J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 3(2):259-266, 2000
Colonization and Immune Responses in Mice to Helicobacter pylori Expressing Different Lewis Antigens
M. R. Suresh, M. B. Fanta, J. Kriangkum,
Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta,
Edmonton, Alberta, Canada
Q. Jiang, D. E. Taylor
Department of Medical Microbiology and Immunology, Faculty of Medicine,
University of Alberta, Edmonton, Alberta, Canada.
Manuscript received January 31, 2000, Revised July 18, 2000; Accepted August 10, 2000.
PDF version for printing
ABSTRACT
BACKGROUND AND AIMS: A mouse model was established to compare colonization by the H. pylori Sydney strain SS1 with several clinical isolates expressing different Lewis antigens on their surface. In addition, both humoral and cell mediated immune responses were determined for different H. pylori strains.
METHODS: Mice were inoculated intragastrically separately with the Sydney strain as well as with five clinical isolates of H. pylori expressing different Lewis (Le) antigen phenotypes. Colonization of the mouse stomach by the bacteria was monitored from two to fourteen weeks post inoculation by four independent methods namely, urease, PCR (using CagA primers), bacterial culture and histology. Antibody titers and cellular immune responses were monitored by ELISA and antigen stimulation test respectively.
RESULTS: Different degrees of colonization were observed in C57, CD1 and Balb/c mice inoculated with H. pylori strain SS1 (Lex, Ley) and clinical isolates UA948 (Lea, Lex), UA861 (α-glucosyl polyLacNAc), UA1258 (Ley), UA802 (Ley) and UA1264 (no Le antigen) starting from week two post inoculation. All three mice strains mounted high immune responses against different H. pylori antigens. Treatment of mice with vancomycin prior to inoculation has no effect either on colonization of the stomach or the immune response of the mice. Histological evaluation established colonization after 10 weeks post inoculation but not gastritis.
CONCLUSIONS: Stomach of mice can be colonized consistently, with H. pylori strain SS1, and colonization was also achieved with all clinical isolates that were not mouse adapted. These strains could be detected more consistently by PCR in the early stages, then by culture only after 8 - 10 weeks. In our study, Lewisx expressing bacterial strain (UA948) failed to colonize Balb/c mice, whereas the Ley expressing strain (UA1258) did not colonize C57/BL6 mice.
Introduction
The role of H. pylori in the development of peptic ulcers has become increasingly clear since it was first reported in 1983(1). It is now recognized as the cause of chronic gastritis in humans, one of the major casual factor in peptic ulcer disease and is associated with an increased risk of gastric adenocarcinoma or gastric lymphoma(2-4). H. pylori is a spiral or curved Gram-negative microaerophilic bacterium which colonizes the human gastric mucosa. The organism is found in the mucus layer and adheres to the surface epithelium of the stomach(4).
To study and develop novel therapeutic and/or preventive agents, animal models which are inexpensive, easily reproducible have been developed including the use of gnotobiotic piglets, dogs, monkeys, cats, mice, and rats(6-17). Problems associated with these models such as low infection rates, establishing germ-free environment coupled with difficulty of handling animals in large numbers hinders the optimization and standardization of the models(7). Work of Lee et al.(16) provided a mouse model of H. pylori infection and introduced the Sydney strain (SS1), a mouse adapted strain. The standardized Sydney strain gives consistent colonization, although the pathology following H. pylori infection does not precisely mimic human gastritis.
H. pylorii express Lewis antigens on their surface as part of their lipopolysaccharide(18). We used immunoelectron microscopy to demonstrate the presence of Lewis X antigen (Galb®4[Fuca1®3] GlcNAc) [Lex] on the H. pylori surface(19). Lewis Y [Ley] and other Lewis structures, Lea and Leb, have also been identified(20). Since gastric epithelial tissue also expresses Lex, Ley, Lea and Leb on its surface, H. pylori mimics these human antigens(21). It has been suggested that this molecular mimicry may relate to efficient colonization of gastric epithelial cells by H. pylori (22,23). The aim of the present study was to compare the ability of H. pylori , expressing different Lewis antigens, to colonize a mouse model. The humoral and cell mediated immune responses to H. pylori expressing different Lewis antigens were also assessed. All studies were performed during early phases of colonization.
Materials and Methods
Bacterial Growth
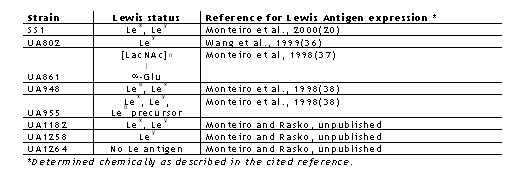
A frozen H. pylori strain SS1 was initially obtained from A. Lee, University of New South Wales, Sydney, Australia and strains UA861, UA802, UA948, UA1258, UA955, UA1182 and UA1264 were from the H. pylori collection of clinical isolates maintained at the University of Alberta, Edmonton, Alberta, Canada (Table 1).
Table 1: Helicobacter pylori strains used in this study.
Strains were plated onto Brain Heart Infusion (BHI) agar plates supplemented with 5% defibrinated sheep blood and were incubated for 2 days as described previously(24). The colonies were removed from the plates, suspended in Brucella broth containing 5% Fetal Bovine Serum (FBS), 15mg/ml vancomycin and 15mg/ml ampicillin and incubated microaerobically (5% carbon dioxide, 10% hydrogen and 85% nitrogen) overnight at 37°C with agitation on a rotary shaker at 100 rpm. The cultures were checked by phase contrast microscopy to ensure their purity, and were centrifuged at 10,000 rpm for 20 minutes and suspended in Brucella broth containing 30% glycerol to an OD660 of 1.
Inoculation of Mice
The C57/BL/6NCr1BR male mice, age 6 to 8 weeks, were obtained from Jackson Laboratory (Bar Harbor, Maine). The CD1 male mice, age 6 to 8 weeks, were obtained from Charles River Canada (St. Constant, Quebec) and the Balb/c male mice, age 6 to 8 weeks, were obtained locally from Health Sciences Laboratory Animals Services (HSLAS) of the University of Alberta, Edmonton. Animal treatment and care were carried out according to the guidelines of the HSLAS of the University of Alberta. All mice were fasted overnight before inoculation and prior to euthanasia. Mice were then given 108 colony-forming units (cfus) of H. pylori in 100ml of Brucella broth containing 30% glycerol through gastric gavage using a 22G gastric gavage needle. The same treatment was repeated after 3 and 5 days. Mouse stomach was removed and opened through the lesser curvature using sterile surgical instruments and cut into small pieces. Colonization of a given mouse was assessed from two to fourteen weeks post inoculation by urease test, bacterial culture, PCR and histology of stomach tissue. To study the effect of vancomycin on the colonization of H.pylori mouse stomach, C57 mice were treated with vancomycin solution in water at a concentration of 15mg/ml for 10 days. The mice were then orally inoculated with H. pylori SS1 strain. Similarly, untreated mice were also inoculated with the same strain.
Polymerase chain reaction (PCR)
DNA was isolated from stomach tissues by using QIAamp Tissue Kit (QIAGEN INC. Santa Clara, CA) following the manufacturer's protocol. For the PCR analysis, H. pylori CagA primers (5'AGTAAGGAGAAACAATCA 3' and 5'AATAAGCCTTAGAGTCTTTTTGGAAATC 3') were used (25). PCR was performed at 94°C for 50 s, at 50°C for 45 s, and at 72°C for 1 min. in a Perkin-Elmer model 480 thermocycler. Each PCR product was subjected to electrophoresis on an agarose gel. Positive samples contained a 1.37kb band that was visualized under UV light.
Bacterial culture
Stomach samples from the inoculated mice were homogenized with PBS and a portion of the homogenates were placed on agar plates and incubated at 37°C under microaerobic conditions. The presence of H. pylori was checked after 3 to 5 days of incubation. The culture was identified as H. pylori based on morphology and production of urease(24).
Humoral immune response
Blood was collected from each mouse before sacrificing, and the antibody titer was measured by direct Enzyme-Linked Immunosorbent Assay (ELISA). Briefly, a 96 well microtiter plate (Nunc, Denmark) was coated with 1 mg/ml mixture of different strains of H. pylori whole bacterial antigens (see Table 1) and incubated overnight at 4°C. The plate was washed and incubated with 3% milk in PBS for 1 hr at room temperature to block nonspecific binding. The plate was washed three times with PBS containing 0.1% Tween-20 (PBST) and incubated with 100ml of the serum samples at a dilution of 1:100 for 1 hr at room temperature. After washing, 100ml of a 1:1000 dilution of goat anti-mouse IgG (whole molecule) HRPO conjugate or 1:16000 dilution of goat anti-mouse IgM (m chain specific) peroxidase conjugate or 1:20000 dilution of goat anti-mouse IgA (a chain specific) peroxidase conjugate (Sigma, St. Louis, Mo.) were separately added and incubated for another 1 hr at room temperature. Following incubation, the plate was washed three times with PBS containing 0.1% Tween-20 (PBST) and 100ml of TMB peroxidase substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD.) was added as a chromogen. Absorbance values at 405 nm were measured in a Vmax kinetic microplate reader (Molecular Device Corp., California, USA).
Cell mediated immune response
Spleens were removed from mice inoculated with H. pylori strain UA948, UA1258 and UA1264 following euthanasia and single cell suspension was prepared by grinding the tissue with a plunger on a cell strainer in the presence of 10 ml RPMI 1640 media (Gibco-BRL, Burlington, Ontario, Canada).The single cell suspension was centrifuged at ~500xg for 7 min and the cell pellet was resuspended in 5 ml of ACK lysing buffer (8.29 g NH4Cl, 1 g KHCO3 and 37.2 mg Na2EDTA in 1 L of water adjusted to pH 7.4) to lyse erythrocytes and then incubated for 5 min at room temperature and washed three times with RPMI 1640 media. The cells were plated in 96-well microtiter plates (Costar, cambridge, MA) in triplicate at a concentration of 105 cells/well in RPMI 1640 media containing 10% FBS (Gibco-BRL) with or without antigen or mitogen. The cells were incubated for 5 days at 37°C and 5% CO2 and pulsed with 1mCi [3H] thymidine (Amersham, Arlington Heights, IL) per well for 18 hrs of incubation. Cells were then harvested on to a Printed Filtermate A (glass fiber filter), size 90 X 120 nm (Wallac Oy, Turku, Finland) using a Harvester (Tomtec, Hamden, CT.). A Melt-on Scintillator Sheet size 73 X 109 nm (Wallac Oy, Turku, Finland) was melted on the filtermate, allowed to cool and sealed into a plastic cover. Thymidine incorporation was measured using a Microbeta Trilux Reader (Wallac, Turku, Finland). The stimulation index was calculated by dividing the CPM counts obtained from the antigen stimulated cells by the CPM counts obtained from the unstimulated cells.
Histology
At 10 week post inoculation, one-half stomach sample of a CD1 mouse and a control mouse stomach were fixed in 10% formalin and embedded in paraffin. Subsequently the samples were processed by standard histochemical techniques and hematoxylin, Eosin, Giemsa and Warthin Starry stained slides were prepared. The stained samples were examined for the presence or absence of H. pylori and/or other bacteria under a microscope(24).
Results and Discussion
Colonization of mice by Strain SS1
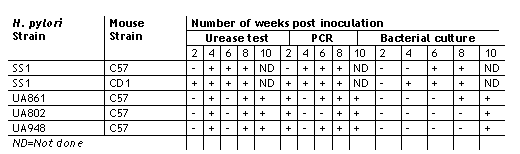
In this study the ability of H. pylori to colonize C57 and CD1 mouse stomachs at a bacterial inoculum of 108 cfu was demonstrated. Colonization of the gastric mucosa was investigated by all of the three methods described above, i.e. urease test, PCR and bacterial culture and was subsequently confirmed by histology. Fasting the mice overnight prior to inoculation appear to facilitate colonization and allowed us to eliminate gastric food contents as a factor in this study. During fasting the acid secretion of the stomach is low. Since the bacteria are sensitive to acid in humans they presumably colonize the stomach during a time period when the stomach is hypochlorhydric which can occur during infection or poor nutrition(26). We found that the urease test is the most rapid detection method, however the sensitivity is low. Consistently positive urease test results were obtained after 4 weeks of inoculation in both C57 and CD1 mice (Table 2). Even though the colonization of the stomach by H. pylori was demonstrated at earlier stages by both the urease test and by PCR (Table 2 and Fig 1), the bacteria were not able to be cultured. Positive bacterial cultures were obtained only after 4 and 6 weeks of inoculation for CD1 and C57 mice respectively.
In case of Balb/c mice consistent colonization was not observed by any of the three methods (data not shown). A fourteen week colonization study of C57 mice by H. pylori SS1 strain was also performed. Ten mice were sacrificed at week 6, 10 and 14 and colonization was assessed as described above. In our experiments, colonization tended to decline after 10 weeks post inoculation (Fig 2).
Table 2: Time course of colonization of C57 and CD1 mice fed with H. pylori SS1 and other H. pylori clinical isolates.
Figure 1: Agarose gel electrophoresis of PCR products from the cagA gene(25): Lane 1, 2, 3 and 4 show DNA from C57 mouse stomach at week 8 post inoculation; Lane 5, 6, 7, and 8 show DNA from CD1 mouse stomach at week 4 post inoculation and Lane 9 and 10 show PCR from DNA of H. pylori SS1 strain not used to infect mice.
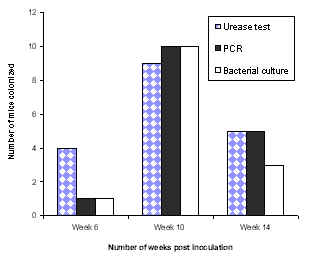
Figure 2: Colonization of C57 mice inoculated with SS1 strain.
Effect of vancomycin treatment
In our early mouse colonization experiments, we observed Lactobacillus-like rod shaped organisms along with H. pylori in histology. At least one report suggests that Lactobacillus can inhibit or interfere with H. pylori infection(27). Hence, we investigated if vancomycin could enhance colonization of the stomach by H. pylori since Lactobacilli were susceptable to this antibiotic. This would also allow us to study the colonization of one species without interference from the other. Vancomycin treatment of mice before inoculation showed no significant effect on colonization ability (data not shown). The pattern of colonization, as shown by the urease test, PCR and bacterial culture was similar in both the treated and untreated groups of mice. In both cases, 80% to 100% of the mice were colonized by the bacteria irrespective of the antibiotic treatment..
Colonization of mice by H. pylori clinical isolates
As shown previously by Lee and co-workers, we have observed consistent colonization of C57 mice by Helicobacter pylori SS1 strain(16). We further investigated to test if other clinical isolates expressing a variety of Lewis antigens can also colonize these mice. We inoculated them with UA948, UA802 and UA861 expressing known surface carbohydrate antigens, which had undergone many passages in vitro. All three strains showed positive urease test results after week 4 post inoculation. Variable PCR positivity was observed 2 weeks after inoculation. Positive bacterial cultures were, however, observed after week 8 (Table 2) consistent with our previous observations with SS1 strain. These results indicate that the mouse stomach can be successfully colonized by several H. pylori strains, with different Lewis blood group status. These isolates are not selected for colonization of the mouse stomach as SS1 has been. In another study the colonization of C57 mice inoculated with clinical isolates of H. pylori was compared with that of Balb/c mice at week 8 post inoculation (Table 3).
The PCR result showed that Balb/c mice were not colonized by H. pylori clinical isolate UA948 (Lea, Lex) whereas 80% colonization was observed in C57 mice. When these mice were inoculated with H. pylori UA1258 expressing Ley surface antigen 80% colonization was observed with Balb/c mice but none in C57 mice. Inoculation of H. pylori UA1264, which does not express either of the complete Lex or Ley antigens, revealed 100% and 80% colonization of C57 and Balb/c mice respectively. In contrast, SS1 expresses Lex and Ley and colonizes both strains of mice(16). This interesting and complex colonization pattern of the Lex , Ley , and Le0 expressing H. pylori in the various strains of mice cannot be easily rationalized. One previous report(28) documents that human oncofetal Lewis antigen (also known as stage-specific embryonic antigen [SSEA]) is highly immunogenic in Balb/c. Our experiments which showed no colonization of Lewisx expressing H. pylori suggesting that immune responses could partially explain the lack of colonization. An alternative explanation is that bacterial colonization bearing the SSEA could be dependent on the homotypic Lex -Lex or glycan-glycan interactions based on several observations(29). For example Lex glycosphingolipid liposomes adhered to plastic plates coated with Lex but not with other related carbohydrates. It is possible that the colonization pattern of H. pylori exhibiting molecular mimicry of various cell surface mammalian carbohydrate antigens is a function of the comparable glycan structures on gastric mucin and mucosal surfaces facilitating adhesion(30,31). However, it must be remembered that it is likely that UA1264 may produce precursor molecules, although neither the complete Lex nor Ley structures are present(32). In addition SS1 has been selected for colonization in the mouse model and other receptors may be of primary importance in this strain of H. pylori.
Table 3: Comparison of the colonization of C57 and Balb/c mice with different H. pylori clinical isolates at 8 weeks post inoculation evaluated by PCR.
Humoral immune response
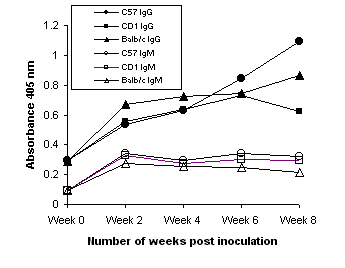
All three strains of mice showed significant immune responses to whole H. pylori antigens in sera two weeks after inoculation with strain SS1 (Figure 3). This immune response detected in terms of IgG levels increased with time. The IgM levels, on the other hand, were detectable in week 2 and remained at the same level. The control mice (week 0), however, showed significantly low antibody levels against H. pylori whole cell antigens both in terms of either IgM or IgG titers. The IgA antibody level of these mice was very low and nearly undetectable throughout the study period (data not shown) indicating that oral inoculation did not elicit serum IgA response in keeping with previous observations(33). There was no significant difference observed in the immune response between the C57 mice treated with vancomycin and those which were not. In both cases the response was shown to decrease after ten weeks (data not shown). Comparable immune responses in terms of IgG were observed when C57 mice were inoculated with strains UA861 (a-glucosyl polyLacNAc), UA802 (Ley) and UA948 (Lea and Lex) (data not shown).
Cellular immune response to Lewis antigens
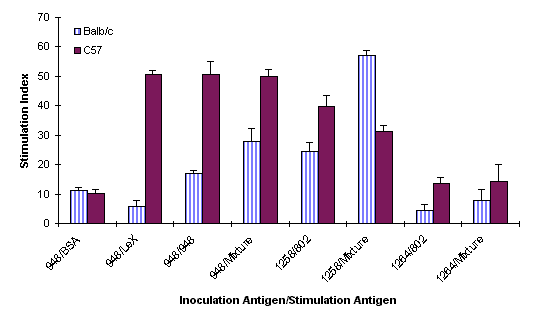
The cellular immune response of C57 and Balb/c mice was studied 8 weeks after oral inoculation with three different H. pylori strains, UA948 (Lea, Lex positive), UA1258 (Ley positive) and UA1264 (no complete Lewis antigen expression). The spleen cells were stimulated with different concentrations of BSA, Synthetic Lex trisaccharide-BSA (V- Labs, Covington, LA), a Lex positive bacterial antigen, and a Ley positive bacterial antigen depending on the nature of the antigen used for inoculation (Figure 4).
Figure 3: Serum IgM and IgG anti- H. pylori antibody levels determined after mice were inoculated intragastrically with 108 cfu of the H. pylori SS1 strain. Mice were bled 2-8 weeks post inoculation and antibody levels estimated by ELISA.
Figure 4: Comparison of cellular proliferation in C57 and Balb/c mice inoculated with H. pylori. The clinical strains UA948, UA1258 and UA1264 were inoculated and spleenocytes stimulated with BSA, Lewisx-BSA, H. pylori strains UA948 (Lex), UA802 (Ley), and a mixture consisting of UA948, UA802, UA955 (Lex, Ley and Leb precursor), and UA1182 (Lex, Ley).
All cells were also stimulated with an antigen mixture containing Lex, Ley, Lea, and Leb -precursor. The proliferative response of the spleen cell was found to be dose-dependent. In all cases maximal stimulation was observed at an antigen concentration of 1 mg/ml (data not shown). Proliferation also depends on the type of antigens used for colonization and stimulation. No significant degree of correlation was observed between colonization and cellular immune responses. Balb/c mice, for instance, were not colonized by Lex positive bacteria after 8 weeks post inoculation. The stimulation index for these mice was found to be comparatively lower than that of C57 mice when stimulated with both Lex positive bacterial antigen and Lex - BSA. But when these mice were inoculated with a Ley positive bacteria 80% colonization was observed. The stimulation index was also high when the cells were stimulated with a Ley positive antigen and a mixture of antigens. The C57 mice on the other hand were 80% colonized by a Lex positive bacterial antigen and showed a very high stimulation index. No colonization of C57 mice was observed when they were inoculated with a Ley positive bacteria but a relatively high proliferation of the splenocytes was observed when stimulated with a similar antigen. Both mice strains (80% of Balb/c and 100% of C57) were colonized by the bacterial strain which does not express complete Lewis antigen. However, the proliferation of the spleen cell of these mice was found to be marginal.
Histology
Microscopic examination of the stomach section of inoculated mouse revealed tightly coiled organisms with the typical morphology of H. pylori colonizing the gastric glands of the test animal (Figure not included). There were no bacteria visible in the gastric glands of the control mouse by standard hematoxylin and eosin (H and E) or Giemsa or Warthin Stary strains (Figures not included). In contrast, numerous bacteria of at least two different types were clearly evident on both the H and E sections and also on the Giemsa and Warthin Stary stains of stomach tissue from the test animal. Both the longer tightly coiled spirals of H. pylori as well as the thicker rod shaped organism, probably Lactobacillus, were visible.
Conclusion
H. pylori colonized the gastric epithelium in mice, although the efficiency of colonization varied with the strain of mouse used and the type of Lewis antigens expressed. The PCR assay was the most sensitive indicator of colonization followed by the urease test. Culture positivity was demonstrable subsequently. Confirmation of the presence of H. pylori was shown by histology, although the sections also show abundant rod shaped Lactobacillus-like bacteria. All H. pylori strains inoculated have shown significant humoral and cellular response after two weeks post inoculation. No correlation was observed between colonization ability of H. pylori and the type of Lewis antigen expressed by the bacterium that was used for inoculation. However, it must be noted that Lewis antigens are subject to antigenic variation(34) and may actually undergo phenotype changes in mice(35). The availability of stable H. pylori strains, which would not be subject to phase variation in vivo, would be a major advance enabling more detailed study of the role that Lewis antigens play in colonization.
Acknowledgments
We thank Dr. N. Nation for performing the histology. This project was supported by the MSI foundation, National Cancer Institute with funds from the NCIC, Terry Fox run and the Canadian Bacterial Diseases Network. DET is a scientist with the Alberta Heritage Foundation for Medical Research. MRS acknowledges MRC and Biomira Inc. for chair support.
References
- Warren, J.R. and Marshall, B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet,1:1273-1275, 1983.
- NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. J AmMed Assoc, 272:65, 1994.
- Parsonnet, J., Hansen, S., Rodriguez, L., Gelb, A.B., Warnka, R.A., Jellum, E., Orentreich, N., Vogelman, J.H. and Friedman, G.O. Helicobacter pylori infection and gastric lymphoma. N Engl J Med, 330:1267-1271, 1994.
- Covacci, A., Censini, S., Bugnoli, M., Petracca, R., Burroni, D., Macchia, G., Masson, A., Papini, E., Cover, T.L. and Blaser, M.J. Helicobacter pylori, a paradigm for chronic mucosal inflammation and prevention. Adv Inter Med; 41:85-117, 1996.
- Engstrand, L. Potential animal models of Helicobacter pylori infection in immunological and vaccine research. FEMS Immunol Med Mic, 10:265-270, 1995.
- Engstrand, L., Gustavsson, S., Jorgensen, A., Schwan, A. and Scheynius, A. Inoculation of barrier-born pigs with Helicobacter pylori: a useful animal model for gastritis type B. Infect Immun, 58:1763-1768, 1990.
- Krakowka, S., Morgan, D.R., Kraft ,W.G. and Leunk, R.D. Establishment of gastric Campylobacter pylori infection in the neonatal gnotobiotic piglet. Infect Immun, 55:2789-2796, 1987.
- Lambert, J.R., Boromeo, M., Pinkard, K.J., Turner, H., Chapman, C.B. and Smith, M.L. Colonization of gnotobiotic piglets with Campylobacter pyloridis - an animal model? J Infec Dis, 155:1344-1348, 1987.
- Radin, M.J., Eaton, K.A., Krakowa, S., Morgan, D.R., Lee, A., Otto, G. and Fox, J. Helicobacter pylori gastric infection in gnotobiotic infection beagle dogs. Infect Immun, 58:2606-2612, 1990.
- Shuto, R., Fujioka, T., Kubota, T. and Nasu, M. Experimental gastritis induced by Helicobacter pylori in Japanese monkeys. Infect Immun, 61:933-939, 1993.
- Dubois, A., Fiala, N., Heman-Ackah Drazek, E.S., Tarnawski, A., Fishbein, W.N., Perez-Perez, G.I. and Blaser, M.J. Natural gastric infection with Helicobacter pylori in monkeys: a model for spiral bacteria infection in humans. Gastroenterol, 106:1405-1417, 1994.
- Fox, J.G., Marini, R., Yan, L., Handt, L., Li, X., Shames, B., Hayward, A., Campbell, J. and Murphy, J.C. Helicobacter pylori- induced gastritis in the domestic cat. Infect Immun, 63:2674-2681, 1995.
- Karita, M., Kouchiyama, T., Okita, K. and Nakazawa, T. New small animal models for human gastric Helicobacter pylori infection: success in both nude and euthymic mice. Am J Gastroentrol, 86:1596-1603, 1991.
- Karita, M., Li, Q., Cantero, D. and Okita, K. Establishment of a small animal model for human Helicobacter pylori infection using germ-free mouse. Am J Gastroentrol, 89:208-213, 1994.
- Marchetti, M., Arico, B., Burroni, D., Figura, N., Rappuoli, R. and Ghiara, P. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science, 265:1655-1658, 1995.
- Lee, A., O'Rourke, J., Corazon, M., Robertson, B., Daskalopoulos, G. and Dixon, M.F. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterol, 112:1386-1397, 1997.
- Fox, J.G., Lee, A., Otto, G., Taylor, N.S. and Murphy, J.G. Helicobacter felis gastritis in gnotobiotic rats: An animal model of Helicobacter pylori gastritis. Infect Immun, 59:785-791, 1991.
- Aspinall, G.O., Monteiro, M.A., Pang, H., Walsh, E.J. and Morin, A.P. Antigen chains in the lipopolysaccharide of H. pylori NCTC 11634. Carbohydr Lett, 1:151-156, 1994.
- Taylor, D.E. and Sherburne, R. Helicobacter pylori expresses a complex surface carbohydrate, Lewis X. Infect Immun, 63:4564-4568, 1995.
- Monteiro, M., Appelmelk, B.J., Rasko, D.A., Moran, A.P., Hynes, S.O., MacLean, L.L., Chan, K.H., St. Michael, F., Logan, S.M., O'Rourke, J., Lee, A., Taylor, D.E. and Perry, M.B. Lipopolysaccharide structures of Helicobacter pylori genomic strains 26695 and J99, mouse model H. pylori Sydney strain, H. pylori P466 carrying sialyl Lewis X, and H. pylori UA915 expressing Lewis B. Eur J Biochem, 267:305-320, 2000.
- Taylor, D.E., Rasko, D.A., Sherburne, R., Ho, C. and Jewell, L.D. Lack of correlation between Lewis antigen expression by Helicobacter pylori and gastric epithelial cells in infected patients. Gastroenterology, 115:1113-1122, 1998.
- Heneghan, M.A., Moran, A.P., Feeley, K.M., Egan, E.L., Goulding, J., Connolly, C.E. and McCarthy, C.F. Effect of host Lewis and ABO blood group antigen expression on Helicobacter pylori colonization density and the consequent inflammatory response. FEMS Immunol Molec Microbiol, 20:257-266, 1998.
- Heneghan, M.A., McCarthy, C.F. and Moran, A.P. Relationship of blood group determinants on Helicobacter pylori lipopolysaccharide with host lewis phenotype and inflammatory response. Infect Immun, 68:937-941, 2000.
- Taylor, D.E., Hargreaves, J.A., Ng, L., Sherbaniuk, R.W. and Jewell, L.D. Characterization of Campylobacter pyloridis from gastric biopsies. Am J Clin Pathol, 87:49-54, 1987.
- Xiang, Z., Censini, S., Bayeli, P.F., Telford, J.L., Figura, N., Rappuoli, R. and Covacci, A. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect Immun, 63:94-98, 1995.
- Gracey, M., Cullity, G. and Suharjono, M. The stomach in malnutrition. Arch Dis Child, 52:325-7, 1977.
- Kabir, A.M.A., Aiba, Y., Takagi, A., Kamiya, S., Miwa, T. and Koga, Y., Prevention of Helicobacter pylori infection by Lactobacilli in gnotobiotic murine model. Gut, 41:49-55, 1997.
- Umeda, M., Diego, I. and Marcus, D.M. The occurrence of anti-3- fucosyllactosamine antibodies and their cross-reactive idiotypes in preimmune and and immune mouse sera. J Immunol, 137:3264-3269, 1986.
- Hakomori, S-I. Lex and related structures as adhesion molecules. Histochem J, 24:771-776, 1992.
- Appelmelk, B.J., Smit, I.S., Negrini, R., Moran, A.P., Aspinall, G.O., Forte, J.G., DeVries, T., Quan, H., Verboom, T., Maaskant, J.J., Ghiara, P., Kuipers, E.J., Bloemena, E., Tadema, T.M., Townsend, R.R., Tyagarajan, K., Crothers, J.M., Monterio, M.A., Savio, A. and DeGraff, J. Potential role of molecular mimicry between H. pylori lipopolysaccharide and host Lewis blood group antigens in autoimmunity. Inf Immun, 64: 2031-2040, 1996.
- Writh, H.-P., Yang, M., Peek, R.M., Tham, K.T. and Blaser, M.J. Helicobacter pylori Lewis expression is related to the host Lewis phenotype. Gastroenterol, 113:1091-1098, 1997.
- Aspinall, G.O. and Monteiro, M.A. Lipopolysaccharides of Helicobacter pylori strains P466 and MO19: structures of the O antigen and care oligosaccharide regions. Biochem, 35:2498-2504, 1996.
- Ferrero, R.L., Thiberge, J.-M., Huerre, M. and Labigne, A. Immune responses of specific-pathogen-free mice to chronic H. pylori (strain SS1) infection. Inf Immun, 66:1349-1355, 1998.
- Wang, G., Zhongming, G., Rasko, D.A. and Taylor, D.E. Lewis antigens in Helicobacter pylori: biosynthesis and phase variation. Molec Microbiol, 36:1187-1196, 2000.
- Janvier, B., Grignon, B., Audibert, C., Pezennee, L and Fauchere, J.L. Phenotypic changes of Helicobacter pylori components during an experimental infection in mice. FEMS Immunol Med Microbiol, 24:27-33, 1999.
- Wang, G., Rasko, D.A., Sherburne, R. and Taylor D.E. Molecular genetic basis for the variable expression of Lewis Y antigen in Helicobacter pylori; analysis of the α(1,2) fucosyltransferase gene. Molec Microbiol, 31:1265-1274, 1999.
- Monteiro, M.A., Rasko, D.A., Taylor, D.E. and Perry, M.B. Glucosylated N-acetyllactosamine O-antigen chain in the Helicobacter pylori strain UA861. Glycobiol, 8:107-112, 1998.
- Monteiro, M.A., Chan, K.H.N., Rasko, D.A., Taylor, D.E., Zheng, P.Y., Appelmelk, B.J., Wirth, H.-P., Yang, M., Blaser, M.J., Hynes, S.O., Moran, A.P. and Perry ,M.B. Simultaneous expression of type 1 and 2 Lewis blood-group antigens by Helicobacter pylori lipopolysaccharides. Molecular mimicry between Helicobacter pylori lipopolysaccharides and human gastric epithelial cell-surface glycoforms. J Biol Chem, 273:11533-11543, 1998.
Corresponding author: Dr. M. R. Suresh, Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta, Edmonton, Alberta, Canada T6G 2N8. msuresh@pharmacy.ualberta.ca
Abbreviations: BHI- Brain Heart Infusion, BSA- Bovine Serum Albumin, CFU- Colony Forming Unit, ELISA-Enzyme-Linked Immunosorbent Assay, FBS- Fetal Bovine Serum, HRPO- Horseradish Peroxidase, PCR- Polymerase Chain Reaction, PBST- Phosphate Buffer Saline with 0.1% Twen-20, TMB- 3, 3', 5, 5' - Tetramethylbenzidine.
JPPS Contents
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps