44.77 |
33.16 |
0.051 |
0.148 |
0.591 |
Dodecenoate |
421.6 |
3.263 |
2.54 |
3 |
2.03 |
0.024 |
0.045 |
0.363 |
Palmitoleate |
477.7 |
4.365 |
1.51 |
1.69 |
1.53 |
0.016 |
0.0256 |
0.275 |
Enhancer pretreatment and permeation studies
Skins were pretreated by placing 1 mL of PG in the donor compartment. The receptor compartment was filled with phosphate-buffered saline containing 0.01 %w/v gentamycin (33 mM phosphate buffer with 0.74% sodium chloride, pH 7.2) and thermostated at 37 °C. After 12 hours, the enhancer solution was removed and the remaining PG on the surface of skin was wiped off. Permeation studies were performed at 37 °C (26-27) in PG pretreated intact, SC stripped and delipidized skins using a mixture of PG: PB-85:15 v/v- final pH 7.4 containing 0.01 %w/v gentamycin as the receptor solution which was stirred at 600 rpm. Skins were equilibrated with receptor solution for 2 hours and 0.5 mL of mixture of esters in PG was used. Samples were collected at 2 hours intervals for 48 hours and analyzed by HPLC.
Skin content analysis
Skin accumulation at the end of permeation study was determined by cutting the effective permeation area of skins, which were then washed with 40 % aqueous methanol solution to remove any surface adhered materials, wiped off, dried at 40 ˚C for 12 hours, minced and homogenized in 3 mL mixture of THF: Water (2:1 v/v), centrifuged at 10000 X g for 10 minutes, 0.45 mL of the clear supernatant was processed and analyzed as per the method mentioned in enzymatic hydrolysis studies.
Drug analysis and data interpretation
Ketorolac and its esters were analyzed by Jasco P987 HPLC system equipped with UV-975 detector at 314 nm (column- C8, 5 µm, 4.6ⅹ150 mm, Inertsil, GL Science; mobile phase flow rate-1.3 mL/min; injection volume-50 µL). Ratio of the mobile phase compositions ( A- Methanol: Water: Acetic Acid-55:43:2 v/v, pH-3.0, B-Methanol 100 %) was controlled to accommodate their retention times. Lower limit of detection was 20 ng/mL, calibration curves were constructed at the concentration range of 0.02-50 µg/mL, and an excellent linearity was observed between the peak area ratios and drug concentrations over this range (r>0.993). Least-squared regression method was used to determine the regression coefficients and the equation for the best fitting line. The accuracy of the method was >90 % and coefficient of variation (CV) did not exceed 10 %. Data were compared for statistical significance by one way analysis of variance (ANOVA). The statistical significance of means was compared by multiple range method of least significant difference.
RESULTS
Capacity factors (K) are represented in Table 1. K values were proportional to the side chain length. Esters (except palmitoleate) were highly stable toward chemical hydrolysis in the tested pH range (Fig 2). They were relatively unstable in liver and skin homogenates (Fig 3). Irrespective of the test media, highest esterase stability was observed for decenoate followed by dodecenoate and palmitoleate. In skin permeation study, unlike parent ketorolac (control), none of the esters were detected in the receptor solution from all skins.
Ester accumulation in all skins was significantly higher compared parent ketorolac (P<0.0033) (Table1, Fig 4), and it decreased with the increment in K values (Fig 5). In all skins, the highest accumulation was observed for decenoate (P<0.05, compared to dodecenoate and palmitoleate). In normal and SC stripped skins, the accumulation of dodecenoate was significantly higher (P<0.004) compared to palmitoleate. There was no significant difference in the accumulation of ketorolac (P> 0.32), dodecenoate (P>0.05) and palmitoleate (P>0.05) in different skins. But the accumulation of decenoate significantly differed (P<0.05) in the order of SC stripped> Normal> delipidized skins.
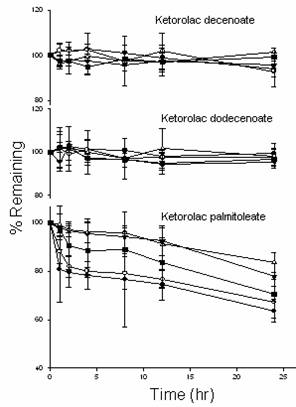
Figure 2 Stability of esters at 37 °C in the mixtures of propylene glycol: phosphate buffer solution (85:15 v/v) at different pH. Data are expressed as mean ± SD (n =3).
In general, the accumulated amounts of parent ketorolac released from the enzymatic hydrolysis of esters was highest in delipidized skin followed by stripped and intact skin, and in the same skin type, the amount of ketorolac generated was in the order of decenoate > dodecenoate > palmitoleate(Fig 4). In intact and SC stripped skins, there was a significant difference in the amount of parent ketorolac released from C10:1 and C12:1 (P<0.021), and C10:1 and C16:1 (P<0.0025), but not from C12:1 and C16:1 (P>061). However, the amount of ketorolac released from esters was not significantly different in delipidized skins (P>0.093). Similarly, the amount of parent keterolac released from C10:1 and C12:1, were significantly different in all skin types (P<0.00081 for C10:1 and P<0.043 for C12:1). But, it was not significantly different in the case of C16:1 (P>0.081).
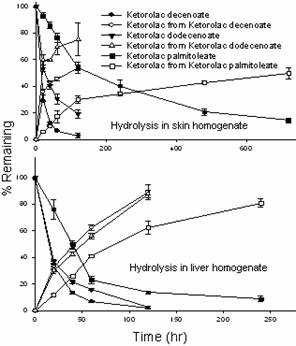
Figure 3 Enzymatic stability of esters in hairless mouse skin/liver homogenate: propylene glycol (80:20 v/v) at 37 °C. Data are expressed as mean ± SD (n =6).
Discussion
Chemical stability of esters in the tested pH range suggested their pharmaceutical stability, and their conversion into parent ketorolac in enzymatic stability tests indicated that they were prodrugs. Contrary to their hydrolysis in skin/liver homogenates, their relative stability in whole skin during permeation study implied that they were susceptible to esterase at high enzyme concentration and were soft prodrugs. This justifies the objective of this study which was to synthesize lipophilic ketorolac fatty ester soft prodrugs to evaluate their accumulation and permeation profiles.
Their relatively higher lipophilicity might have been responsible for their impermeability into receptor solution. Exogenous bulk lipid provided by ester side chains might have contributed to an increment in the total lipid proportion (9-12). Ester side chains because of their structural similarity with the indigenous fatty acids in skin, might have allied with their naturally occurring counter parts in lipid phase contributing to an increment in total lipid content, the major barrier; and/or leading to favorable molecular arrangements and interactions to create order and rigidity rather than disorder of skin lipid layer and consequently a more effective barrier (9-13). However, the exact mechanism for their inability to partition from lipid layer into the relatively lipophilic receptor medium and hence the absence of possible systemic absorption cannot be explained without further works. Their impermeability was in accordance to Goodman et al., 1989; Williams et al., 1992, who reported that the permeability of highly lipophilic molecules was low because of their accumulation in SC (23-24). High dermal accumulation of esters was in accordance to the finding of Fujii et al., 2000. While studying the effect of fatty esters on the permeation of ketoprofen through hairless rat skin, he reported significantly high skin concentrations of octyl isononanoate (C 17), octyl palmitate (C 24) and isocetyl stearate (C 34) (29).
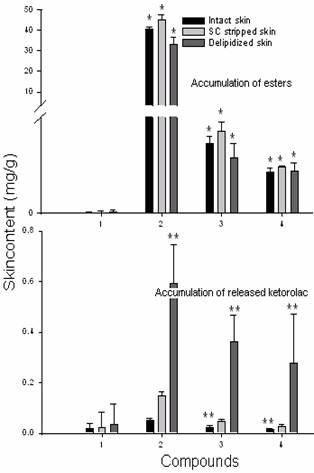
Figure 4 Skin accumulation profiles of esters/ ketorolac released from their enzymatic hydrolysis in hairless mouse whole skins during skin permeation. 1. Ketorolac (control), 2. Ketorolac decenoate, 3. Ketorolac dodecenoate, 4. Ketorolac palmitoleate. Data are expressed as mean ± SD (n =6). P<0.0033 and ** P<0.038 (Compared to parent ketorolac).
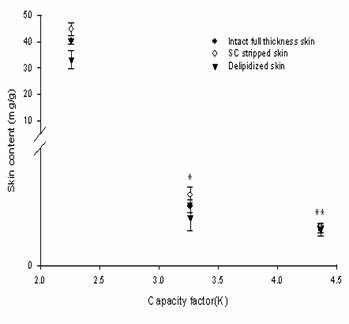
Figure 5 Skin content Vs capacity factor of esters in hairless mouse skins. Data are expressed as mean ± SD (n =6). P<0.033 and **P<0.031 compared to C10:1, (in all skins).
In order to corroborate the assumption of systemic impermeability, permeation studies were performed using the combination of proven permeation enhancing techniques for lipophilic molecules. Lipophilic receptor solution was used to achieve more favorable skin/receptor solution partition of esters (33). PG was preferred because it back diffuses into the dermis to drag the permeant and also acts as a permeation enhancer. It was appropriate to evaluate their chemical stability in a media similar to the receptor solution so that the probability of the chemical hydrolysis of esters permeated into the receptor solution during skin permeation study could be predicted. Thus, the chemical stability test medias also contained a high proportion of PG. Skins were pretreated with PG for its permeation enhancing effect (34). In addition, PG readily permeates the skin and while doing so it transports the drug molecules dissolved in it across the skin (35). Moreover, the use of this co-solvent in combination with enhancers likes fatty alcohols (in this case, the enzymatically released free fatty alcohols) or fatty esters (prodrugs themselves) offer synergistic enhancing effect for ketorolac (22, 36-38).
The order of enzymatic degradation (decenoate> dodecenoate> palmitoleate) indicated that the hydrolysis followed the side chain length. Besides contributing in stability by contributing in lipophilicity (21), increased chain length might have also caused stearic hindrance for esterase. Relative stability in whole intact skin during permeation study could be due to the relatively lower enzyme concentration per substrate (ester) molecule compared to the skin homogenate. Relatively higher rate of hydrolysis in stripped skin might be due to their better accumulation, and the even higher rate in delipidized skin might be because of the fact that they were present in aqueous phase where their interaction with esterase was easier compared to those esters in the lipid phase (intact and stripped skins). The rate of ester hydrolysis was proportional to the capacity factor. Although the exact mechanism of this phenomenon could not be established, it might be because of the facts that the esterase are soluble in aqueous phase, and for hydrolysis to occur, enzyme and substrate must be in the same phase in a close contact. Thus, the more lipophilic compounds were more stable because of their low aqueous solubility. This was in accordance to the finding of Setoh et al., 1995 (28), who reported that the modification of tetragastrin into fatty esters increased its lipophilicity and reduced its degradation in the viable skin. However, the lower rate of ester hydrolysis in whole skins during permeation study generating small amounts of ketorolac suggested that this approach might not be suitable for dermal delivery of parent compound and these esters cannot be used as true prodrugs for dermal delivery. However, on the basis of accumulation pattern of these compounds, it could be possible to design fatty esters of model drugs for dermal delivery provided that their pharmacological activity is not lost on esterification.
Since the degradation of these soft prodrugs results into free fatty alcohol (FFA) enhancer having synergistic enhancing effect with PG for the simultaneously released ketorolac (37-38), absence of ketorolac in receptor solution indicated their permeation retarding effect on the parent drug because of their lipophilicity. In addition, due to their relative stability in whole skins during permeation study, the available amount of released enhancers might have been inefficient to exert any enhancing effect for lipophilic esters or ketorolac. This was in accordance to Goodman et al., 1989; Williams et al., 1992, who reported that the compounds with high affinity to the skin lipids have low self permeation rate and they also retard the penetration of drugs (23-24). Higher accumulation in intact skins indicated their affinity for skin lipids. Similarly, their accumulation in delipidized skins also suggested their higher affinity for other skin components compared to the receptor solution. Since the hairless mouse skin expresses greater esterase activity than human skin (19), these esters would be relatively more stable in human skin and they might not be suitable for transdermal delivery of the parent drug. Though hairless mouse skin is not a precise model for human skin for percutaneous absorption, it can be used to gain insight into the general pattern and mechanisms (21).
Inversely proportional relation between the ester lipophilicity and accumulation suggested that the decrease in lipophilicity caused a favorable partition and hence higher accumulation in the skins. Although, the accumulation of decenoate in SC stripped skin was significantly higher (P<0.012) than that in intact skin suggesting that SC might have a role in its partition from donor solution into the dermal layer, accumulations of dodecenoate and palmitoleate in SC stripped skin were not significantly higher (P>0.05) than that in intact skin implying that the SC was not a major barrier for their permeation. Ester impermeability into receptor solution could be due to their accumulation in and strong affinity for dermal lipids. Thus, considering the vast skin surface area and the esters accumulation pattern, it could be possible to suitably modify the physicochemical property of a drug by synthesizing suitably lipophilic fatty esters for dermal delivery with a minimal potential for their systemic absorption.
For drugs which permeate mainly through the lipid bilayer region (intercellular pathway), similar permeability coefficients should be observed for those transported through the SC-stripped skin and delipidized skin. There was no statistically significant difference (P>0.05) in the skin accumulation of dodecenoate and palmitoleate in SC-stripped and delipidized skins suggesting that the predominant route for their passive penetration across the SC-layer to accumulate in the dermal layer could be the intercellular pathway, but it could be transcellular pathway for dodecenoate (P<0.05) (39). Lower accumulation of these esters in delipidized skin compared to intact or stripped skins suggested that these esters had relatively strong affinity for lipids compared to other components in the skin. However, their lower accumulation in SC (accumulation in Intact skin< SC stripped skin) suggested that they had high affinity for dermal lipids than the SC lipids. Significantly higher accumulation of esters in all skins (P<0.0033) compared to that of ketorolac implied that the esters were highly taken up in both lipid and proteinaceous phases. However, their high uptake in the lipid phase had no positive effects in their skin permeation because of their strong affinity for dermal lipid and other components. Although, their high uptake in the proteinaceous phase seems meaningless in this case because of their impermeability (ketorolac is prescribed for its systemic effect), it could behave as a depot in which esters are trapped making a fair possibility for the designing fatty esters for dermal delivery. Thus, further works using other model drugs that are supposed to accumulate in skin are desirable.
CONCLUSION
Relatively higher dermal accumulation and absence of permeation using the combination of common enhancing techniques, enzymatic stability in all type of whole skins during permeation studies, and chemical stability in various pH of these ketorolac fatty esters prodrugs could delineate a preliminary possibility for designing safer dermal agents with minimum potential for systemic absorption (without the co-application of permeation retarders).
ACKNOWLEDGMENT
This work was supported by Korea Research Foundation Grant (KRF-2004-005-E00003) and the Basic Program of the Korea Science & Engineering Foundation (R01-2006-000-112300-0).