J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 2(3):108-112, 1999
Antitumoral activity of new pyrimidine derivatives of sesquiterpene lactones
Manuscript
Received September 20th 1999, Revised December 16th, 1999; Accepted
December 30th, 1999.
Angelina
Quintero1, Araceli Pelcastre and
José Dolores Solano
Facultad
de Química. Universidad Nacional Autónoma de México. Ciudad
Universitaria 04510 Coyoacan. México D.F., México
Angel
Guzmán and Eduardo Díaz
Instituto
de Química. Universidad Nacional Autónoma de
México. Circuito Exterior
Ciudad Universitaria 04410, México, D.F. México
PDF Version for printing
Abstract
Sesquiterpene lactones display a wide variety of biological effects such as antiviral, anti-inflammatory and cytotoxic activity. In previous studies some derivatives of sesquiterpene lactones were prepared to be tested as antiviral and/or cytoxic agents. In the present report we describe the effects of seven modified sesquiterpene lactones on the proliferation of several cancer cell lines. We demonstrated antitumoral activity of two of them: III (JLNZ-106) and IV (EDAG-IV-Sme) in HeLa, C-33, CALO, INBL, VIPA, SW480, SW620, MCF-7 and CHO cancer cell lines. Compounds III (JLNZ-106) and IV (EDAG-IV-Sme-IV) presented cytotoxic activity (IC50) by inhibiting the incorporation of 14C-thymidine to DNA. These experiments suggest that derivatives III and IV should inhibit DNA replication in cancer cell lines.Introduction
Sesquiterpene lactones are natural products, which show very interesting biological activities (1, 2, 3), for example, methyleneactocine or dehydrolichestenic acid present antibiotic and antitumoral effects. In addition, ivalin acetate has shown antileukemic properties. Some sesquiterpene lactones isolated from Arnica species were tested on human carcinoma cell line as well as on COLO 320, a human colorectal cancer cell lines, where they showed cytotoxic activity (3). Evidence that these compounds exhibit considerable antitumoral activity on Ehrlich ascites, on Walter 256 carcinosarcoma and against leukemia, has been presented which suggest these compounds inhibit DNA synthesis in cancer cells (3). As well, the Michael addition of dipeptide L-Cys-L-Ala-OCH3 to alantolactone yields compounds which shown interesting biological activities has been described (4). Here we describe the cytotoxic activity (5, 6) of seven new derivatives (I-VII) synthesised from dehydrocostus lactone, ivalin acetate and zaluzanine diacetate whose structures were previously published (7, 8).
Materials and Methods
Chemicals:
The following
compounds derived from the corresponding parent sesquiterpene lactones
were used in this study: I (JLNZ-105), II (IVAG-nMe), III (JLNZ-106), IV
(EDAG-IV-Sme), V (JLNZ-110-2), VI (JFG-96), VII (IVCYT-I). DMSO,
tetrazolium (MTT), trypsin, EDTA and taxol were purchased from Sigma (St.
Louis, Mo, USA) and used as received.
Cells lines and media: The cell lines used here were: two human colorectal cancer cell lines: SW-480, SW620; four cervical cell lines: HeLa, C-33, INBL and VIPA¨; the breast cancer cell line: MCF-7; and the ovarian cell line: CHO. VIPA and INBL were maintained in RPMI and all the other cell lines were maintained in D-MEM (both culture media were purchased from GIBCO, BRL, Gaithersburg, MD USA) with non essential amino acids, supplemented with 10% (v/v) heat inactivated fetal calf serum (GIBCO BRL, Gaithersburg, MD USA) and incubated at a humidified atmosphere (5% CO2, 95% air) at 37°C in a Forma Scientific incubator model 3110.
The monolayer cultures were subcultured in a 0.25% of trypsin/EDTA solution. After 24 h in cell culture, the compounds were added using different concentrations taxol (Sigma St. Louis Mo, USA) was used as positive antitumoral control (0.25µM) and was dissolved in DMSO. All experiments were carried out in triplicate at 72 h after the drugs were added.
Cytotoxic
assay: The cytotoxic assays were
performed according to the microculture MTT method (7, 9). Briefly, cells
were harvested (4.5 to 5.0 x 104
cells/mL/well) and inoculated in 24 well microtiter plates. The cultured
cells were then inoculated with and without the compounds, which were
dissolved in DMSO and added in a volume maximum of 2 µL/mL/well. After 72
h incubation, 100 µg/mL of MTT (in PBS, pH 7.2) was added as well as 1 mL
of DMSO to each well, followed by gentle shaking to solubilize the
formazan dye. After centrifugation the extinction coefficient was measured
at 540 nm using a Beckman photometer model DUR-64. Cell growth
inhibition was calculated by means of the formula: % inhibition =
(1-absorbency of treated cells/absorbency of untreated cells) X 100.
14C-thymidine incorporation into DNA: Experiments of incorporation of 14C-thymidine to in vitro DNA synthesis in cancer cells lines with and without studied compounds were carried out according to the technique described by Terrazas et al (10). Briefly, 200 µl of the cell suspensions (approximately 4.5 to 5.0 x 103 ) were placed into a 96 well flat bottom culture plates (Costar, Cambridge, Massachusetts) and incubated for 24 h at 37 oC, in 5% CO2. Then the sesquiterpene lactones were added and incubated for 72 h. Before culture termination, 0.2 µCi of 14C-thymidine (Amersham 56 mCi/ mmol) was added to each well. After incubation, the cells were harvested onto a glass filter paper using Skatron instruments and processed for liquid scintillation counting (Beta-plate scintillation counter Wallac instrument). The experiments were carried out in triplicate.
Results and Discussion
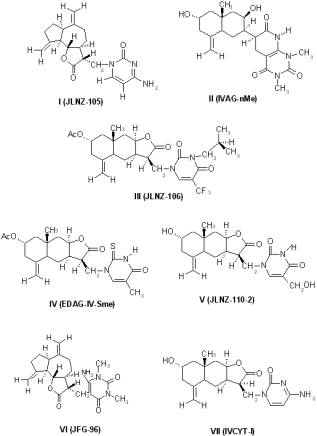
Seven sesquiterpene lactones derivatives were previously synthesised (5, 6). These derivatives show structure modification at: i) the chiral centre at C11 (I, VI); ii) the lactone moiety (removed in II); iii) the pyrimidine moiety (modified at C-13, III, IV, V, VII). (Figure 1).
Figure 1. Structures of new Sesquiterpene lactones: I (JLNZ-105), II (IVAG-nMe), III (JLNZ-106), IV (EDAG-IV-Sme), V (JLNZ-110-2), VI (JFG-96) and VII (IVCYT-I).
In the present work we describe the cytotoxic activity in several cancer cell lines using the MTT technique (7, 8).
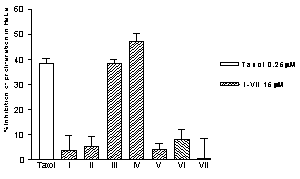
The new compounds were tested for activity in HeLa, C-33, CALO, VIPA, SW 480, MCF-7 and CHO cancer cell lines. Two out of the seven compounds tested, III (JLNZ-106) and IV (EDAG-IV-Sme), showed toxicity against the cancer cell lines used. The inhibition of proliferation of HeLa cells is depicted in Figure 2.
Figure 2. Inhibitory effect of drugs on HeLa cell proliferation. Control: cells with 2µL of DMSO, taxol (0.25 µM) as antitumoral positive control and the compounds assayed. Culture cells (1.0 x 104 cells/ml/well) were inoculated with 15µM of the drugs (in volumes maximum of 0.8µl of DMSO). After 72 h incubation, 100 mg/ml of MTT was added, incubated at 37°C and after the formazan was dissolved, the absorbency was measured at 540 nm. Results are the mean of three determinations and are expressed as % of inhibition of cell proliferation.
The IC50 measurements of the cytotoxic activity for the modified sesquiterpene lactones were 16.4 mM in HeLa cells and 40.0 mM in SW620 cells for compound III and 30.3 mM in HeLa cells and 42.0 mM in SW620 cells for compound IV (Figure 3 and Table 1). From these findings we could observe a low differential activity of compounds III (JLNZ-106) and IV (EDAG-IV-Sme) on the human cancer cell lines used. Derivative III (JLNZ-106) showed a higher toxicity against some of the cancer cell lines tested than drug IV (EDAG-IV-Sme) the in HeLa and C-33 cell lines (Table 1). For the other cell lines tested the cytotoxic activity was almost the same for both compounds; it was about 100 times lower than taxol, the compound used as a positive control in the experiments.
Figure 3. Comparison of IC50 of taxol, compounds III (JLNZ-106) and IV (EDAG-IV-Sme) on HeLa and SW620 cell lines. Each plot is the mean of three determinations.
In spite of the lower activity of these compounds, they are good leads for further chemical modifications, which, we hope, will give us more potent compounds. In addition, preparation of the derivatives is easy and the starting lactones are readily available. These features constitute an important advantage over taxol isolation. So far, we are engaged in the synthesis of other derivatives from other sesquiterpene lactones.
Moreover, we carried out assays of 14C-thymidine incorporation into DNA in the cancer cell lines in order to evaluate whether there is inhibition of cell growth (at the DNA level) by drugs III (JLNZ-106) and IV (EDAG-IV-Sme). The results of these experiments showed inhibition of 14C-thymidine incorporation into DNA (Table 2). From these experiments we concluded that compounds III (JLNZ-106) and IV (EDAG-IV-SMe) inhibit DNA replication.
The precise cellular target affected by sesquiterpene lactones has been previously described (11,12) and is related to the alkylation of different enzymes sites containing sulphydryl groups, which may interfere with their functions. It is known also that sesquiterpene lactones inhibit a large number of enzymes involved in key biological processes such as DNA and RNA synthesis, purine synthesis, glycolisis, and citric cycle and mitochondrial electron transport chain (11,12). Further work is required to fully elucidate the mechanism of action of the new modified compound described herein.
TABLE 1. Cytotoxic activity of the novel compounds III (JLNZ-106) and IV (EDAG-IV-Sme) in human cancer cell lines.
|
Cancer Cell Line |
III(JLNZ-106) |
IV(EDAG-IV-SME) |
Taxol |
|
SW480 |
73.50 |
80.40 |
0.312 |
|
SW620 |
40.90 |
42.70 |
0.25 |
|
HELA |
16.40 |
30.30 |
0.025 |
|
C-33 |
16.90 |
37.60 |
0.045 |
|
INBL |
28.10 |
39.10 |
0.09 |
|
VIPA |
42.89 |
45.80 |
0.022 |
|
MCF7 |
25.16 |
28.25 |
0.03 |
The IC50 is expressed in mM. The result shows the compound concentration producing 50% of growth inhibition assessed by the MTT assay. Each experiment was carried out in triplicate.
TABLE 2. Inhibition of 14C-thymidine incorporation into DNA by compounds III (JLNZ-106) and IV (EDAG-IV-Sme) in cancer cell lines.
|
Cancer Cell Line |
III(JLNZ-106) |
IV(EDAG-IV-SME) |
Taxol |
|
SW480 |
73.55± 2.66 |
65.37±3.69 |
60.00±1.51 |
|
SW620 |
60.19±1.95 |
80.08±1.85 |
79.99±1.04 |
|
HELA |
78.17± 2.77 |
91.46±3.72 |
91.02±1.18 |
|
C-33 |
75.00±3.71 |
61.35±4.80 |
66.36±1.00 |
|
INBL |
66.74±0.70 |
55.39±2.69 |
62.05±2.16 |
|
VIPA |
70.27±2.95 |
61.46±0.19 |
60.52±1.21 |
|
MCF7 |
57.81±1.84 |
79.79±3.58 |
61.00±1.31 |
|
CHO |
61.00± 1.08 |
70.12±1.78 |
66.05±0.61 |
Concentration
of compounds III (JLNZ-106) and IV (EDAG-IV-Sme) (50 µM), and taxol (0.25µM)
in each experiment. The results are expressed as % of the inhibition of 14C-thymidine
incorporation in DNA in relation to the control (without drug). Each
experiment was carried out in triplicate.
Acknowledgements
Thanks to Dr. Ignacio Terrazas for his technical advice with respect to the 14C-thymidine incorporation assay. This work was supported by grant DGAPA IN200598.
We are grateful to Dr. Ignacio Camacho Arroyo for discussion and comments of the manuscript.
References
-
Fei Liou, Y., Hall, I.H., Lee, K., Williams, W.L. and Chaney, S.G. Investigation of sesquiterpene lactones as protein syntheses inhibitors of P-388 lymphocytic leukemia cells. Biochemica et Biophysica Acta 739:190-196, 1983.
-
Lyss, G., Schmidt, T.J., Merfort, I. and Pahl, H.L. Helenalin an anti-inflammatory sesquiterpene lactone from Arnica, selectively inhibits transcription factor NF-kappa. J. Biol. Chem. 378: 951-960, 1997.
-
Woerdenbang, H.J., Merfort, I., Pabreiter, C.M., Schmidt, T.J., Willuhn, G., Van Uden, W., Pras, N., Kampinga, H.H. and Konings, A.W.T. Cytotoxicity of flavonoids and sesquiterpene lactones from Arnica species against the GLC4 and the COLO320 Cell lines. Planta Medica 60: 434-437, 1994.
-
Ginanneschi, M., Chelli, M., Papini, A.M., Pinzani, D. and Rapi, G. Structure of the adduct of Alantolactone with (Z)-L-Cys-Ala-OCH3; H1-C13 assignment of the alantolactone moity by NMR at 14 T. Magnetic Res. Chem. 34:95-97, 1996.
-
Alley, M.C., Scudiero, D.A., Monks, A., Hursey, M.L., Czerwinski, M.J., Fine, D.L., Abbott, B.J., Mayo, J.G., Shoemaker, R.H. and Boyd, M.R., Feasibility of Drug Screening with Panels of Human Tumor. Cell Lines Using a Microculture Tetrazolium Assay. Cancer Res. 48:589-601,1988.
-
Hall, I.H., Lee, K.H., Mar, E.C., Starnes, C.O., Waddell, T.G. Antitumor agents. A proposed mechanism of inhibition of cancer growth by tenulin and helenalin and related cyclopentenones. J. Med. Chem. 20: 33-37, 1977.
-
Diaz, E., Barrios, H., Nava, J.L., Chavez, I. and Guzmán, A., Fuentes, J.F. and Fuentes, A.B. 2D NMR studies of the structures of the stereoselective adducts of the dehydrocostus lactone with pyrimidine derivatives. Spectroscopy Letters 31: 51-61, 1998.
-
Diaz, E., Nava, J.L., Barrios, H., Quiroz, B., Guzmán, A., Leon, L., Fuentes, A. 2D 1H and 13 C NMR evidence for stereoselective formation of new bond C-N, C-S or C-C in the reaction of ivalin acetate with substituted pyrimidines. Spectrochimica Acta 54:567-574, 1998.
-
Carmichael, J., Mitchell, J.B., DeGraff, W.G., Gamson, J., Gazdar, A.F., Johnson, B.E., Glasttein, E. and Minna, J.D. Chemosensivity testing of human lung cancer cell lines using the MTT assay. Br. J. Cancer 57: 540-547, 1985
-
Terrazas, L.I., Bojalil, R., Govezensky, T., and Larralde, C. Shift an early protective TH1-type immune response to a late permissive TH2-type response in murine cystecercosis (Taenia crassiceps). J. Parasitol. 84:74-78, 1998.
-
Beekman, A.C., Woerdenbang, H.J., Vvan uden, W., Pras, N., Konings, A.W.T., Wikstrom, H.V., Schmid, T.J. Structure-cytotoxicity relationships of some Helenanolide-type sesquiterpenes lactones. J. Nat. Prod. 60:252-257, 1997.
-
Page, J.D., Chaney, S.G., Hall, I.H., Lee, K.H. and Holbrook, D.J. Inhibition of inosine monophosphate dehydrogenase by sesquiterpene lactones. Biochemica et Biophysica Acta 926: 186-194, 1987.
¨Correa,
I. et al. 1988. Estradiol effect on p53, E6 of HPV and bcl2 expression
in human cancer cervix cell lines. In: XXII National Congress of the
Mexican Biochemistry Society. Pp 169. México D.F. México
Corresponding author: Angelina Quintero, Facultad de Química. Universidad
Nacional Autónoma de México. Ciudad Universitaria 04510 Coyoacan. México
D.F., México. pache@servidor.unam.mx
Keywords: sesquiterpene lactones pyrimidine derivatives,
cancer
cell lines, antitumoral activity.
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps