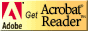J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 3(3):303-311, 2000
Mechanical, Bioadhesive Strength and Biological Evaluations of Chitosan films for Wound Dressing
Tanveer Ahmad Khan, Kok Khiang Peh1 and Hung Seng Ch'ng
School of Pharmaceutical Sciences, University of Science Malaysia, 11800 Penang, MalaysiaReceived October 13, 2000; Revised December 8, 2000; Accepted December 8, 2000
PDF version for printing
ABSTRACT
Purpose. To investigate the suitability of chitosan films prepared using two different solvents, acetic acid (Chitosan-AA) and lactic acid (Chitosan-LA), for wound dressing, in comparison with a commercial preparation, Omiderm®.Methods. The mechanical and in-vitro bioadhesive strength properties of Chitosan-AA, Chitosan-LA, and Omiderm® were investigated using texture analyzer equipment. The vapour permeability of chitosan films was determined using a method for evaluation of moisture permeability of containers and packaging material described in USP XXII. In addition, the biological evaluations were performed via primary skin irritation, intracutaneous, and systemic injection tests.
Results. The three preparations differed significantly in terms of the mechanical and bioadhesive strength properties. Chitosan-LA exhibited a lower tensile strength, but more flexible and bioadhesive than Chitosan-AA. Chitosan film was found to be permeable to water vapour. Chitosan-LA and Omiderm® were non-irritant and did not cause any skin allergic reaction. In contrast, Chitosan-AA films inflicted adverse skin reactions. Nevertheless, no gross sign of toxicity was encountered from the systemic injection of the extracts of the three preparations.
Conclusion. Chitosan films demonstrated significantly different mechanical and bioadhesive strength properties from Omiderm. Chitosan-LA was more soft, flexible, pliable and bioadhesive when compared to Chitosan-AA films. Furthermore, Chitosan-LA did not cause erythema, edema and systemic toxicity. Hence, Chitosan-LA film is suitable to be used in the management of wound healing and skin burn
INTRODUCTION
Chitosan composes of glucosamine and N-acetylglucosamine, which are constituents of mammalian tissues. It is a non-toxic, biocompatible, and biodegradable polymer. Various grades of chitosan are available commercially, which differ primarily in the degree of deacetylation and molecular weight (1). Muzzarelli et al. (2) reported that the degree of deacetylation is one of the important characteristics which could influence the performance of chitosan in many of its applications.
The ability of chitosan to form films may permit its extensive use in the formulation of film dosage forms (3,4) or as drug delivery systems (5-8). Chitosan could be dissolved in organic acids, such as lactic acid and acetic acid, prior to being casted into films. Chitosan is a promising bioadhesive material (9) and it was able to effectively bind and agglutinate a wide variety of mammalian cell types (10). Chitosan has also been advocated as wound healing agent in the form of a bandage (11). Its effect on wound healing in urogenital tissue has also been investigated (12).
For chitosan film to be employed as wound dressing, it should be durable, stress resistant, flexible, pliable, and elastic. It should be easy to apply and remove without incurring any trauma during dressing changes. As such, the mechanical properties of the films are critical and important to be characterized. Chitosan films should possess reasonable tensile properties, which could bear the stresses exerted by different parts of the body having varying contours. Moreover, the dressing must be rapidly and uniformly adherent and conform to wound bed topography and contour to prevent air or fluid pocket formation. Good adherence reduces pain, facilitates decontamination, prevents peripheral channeling into the wound by bacteria, and promotes bonding to tissues. The dressing must be an absolute barrier to bacterial ingress and could prevent egress of wound organisms to the surface of dressing. Furthermore, the dressing is preferably permeable to water vapor to the extent that a moist exudate under the dressing is maintained without pooling, but excess fluid absorption and evaporation leading to desiccation of the wound bed are prevented. In addition, the dressing must be compatible with body tissues, be nontoxic, non-antigenic and non-allergenic (13).
Hence, the objective of the present study was to investigate the mechanical, bioadhesive strength, and water vapour permeability of chitosan films, prepared using two different organic acids, acetic and lactic acids, respectively. In addition, biological studies were conducted to investigate the skin irritation and systemic toxicity of the films. Omiderm®, a commercially available product for wound dressing was selected for comparison. Omiderm® is a highly permeable, transparent, flexible and non-adherent polyurethane film for wound dressing. Additionally, it is non-toxic, non-pyrogenic and hypoallergenic. It possesses the necessary attributes to be employed as wound dressing and furthermore has been effective in the management of wounds.
MATERIALS AND METHODS
Materials
Chitosan (practical grade) was purchased from Sigma Chemical, St. Louis, USA. N-Acetyl Glucosamine and D-Glucosamine hydrochloride were purchased from Fluka, Buchs, Switzerland. Omiderm® was purchased from ITG Laboratories, CA, USA. Lactic acid and absolute alcohol were obtained from Ajax Chemicals, Australia. Acetic acid was supplied by R & M Marketing, UK. Sodium chloride solution (0.9% w/v) was a gift from B. Braun Pharmaceuticals, Penang, Malaysia. Sesame oil, polyethylene glycol 400 and sodium tetraborate were obtained from Sigma Chemical, St. Louis, USA. All other chemicals used were of reagent grade. The materials were used as received.
Determination of the Degree of Deacetylation
The First derivative UV-Spectrophotometry method reported by Tan et al. (14) was used after slight modification for the determination of the degree of deacetylatioin of chitosan. The degree of deacetylation (DD) of the chitosan was determined using the following formula:
DD = 100 - ([A / (W-204A) / 161 + A] x 100)
where A is the amount of N-acetylglucosamine determined/204 and W is the mass of chitosan used.
Molecular Weight Determination
The viscosity-average molecular weight was calculated using Mark-Houwink equation relating to intrinsic viscosity.
[h] = Km Mva
where Km = 1.81 X 10-3 and a = 0.93 are the empirical viscometric constants that are specific for a given polymer, solvent and temperature (15).
Preparation of Chitosan Films
Chitosan (1.4%w/v) in 1%w/v lactic acid or 2%w/v acetic acid solutions was prepared by stirring overnight using magnetic stirrer. The resulting solution was filtered through a sintered glass filter to remove the extraneous matter, followed by casting on glass plate and dried in an oven (Memmert, Germany) at 60°C for 24 hours. After drying, the transparent film was carefully peeled off from the glass plate. The films were stored in an air tight glass container maintained at room temperature of 25 ± 1°C and relative humidity of 60-65% until further investigations. The thickness of the film sample was measured using a micrometer (Digimatic Micrometer, Mitutoyo, Tokyo, Japan) at five locations (center and four corners), and the mean thickness calculated. Samples with air bubbles, nicks or tears and having mean thickness variations of greater than 5% were excluded from analysis.
Mechanical Property Measurements
The mechanical properties of chitosan films were evaluated using a texture analyzer (TA.XT2, Stable Micro System, Haslemere, Surrey, UK) equipped with a 5 kg load cell. Film strip in dimension of 10 mm by 50 mm and free from air bubbles or physical imperfections, was held between two clamps positioned at a distance 3 cm. During measurement, the film was pulled by top clamp at a rate of 0.5 mm/s to a distance of 5 cm before returning to the starting point. The force and elongation were measured when the films broke. Measurements were run four times for each film. The tensile strength and elongation at break were calculated as below:
Tensile strength (N/mm2) = Breaking force (N)/Cross-sectional area of sample (mm2)
Elongation at break (%) = Increase in length at breaking point (mm)/Original length (mm) x 100%
In-vitro Bioadhesive Strength Measurement
The bioadhesive strength of chitosan films was evaluated employing method described by Peh and Wong (16) with slight modification. The measurement was conducted using a texture analyzer equipment (TA.XT2, Stable Micro System, Haslemere, Surrey, UK) equipped with a 5 kg load cell with chicken pouch as the model tissue. The chicken pouch taken from freshly slaughtered chicken was used after the removal of all the fats and debris. The chicken pouch membrane was affixed on the cylindrical perspex support (diameter, 2 cm; length, 4 cm; surface area, 3.14 cm2) and secured with an aluminum cap with a hole having a surface area of 148.3 mm2. The perspex support was then positioned at the bottom of the measuring system and held in place by the clamp. Chitosan film cut into circular shape with diameter of 13.7 mm was affixed to another perspex support of similar dimension using a double-sided adhesive tape. The perspex support was then screwed onto the upper probe of the instrument. The two perspex supports were aligned to ensure that the film came into direct contact with the surface of the chicken pouch when the upper perspex support was lowered.
During measurement, 100 ml of distilled water was evenly spread on the surface of the tissue. The probe was lowered at a speed of 1 mm/s to contact with the tissue at a force of 1N for a contact time of 30 s. It was then withdrawn at a rate of 1 mm/s to a distance of 5 mm. An acquisition rate of 200 points/s was chosen for the analysis. Data collection and calculation were performed using the XTRA Dimension software package of the instrument. Work of adhesion and peak detachment force were used to evaluate the bioadhesive strength of the films. The work of adhesion was calculated from the area under the force-distance curve, and the peak detachment force was taken as the maximum force required for detaching the film from the tissue. Measurements were performed in four replicate.
Water Vapour Permeability of Chitosan Films
The rate of water vapour permeability of chitosan film was estimated using the method described in USP XXII (17) for the evaluation of moisture permeability of containers and packaging materials. Chitosan-AA was selected for the study. Chitosan films were tied onto the mouth of small glass bottles of the same size and type, with an average volume of 29.0 ± 0.5 ml. 10 glass bottles were used for samples and 2 for the control. Glass bottle for sample was filled with anhydrous calcium chloride, while glass bottle for the control with sufficient amount of small glass beads attaining a weight near to that of the test container. The weight of the individual containers was recorded and kept in a desiccator maintained at 75 ± 3%RH using saturated solution of sodium chloride at a temperature of 25 ± 2°C. The average area available for vapor permeation was 3.71 cm2. After 14 days, the weight of the individual container was recorded. The following equation was employed to calculate the rate of moisture permeability:
Rate of moisture permeability (mg/day/liter) = (100/14v) [(Tf - Ti) - (Cf - Ci)]
where V is volume (ml) of the container, (Tf - Ti) is the difference (mg) between the final and initial weights of each test container, (Cf - Ci) is the average of the difference (mg) between final and initial weights of two containers (control).
Primary Skin Irritation Test
The chitosan films were assessed for skin irritation using the test method of ASTM F 719 - 81 (18) with slight modification. Six healthy male albino New Zealand rabbits weighed between 2 - 2.5 kg were selected for the study. Each rabbit was caged individually and there was no restriction on food and water supply during the test. 24-hr prior to the test, the hair from the back of each rabbit was clipped using an electric clipper on both sides of the spine to expose sufficiently large test areas, which could accommodate three test sites on each side of the spine. The test sites were wiped with alcohol. One side of the spine was abraded using a sterile blade to break partially the stratum corneum while the other side of the spine remained intact. The test sites were moistened with normal saline before films with surface area of 2.54 cm2 were placed on the abraded and the intact sites. Three preparations were evaluated, namely, Chitosan-AA, Chitosan-LA and Omiderm®. The test sites were covered with gauze to hold the films in place and further occluded with adhesive tape. The preparation, gauze and adhesive tape were removed 24- hr after application. The test sites were observed for erythema and edema at 1, 24, and 48 hr after removal of the test preparation and given the scores according to Table 1.
Table 1: Grading values for the primary skin irritation and intracutaneous tests.
The scores for erythema and edema of the abraded and intact sites at the three time intervals for each preparation were totaled and divided by six to obtain the primary irritation index (PII) for each test rabbit. The average primary irritation index was then calculated by dividing to the sum of the PII for each preparation for all test animals with the number of test animals (6 in the present experiment).
Intracutaneous Test
The intracutaneous test was performed according to the method of USP XXII (19). Chitosan-AA, Chitosan-LA, and Omiderm® films (surface area, 120 cm2) were extracted with 20 ml of extracting medium, namely, normal saline (0.9%), ethanol:normal saline (1:20), polyethylene glycol 400, and sesame oil, by heating in an autoclave at 121 °C for 1 hr. The extracts were allowed to cool to room temperature and filtered through a 0.45-mm diameter sieve. Two rabbits were used for each film preparation. 24-hr prior to the test, the hair from the back of each rabbit was clipped using an electric clipper on both sides of the spine to expose sufficiently large test areas which could accommodate five injection sites on each side of the spine. The sites were swabbed with alcohol and dried prior to the injection. A 0.2 ml of the extracts of each sample was injected intracutaneously at 5 sites on one side of the spine, while 0.2 ml of the corresponding extracting medium (as blank) was injected at 5 sites on the other side of the spine of rabbits. All the injection sites of the extract and blank were examined at 24, 48, and 72-hr intervals for gross evidence of tissue reactivity. The tissue reaction, erythema and edema, for all the injection sites were graded for sample and blank at every scoring period for each rabbit according to Table 1. The total scores and the mean value for erythema and edema of each preparation were calculated.
Systemic Injection Test
The extracts of chitosan films were evaluated using the systemic injection test of USP XXII (20). Five albino mice in both sexes having weight between 18-23 g were used for the test. Chitosan-AA, Chitosan-LA, and Omiderm® films (surface area, 120 cm2) were extracted with 20 ml of extracting medium consisting of normal saline (0.9%), ethanol:normal saline (1:20), polyethylene glycol (PEG) 400, and sesame oil, by heating in an autoclave at 121 °C for 1 hr. The sample extracts were cooled to room temperature and filtered through a 0.45-mm diameter sieve. Each mouse was weighed prior to the injection and a calculated dose of the extract was administered intraperitoneally or intravenously to the mice in accordance to Table 2.
Table 2: Amount and routes of systemic injection of extracts or blank.
The mice were observed for gross signs of toxicity at 0 (immediately after injection), 4, 24, 48 and 72 hr after injections according to the classification of toxic symptoms given in Table 3.
Table 3: Classification of toxic symptoms for systemic injection test.
RESULTS AND DISCUSSIONS
Degree of Deacetylation and Molecular Weight of Chitosan
The degree of deacetylation and molecular weight are important parameters, which could influence the performance of chitosan in many of its applications. Since this information was not provided by the supplier of the chitosan (practical grade) used in the present study, we therefore estimated these two parameters. The degree of deacetylation of chitosan was found to be 84.05 ± 0.17%. On the other hand, the viscosity-average molecular weight was estimated to be 1.00 ± 0.04 x 106 Da.
Mechanical Properties
The tensile testing provides an indication of the strength and elasticity of the film, which can be reflected by tensile strength and elongation at break. It is suggested that films suitable for wound dressing should preferably strong but flexible. The mechanical properties of Chitosan-AA, Chitosan-LA, and Omiderm® films are given in Table 4.
Table 4: Mechanical and in vitro bioadhesive strength properties of the films (Mean±SD, n= 4).
Statistical significance Tukey-HSD (Statistical significance)
The three film preparations differed significantly (p<0.05) in tensile strength. It was noted that the highest tensile strength was obtained for Chitosan-AA, followed by Chitosan-LA, and lastly Omiderm®. The difference in tensile strength was statistically significant between Chitosan-AA and Chitosan-LA. On the contrary, no statistically significant difference in tensile strength between Chitosan-AA and Omiderm® (p=0.056) as well as between Chitosan-LA and Omiderm® (p=0.977).
Similarly, the three films also differed significantly (p<0.05) in the elongation at break. Chitosan-LA exhibited highest value, followed by Chitosan-AA and lastly Omiderm®. The difference in elongation at break was found to be significant between Chitosan-AA and Chitosan-LA (p<0.05), Chitosan-AA and Omiderm® as well as Chitosan-LA and Omiderm®. These results indicated that Chitosan-AA was tough and hard, whereas Chitosan-LA more soft, and flexible.
Lactic acid could be used as a universal solvent for chitosan (21) and has been employed as a plasticizer to improve the flexibility of Zein films (22). Hence, incorporation of lactic acid as a solvent cum plasticizer was anticipated to render the Chitosan-LA film more elastic. Also, increase in elasticity invariably reduced the tensile strength as the film because less strong. As such, Chitosan-LA film exhibited a comparatively higher elongation at break but lower tensile strength than chitosan-AA film.
Bioadhesion Evaluations
Referring to Table 4, there was a statistically significant difference in both the peak detachment force and work of adhesion among the three different films. It was noted that the peak detachment force was highest for Omiderm®, followed by Chitosan-LA, and lastly Chitosan-AA films. On the other hand, Chitosan-LA demonstrated significantly highest work of adhesion, followed by Omiderm®, and lastly Chitosan-AA films. Although the ranking for the three films was different when compared using peak detachment force and work of adhesion, Chitosan-LA consistently exhibited a significantly higher adhesion than Chitosan-AA in both the parameters. Utilization of lactic acid as a solvent cum plasticizer has produced a more soft and elastic film as described earlier. The increase in flexibility of the Chitosan-LA film could have improved the contact between the film and the tissue, hence promoting penetration of the polymeric chains into the tissue to form a strong bonding, leading to an increase in the adhesion strength (23).
Water Vapor Permeability
Permeability of moisture and gases through the film for wound dressing is important to keep the wound comfortable and help in the healing process. It was reported that the film must be permeable to the extent that a moist exudate under dressing was maintained, preventing excess fluid absorption and evaporation leading to desiccation of the wound bed (13). In order to investigate if chitosan film was permeable to moisture, the water vapour permeability study was conducted using Chitosan-AA as an example. The rate of moisture permeability was 4435.83 mg/day/liter for film with a mean thickness of 48 um.
The selected test method was meant for the determination of moisture permeability of tight and well-closed containers utilized for drug (17). The containers were categorized as tight containers if results showed that no more than one of the 10 test containers exceeded 100 mg/day/liter in moisture permeability, and categorized as well-closed containers if not more than one of the 10 test containers exceeded 2000 mg/day/liter. As such, the results obtained suggested that chitosan film was highly permeable.
Primary Skin Irritation Test
Figure 1 shows the values of average primary irritation index of Chitosan-AA, Chitosan-LA, and Omiderm®. This test was conducted to evaluate the irritancy of chitosan films through contact with abraded and intact skin of rabbits. The average primary irritation index (PII) values obtained for Chitosan-LA and Omiderm® were noted to be closely similar. In comparison, the mean PII value of Chitosan-AA film was about 16 times higher. These results suggested that Chitosan-LA and Omiderm® films were non-irritant to the skin of rabbits. In contrast, Chitosan-AA inflicted erythema and edema to the skin of rabbits, indicating that this film is not suitable to be used for wound dressing.
Figure 1: Average Primary Skin Irritation Index Values For Chitosan-AA, Chitosan-LA and Omidern. Mean + SD, N=6.
Intracutaneous Test
This test was conducted to evaluate the responses of rabbits to the extracts of chitosan films and Omiderm® following inctracutaneous injections. The irritation scores of the film extracts are depicted in Figure 2. It was observed that the scores for Chitosan-LA extracts in the four different media were closely similar to those of the control. On the contrary, the tissue response of Chitosan-AA extracts was significantly greater than the control, Chitosan-LA and Omiderm®. The higher numerical scores for Chitosan-AA extracts could be ascribed to acetic acid, causing severe skin reactions to the rabbits. This finding is in good agreement with the results of primary skin irritation test.
The results obtained indicated that lactic acid is safe and non-irritant to the skin of rabbits. It has been reported that lactic acid is biologically safe and is a natural constituent of the human body (24, 25). It occurs in appreciable quantities in various organs, blood, and the skin. Moreover, a 1% solution of lactic acid was harmless when applied to the skin (26). These reports are advocating the safety of lactic acid at the levels used as an excipient in the skin preparations.
Figure 2: Intracutaneous Skin Response of the Samples Extracted Using Four Different Media and the Blank. Mean + SD, N=10.
Systemic Injection Test
As chitosan film is advocated for wound dressing, materials used for fabrication of the films are critical to be evaluated as these materials may penetrate into the blood stream, eliciting untoward reactions. Hence, this test was performed to evaluate the systemic response of mice to the extracts of chitosan films and Omiderm®, to ascertain that there was no adverse systemic response from the films. The results of the test are tabulated in Table 5. It was observed that none of the mice died during the observation period. Therefore, the three film preparations could be considered non-toxic.
CONCLUSION
Chitosan-LA was more soft, flexible, pliable and bioadhesive than Chitosan-AA films. Furthermore, Chitosan-LA was non-allergic and non-toxic. Hence, Citosan-LA film is more suitable than chitosan-AA film to be used in the management of wound healing and skin burn.
REFERENCES
- Genta, I., Perugini, P. and Pavanetto, F., Different molecular weight chitosan microspheres: Influence on drug loading and drug release. Drug Dev Ind Pharmacy, 24:779-784, 1998.
- Muzzarelli R. A. A., Chitin, Pergamon Press, Oxford, pp 69, 1977.
- Kubota, N., Ohga, K. and Moriguchi, M., Permeability properties of glycol chitosan membrane modified with thiol Groups. J Appl Polym Sci., 42:495-501, 1991.
- Li, Q., Dunn, E.T., Grandmaison, E. W. and Goosen, M.F.A., Application and properties of Chitosan. J Bioact Compat Polym, 7:370-397, 1992
- Yaku, T. and Yamashita I., Japan Patent, 19213, 1973.
- Muzzarelli R. A. A., Chitin, Pergamon Press, Oxford, pp 255, 1977.
- Miyazaki, S., Yamaguchi, H. and Takada, M., Pharmaceutical application of biomedical polymers. XXIX. Preliminary study on film dosage form prepared from chitosan for oral drug delivery. Acta Pharm Nord, 2(6): 401- 406, 1990.
- Nakatsuka, S. and Andrady, L.A., Permeability of vitamin-B-12 in chitosan membranes. Effect of crosslinking and blending with poly(vinyl alcohol) on permeability. J Appl Polym Sci., 44: 7-28, 1992.
- Henriksen, I., Green, K.L., Smart, J.D., Smistad, G. and Karlsen, J., Bioadhesion of hydrated chitosans: An in vitro and in vivo study. Int J Pharm., 145:231-240, 1996.
- Evans, E.E. and Kent, S.P., The use of basic polysaccharides in histochemistry and cytochemistry. IV. Precipitation and agglutination of biological materials by Aspergillus polysaccharide and deacetylated chitin. J Histochem Cytochem., 10:24-28. 1962.
- Sathirakul, K., How, N.C., Stevens, W.F. and Chandrkrachang, S., Application of chitin and chitosan bandages for wound healing. Adv Chitin Sci, 1:490-492, 1996.
- Bartone, F.F. and Adickes, E.D., Chitosan: Effects on wound healing in urogenital tissue: Preliminary report. J Urol., 140:1134-1137, 1988.
- Widra, A., Skin, Synthetic, in Mark, H.F., Bikales, N.M., Overberger, C.G. and Menges, G., (eds), Encyclopedia of Polymer Science and Engineering, 2nd edition, Vol 15, John Wiley and Sons, USA, pp 335-344, 1989.
- Tan, S.C., Khor, E., Tan, T.K. and Wong, S.K., The degree of deacetylation of chitosan: advocating the first derivative UV-spectrophotometry method of determination. Talanta, 45:713-719, 1998.
- Maghami, G.G. and Roberts, G.A.F., Evaluation of the viscometric constants for chitosan. Makromol Chem., 189:195-200, 1988.
- Peh, K.K. and Wong, C.F., Polymeric film as vehicle for buccal delivery: Swelling, mechanical, and bioadhesive properties. J Pharm Pharmaceut Sci., 2(2):53-60, 1999.
- United States Pharmacopeia, XXII and National Formulary, XVII, Ninth supplement, The United States Pharmacopeial Convention, Rockville, MD, pp 3594-3595, 1993.
- A.S.T.M., "Standard Practice for Testing Biomaterials in Rabbits for Primary Skin Irritation," A.S.T.M Designation F 719 - 81 (Reapproved 1996); American Society for Testing and Materials, Philadelphia. pp 178-179, 1998.
- United States Pharmacopeia, XXII and National Formulary, XVII, Ninth supplement, The United States Pharmacopeial Convention, Rockville, MD, pp 3577-3578, 1993.
- United States Pharmacopeia, XXII and National Formulary, XVII, Ninth supplement, The United States Pharmacopeial Convention, Rockville, MD, pp 3576-3577, 1993.
- Knapczyk, J., Chitosan hydrogel as a base for semisolid drug forms. Int J Pharm., 93: 233-237, 1993.
- Lai, H.M. and Padua, G.W., Properties and microstructure of plasticized Zein films. Cereal Chem., 74 (6):771-775, 1997.
- Peppas, N.A. and Buri, P.A., Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues. J Controlled Rel., 2:257-275, 1985.
- Tsai, C.J. Hsu, L.R. Fang, J.Y. and Lin, H. H., Chitosan hydrogel as a base for transdermal delivery of Berberine and its evaluation in rat skin. Biol Pharm Bull., 22(4): 397-401, 1999.
- Gijsen, R., Lactic acid and the fight against tooth decay. Manuf Chem., 66 (12): 37, 1995.
- Lee, M.G., Lactic Acid, in Wade, A. and Weller, P. J. (eds), Handbook of Pharmaceutical Excipients, The American Pharmaceutical Association and the Pharmaceutical Press, London, pp 250-251, 1994.
Corresponding Author: Kok Khiang Peh, School of Pharmaceutical Sciences, University of Science Malaysia, 11800 Penang, Malaysia, kkpeh@usm.my
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps