J Pharm Pharmaceut Sci (www.cspscanada.org) 10(3):288-298, 2007
Transdermal delivery of hydrophobic and hydrophilic local anesthetics from o/w and w/o Brij 97-based microemulsions
Varaporn Buraphacheep Junyapraserta,*, Prapaporn Boonmea,b, Sarunyoo Songkrob, Karen Krauelc, Thomas Radesc
aDepartment of Pharmacy, Faculty of Pharmacy, Mahidol University, Bangkok 10400, Thailand bDepartment of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, Prince of Songkla University, Songkhla 90112, Thailand cSchool of Pharmacy, University of Otago, Dunedin 9001, New Zealand
Received, January 5, 2007; Revised, April 15, 2007; Accepted, May 8, 2007; Published, May 18, 2007
Corresponding Author: Assoc. Prof. Dr. Varaporn Buraphacheep Junyaprasert, Department of Pharmacy, Faculty of Pharmacy, Mahidol University, Bangkok 10400, Thailand; Phone: +66-26448677 ext. 5730; Fax: +66-26448694; E-mail address: pyvbp@mahidol.ac.th
ABSTRACT - Purpose: To characterize the physicochemical properties of drug-loaded oil-in-water (o/w) and water-in-oil (w/o) Brij 97-based microemulsions in comparison to their blank counterparts and to investigate the influence of microemulsion type on in vitro skin permeation of model hydrophobic drugs and their hydrophilic salts. Methods: The microemulsion systems were composed of isopropyl palmitate (IPP), water and a 2:1 w/w mixture of Brij 97 and 1-butanol. The samples were characterized by visual appearance, pH, refractive index, electrical conductivity, viscosity and determination of the state of water and IPP in the formulations using differential scanning calorimetry (DSC). Transdermal flux of lidocaine, tetracaine, dibucaine and their respective hydrochloride salts through heat-separated human epidermis was investigated in vitro using modified Franz diffusion cells. Results: The physicochemical properties of drug-loaded microemulsions and their blank counterparts were generally similar; however, slight changes in some physicochemical properties (apparent pH and conductivity) were observed due to the intrinsic properties of the drugs. The o/w microemulsions resulted in the highest flux of lidocaine, tetracaine and dibucaine as compared to the other formulations with in the same group of drugs. Conclusions: The characterization results showed that incorporation of the model drugs into the microemulsions did not change the microemulsion type. The permeation data exhibited that the nature of the microemulsions was a crucial parameter for transdermal drug delivery. The o/w microemulsions containing hydrophobic drugs provided the highest skin permeation enhancement. In addition, skin permeation was depended on the molecular weight of the model drugs
Introduction
Microemulsions are amongst the most useful and interesting drug delivery systems. They are optically transparent, low viscous and thermodynamically stable dispersions of oil and water stabilized by an interfacial film of a surfactant, usually in combination with a cosurfactant such as a medium-chain alcohol. In pharmaceutics, microemulsions are used as drug delivery vehicles because of their good appearance, thermodynamic stability and ease of preparation (1, 2). Numerous studies have reported that microemulsions increase skin permeation of incorporated drugs and cosmetic substances (3-16).
In a previous study by our group (17), the colloidal structures of isopropyl palmitate (IPP)/water/Brij 97:1-butanol (2:1) systems were investigated and characterized. It was found that the transition point from water-in-oil (w/o) to oil-in-water (o/w) microemulsions occurred at a water concentration of 30% to 35% w/w when the concentration of the surfactant mixture (2:1 Brij 97:1-butanol) was kept constant at 45% w/w. Therefore, it was expected that formulations containing less than 30% and more than 35% w/w water would be w/o and o/w microemulsions, respectively. However, addition of the drug into the microemulsion formulation might affect the microstructure of the system. Thus, the drug-loaded microemulsions should be characterized and compared with their blank counterparts to ascertain the microemulsion type before further investigations. In contrast to the ease of preparation, the characterization of microemulsions is complicated and requires a combination of several experimental techniques. The techniques reported for characterizing microemulsion microstructures include electrical conductivity, viscometry, differential scanning calorimetry (DSC), small-angle X-ray scattering (SAXS), nuclear magnetic resonance spectroscopy (NMR), freeze-fracture transmission electron microscopy (FF-TEM) and cryo-field emission scanning electron microscopy (cryo-FESEM) (18-28). Each technique provides different information which can be used to complement each other to determine the microemulsion type.
Although the skin permeation enhancement by microemulsions has been widely studied, the correlations between microemulsion microstructures and the efficiency of skin permeation are not fully elucidated (29). Few studies had focused on the influence of microemulsion type on the permeation of drugs having different hydrophilicity; moreover, the components in the studied microemulsions were not identical. It is acknowledged that the compositions play an important role in permeation enhancement of microemulsions. The skin permeation results of drugs from the systems having differences in drug molecular structures and/or differences in microemulsion components may not be comparable. In this study, microemulsion formulations with similar constituents but of different types (o/w or w/o) were produced and their effect on the skin permeation of three hydrophobic anesthetic bases and their respective hydrophilic salts was examined.
Three local anesthetic drugs, i.e. lidocaine, tetracaine and dibucaine and their hydrochloride salts were used as model hydrophobic and hydrophilic drugs, respectively. For topical administration, local anesthetic drugs are used to alleviate unpleasant sensations by preventing or diminishing the conduction of sensory nerve impulses near to the site of their application (30-32). Different attempts have been made to develop formulations for delivering local anesthetics to the skin including solution, microemulsion, liposome and surfactant-vesicle formulations (8, 9, 11, 33-39) in order to produce an effective nerve block in a reasonable time.
The aims of this study were to investigate the effects of incorporation of model hydrophobic anesthetic free bases and their hydrophilic hydrochloride salts on the physicochemical characteristics of o/w and w/o Brij 97-based microemulsions and to determine the skin permeation of the model drugs from the microemulsions to observe the effects of microemulsion type on the permeation of drugs with different hydrophilicity.
Materials and METHODS
Materials
All local anesthetic drugs, i.e. lidocaine (L), tetracaine (T) and dibucaine (D) and their respective hydrochloride salts (L-H, T-H and D-H) were purchased from Sigma-Aldrich (MO, USA). Brij 97 (polyoxyethylene-10-oleyl ether) and IPP were obtained from Uniquema (DE, USA). 1-Butanol was obtained from BDH Chemicals (Poole, UK). Isotonic phosphate buffer pH 7.4 (IPB) was composed of disodium hydrogen phosphate (Clarlo Erba, Rodano, Italy), sodium dihydrogen orthophosphate (BDH Chemicals, Poole, UK) and sodium chloride (Clarlo Erba, Rodano, Italy). Glacial acetic acid and methanol, which were used in the high performance liquid chromatography (HPLC) assay, were supplied from Lab-Scan Analytical Science, Bangkok, Thailand. Distilled water was used throughout the experiments. All chemicals were pharmaceutical grade and used without further modification.
Microemulsion Preparation
A pseudoternary phase diagram was constructed using the water titration method as described in a previous study (17). After the microemulsion region in the phase diagram was obtained, formulations were selected at a concentration of the surfactant mixture of 45% w/w which were likely to produce both an o/w and w/o microemulsions. For the o/w microemulsion, the concentration of IPP was 15% w/w and that of water was 39% w/w. The concentrations of IPP and water were 39% and 15% w/w , respectively for the w/o microemulsion. The o/w and w/o microemulsion formulations were designated as ME1 and ME2, respectively. The model drugs were incorporated at a concentration of 1% w/w to the microemulsion formulation. Hence, twelve drug-loaded microemulsions were prepared by combining appropriate amounts of each component (Table 1) followed by vigorous mixing with a vortex mixer for 5 min. Samples were then characterized and compared with their blank counterparts.
Table 1. Microemulsion formulations (% w/w).
|
ME1 |
ME2 |
Model drug* |
1 |
1 |
Brij 97:1-butanol (2:1) |
45 |
45 |
IPP |
15 |
39 |
Water |
39 |
15 |
*Model drugs used in this study were lidocaine (L), tetracaine (T) and dibucaine (D) and their hydrochloride salts (L-H, T-H and D-H, respectively).
Centrifugation
Thermodynamic stability of the samples was studied by ultracentrifugation using a 70Ti rotor (Optima L-70, Beckman Instruments, USA) at 40,000 rpm (or 117,734 × g) for 30 min at 4°C.
pH Measurements
The apparent pH of the formulations was measured by a pH meter (Accument AB15, Fisher Scientific, USA) in triplicate at 25°C.
Refractive Index (RI) Measurements
The refractive indices of the formulations were determined using a refractometer (Fisher Scientific, Japan) in triplicate at 25°C.
Conductivity Measurements
Electrical conductivity of the samples was measured using a Riac CM/100 conductivity meter fitted with an YSI 3418 electrode (Yellow Springs Instruments, USA), having a cell constant of 0.11 cm-1. The measurements were carried out in triplicate at 25°C.
Viscosity Measurements
Viscosity of the samples was measured using a Brookfield DV-III programmable cone and plate rheometer (Brookfield Engineering Laboratories, USA) fitted with a CP-42 cone spindle. Brookfield Rheocalc operating software was used to control the rheometer. The jacketed sample cup was connected to a circulating water bath operating at 25°C. A sample volume of 1 ml was used for viscosity measurements and the experiment was repeated in triplicate.
Differential Scanning Calorimetry (DSC)
DSC measurements were performed with a differential scanning calorimeter TA Q100 equipped with a refrigerated cooling system (TA Instruments, USA). Nitrogen with a flow rate of 50 ml/min was used as a purge gas. Approximately 4 to 13 mg of sample was weighed precisely into hermetic aluminum pans. An empty hermetically sealed pan was used as a reference. Samples were cooled from
25°C to -50°C at a cooling rate of 5°C/min, held for 3 min at -50 °C and then heated to 25 °C at a heating rate of 10°C/min. All measurements were preformed at least in triplicate.
In Vitro Skin Permeation Studies
Human skin tissue was kindly provided by Yanhee General Hospital, Bangkok, Thailand. Skin samples were obtained from abdominoplasty surgeries from Asian healthy women ranging in age from 26 to 57 years. All obtained skin samples were abdominal. Patient donor details, age and nationality, were recorded upon receipt of the skin. The study was carried out with the approval of the Committee on Human Rights Related to Human Experimentation, Mahidol University, Bangkok, Thailand.
After subcutaneous fat was removed with surgical scissors, the epidermis was separated from the underlying dermis using a heat separation technique (40). The skin was immersed into warm water at 60°C for 2 min. Consequently, the epidermal layer was carefully separated from the dermis using blunt forceps. The obtained epidermis was wrapped with aluminum foil and stored at -20°C until used. The stored epidermis was allowed to thaw, cut into 4.5 x 4.5 cm2 pieces and hydrated by placing in IPB overnight before use (41).
In vitro skin permeation studies were performed using modified Franz diffusion cells (Crown Bioscientific, USA). The diffusion area of the cells was 1.77 cm2 and the receptor compartment volume was 12 ml. The diffusion cells were connected with a circulating water bath and the temperature was controlled at 37°C. IPB was used as a receptor fluid and stirred by an externally driven Teflon-coated magnetic bar. The heat-separated human epidermis was placed on the receptor compartment with the stratum corneum facing upwards and then the donor compartment was connected with a clamp. One ml of microemulsion formulation was applied onto the skin surface of each donor compartment. The donor compartment was covered with a glass lid and the sampling arm was covered with the aluminum foil. At suitable time intervals (0, 1, 2, 3, 4, 6, 8, 10, 12, 24 h), an accurate amount (0.5 ml) of each sample was withdrawn from the center of the receptor compartment with a syringe connected with a needle. An equal volume of fresh IPB (37°C) was replenished immediately. The amount of drug in the receptor fluid was assayed by HPLC. At least six replicates using skin samples from different donors were performed for each drug-loaded microemulsion.
The flux of drug from each microemulsion was calculated and compared with that from the other microemulsions. The cumulative drug permeation (Qt) was calculated from Equation 1:
![]() (1)
(1)
where Ct is the drug concentration of the receptor fluid at each sampling time, Ci is the drug concentration of the ith sample, and Vr and Vs are the volumes of the receptor fluid and the sample, respectively. Data were expressed as the cumulative drug permeation per unit of skin surface area, Qt/S (S = 1.77 cm2). The steady-state fluxes (JSS(6 – 24 h)) were calculated by linear regression interpolation of the experimental data at steady state (between 6 and 24 h, DT) as shown in Equation 2:
![]() (2)
(2)
HPLC Assay
The concentrations of model drugs permeated to the receptor fluid were quantitatively analyzed by HPLC (Shimadzu, Japan) consisting of an auto injector SIL-10A (Shimadzu) at a flow rate of 0.8 ml/min of mobile phase, a pump (Shimadzu, LC-10AD) and peak detection by UV (Shimadzu, SPD-10AV). A reversed phase Chrompack Inertsil ODS-3 column, 5 mm particle size, 250 x 4.6 mm (The Netherlands) was used as stationary phase. A mixture of 10% acetic acid in water and methanol (75:25 v/v, 65:35 v/v and 50:50 v/v for L/L-H, T/T-H and D/D-H, respectively) was used as a mobile phase. The injection volume was 20 ml. The samples were detected at 254 nm and integrated using the RF 10A (version 1.1) LC software program. The calibration curve (peak area versus drug concentration) was constructed by running standard solutions of each model drug in IPB for every series of samples. Validation of the method was performed to ensure that the calibration curve between 1 and 50 mg/ml of all model drugs was in the linearity range of the assay and coefficients of variation were less than 5%, both intra-day and inter-day.
Data Analysis
The characterization data were expressed as the means of three experiments ± S.D. The skin permeation data were expressed as the means of at least six experiments ± S.E.M. One-way analysis of variance (ANOVA) and Tukey’s multiple comparison test were used to compare fluxes of different formulations and a P value of 0.05 was considered to be statistically significant.
Results
In Figure 1, the shaded area of the pseudoternary phase diagram of IPP/water/Brij 97:1-butanol (2:1) refers to the microemulsion region while the outside area indicates multiphase turbid regions. Based on the phase diagram, two representative microemulsion formulations (referred to as ME1 and ME2, Table 1) were selected from the microemulsion region to be incorporated with the model drugs for further studies.
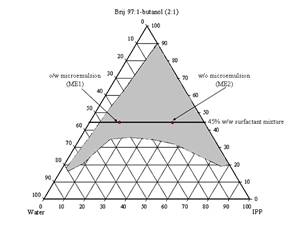
Figure 1. Pseudoternary phase diagram of the IPP/water/Brij 97:1-butanol (2:1) and representative microemulsion formulations.
Table 2. Physicochemical characteristics of the microemulsions; n = 3.
Formulation |
pH |
Conductivity |
Refractive |
Viscosity |
Rxy |
ME1 |
5.94 ± 0.04 |
191.69 ± 0.34 |
1.4044 ± 0.0005 |
9.47 ± 0.11 |
0.9998 |
ME1_L |
7.69 ± 0.03 |
237.60 ± 2.20 |
1.4042 ± 0.0004 |
10.57 ± 0.23 |
1.0000 |
ME1_T |
8.16 ± 0.01 |
200.75 ± 0.22 |
1.4050 ± 0.0006 |
10.30 ± 0.17 |
1.0000 |
ME1_D |
8.09 ± 0.02 |
189.05 ± 0.23 |
1.4052 ± 0.0016 |
14.27 ± 0.06 |
1.0000 |
ME1_L-H |
5.23 ± 0.04 |
548.90 ± 7.70 |
1.4034 ± 0.0002 |
8.65 ± 0.31 |
0.9999 |
ME1_T-H |
5.46 ± 0.03 |
500.50 ± 1.10 |
1.4047 ± 0.0005 |
8.66 ± 0.19 |
1.0000 |
ME1_D-H |
5.41 ± 0.01 |
561.37 ± 1.68 |
1.4051 ± 0.0002 |
9.44 ± 0.89 |
0.9999 |
ME2 |
6.32 ± 0.12 |
23.91 ± 0.23 |
1.4289 ± 0.0007 |
6.52 ± 0.04 |
0.9999 |
ME2_L |
7.54 ± 0.03 |
27.13 ± 0.17 |
1.4260 ± 0.0005 |
11.63 ± 0.29 |
1.0000 |
ME2_T |
8.11 ± 0.03 |
25.37 ± 0.06 |
1.4275 ± 0.0006 |
11.37 ± 0.23 |
1.0000 |
ME2_D |
7.99 ± 0.05 |
22.62 ± 0.72 |
1.4266 ± 0.0006 |
10.83 ± 0.12 |
1.0000 |
ME2_L-H |
5.18 ± 0.01 |
81.29 ± 0.33 |
1.4268 ± 0.0005 |
10.16 ± 0.33 |
1.0000 |
ME2_T-H |
5.57 ± 0.04 |
82.46 ± 0.34 |
1.4269 ± 0.0002 |
10.28 ± 0.33 |
0.9999 |
ME2_D-H |
5.66 ± 0.04 |
79.49 ± 0.28 |
1.4265 ± 0.0003 |
9.43 ± 0.43 |
0.9999 |
All twelve drug-loaded Brij 97-based microemulsions and their blank counterparts were clear yellowish liquids. No birefringence was detected under the cross-polarized light microscope and no phase separation was found after ultracentrifugation at low temperature.
Table 2 exhibits the physicochemical characteristics (apparent pH, refractive index, conductivity, apparent viscosity and correlation coefficients (Rxy) for Newtonian flow behavior) of the drug-loaded Brij 97-based microemulsions and their blank counterparts. Incorporation of the model drugs affected the apparent pH of each formulation due to the acid-base properties of each drug. It can be seen that incorporation of the model drugs in base form slightly increased pH values while incorporation of their respective salts slightly reduced pH values. The refractive indices of all drug-loaded microemulsions were similar to those of their blank counterparts. The refractive indices of o/w microemulsions were lower than those of w/o microemulsions due to the lower refractive index of water as external pseudophase (1.3336 ± 0.0004) compared to that of IPP as external pseudophase (1.4378 ± 0.0003). The o/w microemulsions provided higher conductivity values than the w/o microemulsions due to the conductivity properties of the aqueous external pseudophase. The model drugs in free base form did not affect the conductivity as compared to the blank counterparts while the drugs in salt form led to an increased conductivity. The salt forms were expected to dissociate in the presence of water and thereby causing an increase in conductivity of the nonionic microemulsions. The apparent viscosity values of the microemulsions were generally low and correlation coefficients (Rxy) between shear rate (x) and shear stress (y) were close to or equal to 1 indicating Newtonian flow behavior, as expected for microemulsions (19, 42). Incorporation of the model drugs slightly affected the viscosity of the microemulsions; however, it did not change the general flow behavior.
When water is present in a microemulsion system, it can be either free or bound water depending on the state of the system. Bulk (free) water is assumed to have physicochemical properties similar to those of pure water. Bound water on the other hand is strongly influenced by the surfactants present in the samples and its properties will differ from those of pure water, i.e. the presence of a nearby surfactant alters its thermodynamic properties such as freezing point, melting point, enthalpy and heat capacity (24, 43). In the ME1 group, the freezing peak onsets of water were observed at -28 to -21 °C and the melting peak onsets of water were observed at -7 to -4 °C, indicating free water in o/w microemulsions as shown in Figure 2. In the ME2 group, the freezing peak onsets of IPP were found at 5 to 6 °C and the melting peak onsets of IPP were found at 6 to 8 °C as shown in Figure 3, and samples are referred to w/o microemulsions. The DSC curves of drug-loaded microemulsions and their blank counterparts were generally similar; however, slight shifts in the thermal events were found. The drugs might have had some effects on the surfactant film or on interaction between surfactant and vehicle molecules; however, they did not cause a change in microemulsion type. In addition, it could also be noted that no samples showed a freezing exotherm or a melting endotherm at the same positions of those of pure water or pure IPP. The partial miscibility of surfactants and 1-butanol with water or IPP is a likely reason for this (17, 44).
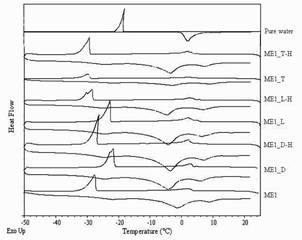
Figure 2. DSC curves of pure water, drug-loaded and blank o/w Brij 97-based microemulsions.
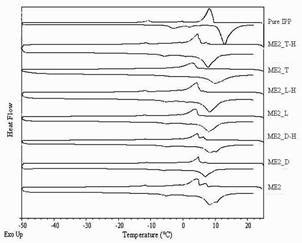
Figure 3. DSC curves of pure IPP, drug-loaded and blank w/o Brij 97-based microemulsions.
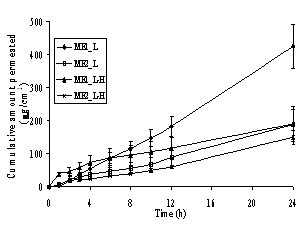
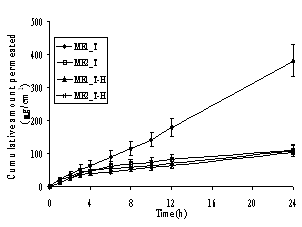
In Figure 4, a steady increase in the amount of permeated drug in the receptor compartment with time was observed in the in vitro permeation profiles of the model drugs through heat-separated human epidermis from the Brij 97-based microemulsion vehicles. Interestingly, all three groups of the model drugs provided similar patterns of skin permeation profiles. The o/w microemulsions of model hydrophobic drugs gave the highest amount of permeated drug whereas the other three formulations gave similar and lower amounts of permeated drug.
In Table 3, the flux values demonstrate that the o/w microemulsions of the model drugs in base form provided significantly the highest flux (P<0.05) when compared within the same group of drugs. The fluxes of other three formulations were not significantly different (P>0.05), but lower than for the o/w microemulsion of the base form. In addition to the nature of the microemulsions, the molecular weight of the model drugs also affected the skin permeation fluxes from Brij 97-based microemulsions. The molecular weights of the model drugs are in the rank order of L (MW 234.3) < T (MW 264.4) < D (MW 343.5) and L-H (MW 270.8) < T-H (MW 300.8) < D-H (MW 379.9) (32). In Table 3 it can be seen that for the results of the permeation studies within the same type of microemulsion (o/w or w/o), the means of fluxes of the investigated drugs tended to be in the rank of L > T > D and L-H > T-H > D-H. Although the differences did not reach statistical significance (P>0.05), it should be noted that the fluxes appeared to be inversely related to the molecular weight of the drugs.
(A)
(B)
(C)
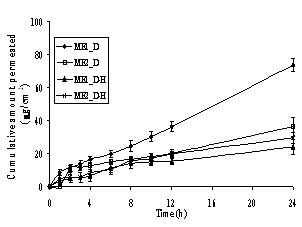
Figure 4. Permeation profiles of the model drugs through human epidermis from the o/w (ME1) and w/o (ME2) Brij 97-based microemulsions: (A) lidocaine (L) and its salt (L-H), (B) tetracaine (T) and its salt (T-H) and (C) dibucaine (D) and its salt (D-H). Each data point is the mean (± S.E.M.) of 6 to 8 experiments
Table 3. Skin permeation fluxes of the model anesthetic drugs; 6 ≤ n ≤ 8.
Microemulsion |
Jss (mg/h/cm2)* |
ME1_L |
21.45 ± 2.24 (7) |
ME2_L |
8.11 ± 2.36 (8) |
ME1_L-H |
5.82 ± 1.11 (7) |
ME2_L-H |
6.68 ± 1.19 (8) |
ME1_T |
16.42 ± 2.25 (7) |
ME2_T |
2.80 ± 0.81 (6) |
ME1_T-H |
3.08 ± 0.44 (7) |
ME2_T-H |
3.44 ± 0.35 (7) |
ME1_D |
3.04 ± 0.20 (8) |
ME2_D |
1.21 ± 0.28 (8) |
ME1_D-H |
0.65 ± 0.13 (7) |
ME2_D-H |
0.98 ± 0.08 (7) |
Discussion
It was observed that the area of IPP/water/Brij 97:1-butanol (2:1) microemulsion in the pseudoternary phase diagram (Figure 1) was quite large since 1-butanol acted efficiently as a cosurfactant interacting with the surfactant monolayer and increasing the flexibility of the interfacial film (45).
One of the methods that is generally used to differentiate a microemulsion system from a liquid crystal is to observe the samples under the cross-polarized light microscope. Birefringence is not found in microemulsion but is observed in lamellar and hexagonal liquid crystals (46, 47). In addition, thermodynamic stability is one of important properties of microemulsions. In this study, the results indicate that all samples did not show birefringence and remained transparent single-phase liquids after centrifugation, indicating presence of microemulsion systems.
From the study of physicochemical properties of drug-loaded microemulsions compared with their blank counterparts (Table 2), the results from different experimental techniques agreed well and indicated that the types of Brij 97-based microemulsions were not altered after incorporated with 1% w/w of the model drugs. However, slight changes in some physicochemical characteristics, i.e. pH and conductivity, were observed because of the intrinsic properties of the model drugs.
In the in vitro skin permeation studies, it was demonstrated that the amount and the fluxes of all hydrophobic free bases released from the o/w microemulsions were the highest, while those of other three formulations in the same group of drugs were almost equal and significantly lower (Figure 4 and Table 3). Previously, some possible mechanisms of action of microemulsions in the increment of flux of a drug into skin have been proposed. Three possible mechanisms of action of microemulsions in estradiol flux enhancement were discussed (12). Firstly, microemulsions act as drug reservoirs where loaded drug is released from the inner pseudophase to the outer pseudophase and finally further into the skin. Secondly, microemulsion droplets might break down on the surface of the stratum corneum and then release their contents into the skin. Thirdly, permeation of loaded drug occurs directly from the droplets to the stratum corneum without microemulsion fusion at the stratum corneum. The last mechanism has been frequently supported by findings of other groups (13, 16) and indicates that the enhancement effect of microemulsions is caused by the nano-sized droplets dispersed in the continuous phase which can move easily into the stratum corneum and carry the drug through the skin barrier. The hypothesis may also explain the findings in the present permeation study. According to the permeation of drug-loaded microemulsion droplets attributing to the permeation enhancement effect, the oil droplets of the o/w type might permeate into the epidermis easier than the water droplets of the w/o type at the same surfactant concentration owing to the lipophilic nature of the stratum corneum. The oil can enter the hydrophobic tail of the stratum corneum bilayer, perturb it by creating separate domains, and induce highly permeable pathways in the stratum corneum (48).
In addition, a hydrophobic drug is preferentially encapsulated in the oil droplet and the highly drug loaded droplets favor partitioning into the epidermis, resulting in the highest flux. This phenomenon confirms that the oil droplet nature of an o/w microemulsion is a crucial factor for flux of drugs, especially hydrophobic substances. The results were in agreement with previous reports which indicated that the o/w microemulsions provided higher membrane fluxes of diclofenac diethylamine (42) and ketoprofene (44) than the w/o microemulsions while bicontinuous microstructure hampered the drug release.
Furthermore, it was found that drugs with lower molecular weight could permeate from microemulsions into the epidermis to a greater extend than those with higher molecular weight. In general, the drug with lower molecular weight have a higher drug mobility or diffusion coefficient and subsequently higher permeation rates through intact epidermis (49). A predictive rule is that the maximum flux of drug through the skin should decrease by a factor of 5 for an increase of 100 Daltons in molecular weight (50).
The results from the present study lead to the conclusion that both microemulsion types and intrinsic properties of drugs affected the skin permeation of the drugs from the Brij 97-based microemulsions.
ACKNOWLEDGMENT
The authors are grateful for financial support received from the Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (PHD/0146/2544). Yanhee General Hospital, Bangkok, Thailand is thanked for supply of skin tissues used for the in vitro skin permeation studies.