J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 3(3):317-324, 2000
In-Vitro Characterisation Of Metered Dose Inhaler Versus Dry Powder Inhaler Glucocorticoid Products: Influence Of Inspiratory Flow Rates
Majid R. Feddah, Kenneth F. Brown, Elizabeth M. Gipps, Neal M. Davies1
Faculty of Pharmacy, The University of Sydney, New South Wales, AustraliaReceived October 25th, 2000, Revised December 31st, 2000, Accepted December 31st, 2000
PDF version
ABSTRACT
Purpose: To study the influence of inspiratory flow rate on the fine particle mass and the particle size distribution for metered dose inhaler (MDI) and dry powder inhaler (DPI) glucocorticoid products, in vitro. To compare the performance of MDI and DPI inhalers containing the same drug and strength at an impaction flow rate of 60 L/min. Methods: The Marple Miller cascade impactor model 150 and 160 were used to characterise several glucocorticoid MDI and DPI products at different simulated inspiratory flow rates (30 L/min, 60 L/min and 90 L/min). Following the actuation of one single inhaler puff the amount of drug deposited in each stage of the impactor was quantified using high performance liquid chromatography with UV detection at 242 nm. The size distribution of the primary particles of DPI products was measured by laser diffraction. Results: DPIs were significantly more dependent on impaction flow rate than MDIs. Except for Pulmicort®, the fine particle mass FPM delivered from the MDI products was significantly higher than that delivered from the DPI aerosols. Conclusions: Although the metered dose inhaler is the older technology it exhibits greater respirable dose in vitro than newer dry powder inhaler devices. Care should be taken when shifting from one inhaler dosage form to another because this may affect the actual dose delivered to the lung. Further in vivo studies may be warranted in light of these findings.

INTRODUCTION
Inhalation of aerosols as a route of pulmonary drug delivery has been used for many years to deliver xenobiotics to the lung for both systemic and local effects. The ability to deliver active substances directly to the site of action enables lower doses compared to other routes of administration with an equivalent therapeutic response and a lower systemic exposure. Pulmonary inhalation is also becoming an attractive route of administration for medications which are difficult to formulate orally such as proteins and peptides (1).
Inhalation aerosols for treatment of lung diseases are delivered largely using either pressurised MDIs or DPIs. Although MDIs are one of the main respiratory delivery systems many problems are still associated with their use. There is often poor synchronisation between actuation and inhalation "hand to lung coordination" especially in elderly and pediatric patients (2-5), leading to a failure to continue inhaling when the propellant spray hits the back of the throat (6, 7). In addition the high velocity of the inhaled particles leads to high oropharyngeal deposition which induces local and systemic side effects (8-10). The respiratory spacer devices eliminates the need for coordination of actuation and inspiration and reduces the primary droplet size by providing extra time for complete evaporation of propellant and reduces the velocity of the aerosol particles passing through the device (11, 12). Consequently, large particles will sediment or impact on the holding chamber walls. Hence, deposition of these large particles in the oropharyngeal cavity should be reduced. There are also concerns about the adverse effects of chloroflurocarbon (CFC) on the ozone layer and the environment resulting in restrictions on the continued production of CFC aerosol formulations throughout the world (13, 14). Due to these factors, several new devices and approaches for aerosol inhalation are being developed. DPIs were introduced to overcome problems associated with the use of MDIs, however, the DPIs are breath activated so that drug particles delivered to the lung from the device depend on the inspiratory efforts of the patient. This effort will determine the degree of emitted dose and the respirable fraction and hence no breathing coordination with actuation is needed with these formulations. In addition, DPIs are devoid of CFC and it has been suggested that they may be more effective for pediatric patients (15).
The inhalation flow rate, the particle size distribution and the aerosol device employed are important parameters in drug delivery to the respiratory tract, because they influence the actual dose delivered to the lung. The vast majority of DPIs consist of either micronised drug particles mixed with a carrier substance, normally lactose (i.e. Accuhaler® Rotahaler®) or as pure drug (i.e. Turbuhaler®). An effort while inhaling is required from the patient to deaggregate and to aerosolize the drug particles from the carrier surface and to facilitate their deposition in the respiratory tract. Inspiratory flow often varies between individuals and between doses and is influenced by the device employed (16-18). The resistance of the inhalation device controls the flow inside the device and hence the amount of effort required to generate the aerosol particles (19). As a consequence differential flow in each device occurs between individual patients and within individual patients during the course of their disease. Thus it is important for physicians before prescribing inhalation aerosols to know in detail the peak flow rate of the patient, and the lowest inspiratory flow at which a specific inhaler will work most effectively (20).
Inhalation of glucocorticoids for treatment of asthma has increased in the last decade with clinical evidence that early treatment of asthma with inhaled steroids has resulted in greater lung function improvement in adults and children (21, 22). These glucocorticoids are marketed both as MDIs and as DPIs which are assumed to provide therapeutic equivalence when the same drug with an equivalent daily dose recommended by the manufacturers. Particles intended to be delivered to the lung by inhalation should have aerodynamic diameter smaller than 5 mm to increase the probability of penetrating deep into the lung and to facilitate the therapeutic activity of the drug. There have been no previous in vitro studies investigating the influence of inspiratory flow rate of glucocorticoid MDIs and DPIs at 30L/min, 60L/min and 90 L/min using a single actuated aerosol puff. In addition, many of the previous in vivo studies evaluating glucocorticoids DPI vs. MDI have shown contradictory results.(23-27) In this study the influence of three simulated inspiratory flow rates 30 L/min, 60 L/min and 90 L/min on several glucocorticoid DPI and MDI products was evaluated. The aerosol performance of MDI and DPI products of fluticasone propionate, budesonide and beclomethasone dipropionate was also evaluated at 60L/min simulated inspiratory flow rates.
MATERIALS AND METHODS
Materials
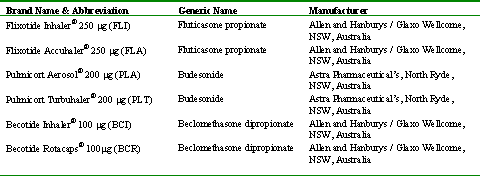
Beclomethasone dipropionate and dexamethasone 21-acetate (analytical standards) were purchased from Sigma Chemicals Co., (St. Louise, MO, USA). Budesonide was obtained by emptying a commercial Pulmicort Turbuhaler® Astra Pharmaceutical's (North Ryde, NSW, Australia). Fluticasone propionate was kindly donated by Glaxo Wellcome Group research (UK). Methanol and acetonitrile HPLC grade were purchased from Biolab scientific, (Clayton, South VIC, Australia). Analytical grade acetic acid was purchased from Rhone-Poulenc Chemicals (Clayton, South VIC, Australia). Glucocorticoid products used in this study are indicated in Table 1.
Table 1: Commercial Glucocorticoid Products Utilized in this Study
Devices used in this study were Accuhaler® (Diskus), it is a multi-dose dry powder inhaler which contains 60 doses of fluticasone propionate mixed with lactose as a carrier sealed in a foil strip. The dose is available for inhalation by sliding the lever until it clicks. Turbuhaler ® is a multi-dose DPI, containing 200 doses of pure budesonide kept in a small reservoir on the bottom of the inhaler. The metered dose is prepared by twisting the turning grip at the bottom of the inhaler, and by inhaling the drug particles are dispersed into the air stream, through the spiral path mouthpiece of the Turbuhaler®. Rotahaler® is a single dose DPI, the drug particles (beclomethasone dipropionate) along with the carrier lactose is kept in a gelatin capsule. The capsule is inserted at the end of the inhaler, as the one end is turned, the capsule is broken into two halves, the drug particles are dispersed into the air stream as the patient inhales through the device.
Methods
Characterisation By Impaction
The Marple-Miller cascade impactors (MSP Corp. Minneapolis, MN, USA) Model 150 which were designed for operation at 30L/min and the 160 model for operation at 60 L/min and 90 L/min were used. Upon impaction the aerosol is divided into five size categories according to its aerodynamic diameter. The cutoff for the two impactors at different flow rates is shown in Table 2.
Table 2: Particle size cut-off point for each impactor stage at different flow rates

To simulate the inspiratory flow rate, the impactor was connected to a vacuum pump (Erweka GmbH serial no 70018 Heusenstamm, Germany). The flow rate was measured by a mass flow meter (Model 822S-H-4-OV1-V1, Sierra Instruments Monterey, CA, USA) connected to the impactor through a stainless steel 90-degree induction port. A fiberglass filter (Gelman Sciences, Ann Arbor, MI, USA) was used. The respirable fraction was expressed in terms of FPM smaller than "5 m (which is defined as the total mass collected on cascade impactor stages 3, 4, 5 and the filter)." The Mass Median Aerodynamic Diameter (MMAD) is the particle diameter below which 50% of the particles enter the impactor reside (particle < 10 m) (28).
Sampling
The five stages, and the induction port of the Marple Miller cascade impactor were coated with propylene glycol : methanol (50 : 50), to control particle bounce when testing dry powder inhalers.
The MDI and the DPI products were tested at three different simulated inspiratory flow rates 30 L/min, 60 L/min and 90 L/min. Each MDI device was shaken for 15 seconds and actuated to waste three times before it was introduced to the impactor through the induction port. One single puff was introduced into the impactor at each nominated flow rate. Flow was allowed to continue for a further 20 seconds before the pump was switched off for both MDI and DPI products. The temperature during the study was maintained at 22°C ± 1°C and the relative humidity at 49% ± 2%.
Sample Preparation And HPLC Analysis
The cascade impactor was dismantled, followed by washing separately each of the five stages and the filter with methanol to collect the deposited drug. In addition, the inhaler mouthpiece of the MDIs and the induction port for both the MDI and the DPI was washed with methanol and each fraction was collected for individual analysis. An aliquot of the methanolic internal standard dexamethasone 21-acetate was added to each sample, and dried at 35șC under nitrogen. Except those from the mouthpiece and the throat piece, all the dried residues were reconstituted in the mobile phase, and injected directly into the HPLC chromatograph. The mouth piece and induction port samples of the DPI products were centrifuged at 15000 RPM before being injected into to the chromatograph using Beckman Microfuge E (Beckman Instruments, CA, USA) to remove the lactose carrier. High performance liquid chromatography was performed on a Beckman System Gold HPLC apparatus (Beckman Instruments, Fullerton, CA, USA) with an ultraviolet detection at 242nm. HPLC column Altima C18 (250 x 4.6 mm i.d) from Alltech was used for determination of the amount of drug in each stage of the impactor. The mobile phase consisted of methanol: acetonitrile: water: acetic acid (60:10:28.3:1.7) at a flow rate of 1.7 ml/min, injection volume 100ml and quantification was by peak area ratio, using a standard curve in the range of 1.0mg/ml-100mg/ml in methanol.
Characterisation by Laser diffraction
Particle size distribution of the original powders was determined using laser diffraction (Mastersizer, Malvern, UK) (29). One single dose from each of the dry powder inhalers was suspended in a 5ml of distilled water. The suspension was sonicated for 30 seconds before being loaded into a stirred sample cell containing water. The concentration of the suspension was adjusted to obtain optimal obscuration. Measurement was repeated five times, each of one minute apart, to ensure that no dissolution or agglomeration of the particles occurred. The size distribution was expressed in terms of the volume median diameter (VMD), which is the value of the particle size that divides the population into two equal halves. The VMD is directly related to the mass median diameter (MMD) by the density of the particles. Hence, the MMD of the powders could be calculated from the product of MMD and the square root of particle density. The particle size distribution of the power was determined using a density value of 1.26 g/ml. The true density of the glucocorticoid powders was determined by buoyancy method (30). Powder samples (1-2 mg) were placed in a density gradient liquid and centrifuged (Jouan CT422, Saint Herblain Cedex, and France) at 3500 rpm and 5°C for 30 min. The particle density was equal to the liquid density when the particles remained stationary in the liquid after centrifugation.
Statistical Analysis
Statistical evaluation of the results was performed using analysis of variance (JMP Statistics Software, V3.1 SAS Institute Inc., Cary, NC, USA). Significant differences between products were further analysed using unpaired t test and p values of < 0.05 were considered to be significant.
RESULTS AND DISCUSSION
Examining the influence of inspiratory flow rate on the FPM and the MMAD for the DPI products revealed that by increasing the inspiratory flow rate from 30 L/min to 60 L/min to 90 L/min, FPM significantly increased by 17% and 75% for FLA and by 1.2 and 2.2 fold for PLT and by 43% and 129% for BCR respectively (Fig. 1).
Figure 1: Influence of inspiratory flow rate 30 L/min, 60 L/min and 90 L/min on the Fine Particle Mass (FPM) for FLA 250g, PLT 200g and BCR 100g (*Significant difference p < 0.05), Mean ± SD for five replicates.
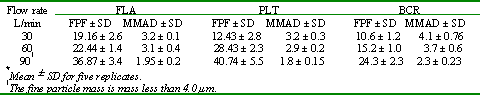
Furthermore, a significant decrease in the MMAD was observed on the aerosolized particles calculated from the particles entering the impactor (28) by increasing the flow rate from 60 L/min to 90 L/min for all the DPI products tested (Table 3).
Table 3: Fine Particle Fraction (FPF)v from the labeled dose and the Mass Median Aerodynamic Diameter of Flixotide Accuhaler® (FLA), Pulmicort Turbuhaler® (PLT) and Becotide Rotahaler® (BCR) at different flow rates.
The results are in good agreement with a previous in vivo study on lung deposition of PLT, which showed that the lung deposition was increased from 14.8% to 27.7% when the inspiratory flow rate was increased from 36 L/min to 58 L/min.(31) However, an in vitro study by Ross and Schultz showed an apparent increase in the FPM of BCR and PLT by increasing the flow rate from 30 L/min to 60 L/min.(32) The results could be explained by the fact that the DPIs products consist of micronised drug particles blended with lactose as a carrier (FLA and BCR) or as a pure drug (PLT), and the efficiency of the micro-particles deaggregation is directly related to the impaction flow rate. Higher flow rates produce more particles in the respirable fraction of the drug excipient mixture.(33,34) The particle size distribution of the original powders of the DPI products was measured by Laser diffraction. The results showed that there is no significant difference in the MMD of the original powders which is approximately 2 m. (Fig 2).
Figure 2: Comparison of the particle size distribution of the primary particles of BCR 100g (1), FLA 250g (2), and PLT 200g (3) measured by laser diffraction and by impaction at 60 L/min using Marple Miller Cascade Impactor Model 160, Mean for five replicates.
However, the aerodynamic particle size distribution of the dispersed powders (measured by impaction technique) showed that the MMAD of the powders was significantly different among the products and considerably higher than that of the original powders. The large MMADs observed in the generated particles from the different devices indicate that all the devices were not efficient enough to deaggregate and to aerosolize the original particles. However, the percentage of the FPM collected from the different products at 60 L/min showed that Turbuhaler® device was more efficient in the generation of the FPM than the other devices (Table 3). These differences in FPM could be due to a further disintegration of the powder agglomerates through the spiral mouthpiece of the Turbuhaler®. The influence of inspiratory flow rate on the FPM and the MMAD for the MDI products was also evaluated (Fig 3) and Table 4.
Figure 3: Influence of inspiratory flow rate 30 L/min, 60 L/min and 90 L/min on the fine particle mass (FPM) for FLI 250g, PLA 200g and BCI 100g (*Significant difference p < 0.05), Mean ± SD for five replicates.
Table 4: Fine Particle Fraction (FPF)v From the labeled dose and the Mass Median Aerodynamic Diameter of Flixotide Inhaler® (FLI), Pulmicort Aerosol® (PLA) and Becotide Inhaler® (BCI).
/N.Davies/Table4.gif)
Increasing the flow rate from 30 L/min to 60 L/min was found to increase the FPM significantly by 53% for FLI and 42% for PLA and BCI. However there is no significant increase in the FPM when the flow rate increased from 60 L/min to 90 L/min. The increase in the FPM with a decrease in the MMAD found in the MDI products could be due to less turbulent deposition in the induction port at higher flow rate due to less mismatch between the velocity of the MDI plume and the surrounding sheath air. Our results are consistent with a previous in vitro study on b-agonists which demonstrated a 40% increase in the FPM of salbutamol sulphate when the flow rate was increased from 30 L/min to 55 L/min (35). Furthermore, the MMAD of the aerosolized particles was affected by the flow rate (Table 4), while the MMAD of FLI and the BCI decreased significantly when the flow rate increased from 30 L/min to 60 L/min, a significant decrease in the MMAD of PLA was demonstrated when the flow rate increased from 60 L/min to 90 L/min.
The aerosol performance of the MDI vs. DPI for the same drug and strength was evaluated at a simulated inspiratory flow rate of 60 L/min, which has been suggested as an optimum flow rate for DPIs (36). In addition the mean peak inspiratory flow rate (PIFR) in healthy humans was found to be 333 L/min in males and 214 L/min in females (18). While in asthmatic patients this PIFR is reduced to 59 (51-93) and 60 (26-103) as reported by Engle et al and Brown et al.(17,37). The FPM delivered from FPI and BCI were 1.2 and 1.4 fold greater respectively than corresponding DPIs, while no significant difference was demonstrated between the two dosage forms of budesonide (Fig 4).
Figure 4: Comparison of the fine particle mass (FPM) delivered from dry powder inhalers (DPI) with that delivered from metered dose inhalers (MDI) (*Significant difference p < 0.05), Mean ± SD for five replicates.
The MMAD generated from the MDIs was smaller than those generated from the DPIs except for budesonide (Fig 5).
Figure 5: Comparison of the mass median aerodynamic diameter (MMAD) delivered from FLA 250g (3), PLT 200g (4) and BCR 100g (2) as DPI products with FLI 250g (6), PLA 200g (1) and BCI 100g (5) as MDI products, Mean (SD) for five replicates.
Budesonide results are in agreement with previous in vitro studies which reported no significant difference between the FPM delivered from the PLT and PLA. For beclomethasone dipropionate there is no significant difference between BCR and BCI demonstrated at 60L/min (32). The results in the deposition of beclomethasone dipropionate products are at variance with a previous study and could by due to the difference in the methodology between studies.
CONCLUSIONS
Although the metered dose inhaler is the older technology it exhibits greater respirable dose in vitro and less variability in dose delivered than newer dry powder inhaler devices except for Turbuhaler®.
Care should be taken when shifting from one inhaler dosage form to another because this may affect the actual dose delivered to the lung. Further in vivo studies may be warranted in light of these findings.
ACKNOWLEDGMENTS
The authors wish to thank 3M Pharmaceuticals Pty Ltd., Thornleigh, NSW, Australia, for the support of a postgraduate student scholarship to Majid Feddah.
REFERENCES
- Adji, A. L., and Gupta, P. K.: Inhalation delivery of therapeutics peptides and proteins, New York, 1997.
- Orehek, J., Gayrard, P., Grimaud, C., and Charpin, J.: Patient error in use of bronchodilator metered aerosols. British Medical Journal 1: 76, 1976.
- Coady, T. J., Stewart, C. J., and Davies, H. J.: Synchronization of bronchodilator release. The Practitioners 217: 273-275, 1976.
- Newhouse, M. T., and Ruffin, R. E.: Deposition and fate of aerosolized drugs. CHEST 6: 936-943, 1978.
- Newman, S. P., Pavia, D. P., and Clarke, S. W.: Simple instruction for using pressurized aerosol bronchodilatiors. Journal of the Royal Society of Medicine 73: 776-779, 1980.
- Crompton, G. K.: Problems patients have using pressurised aerosol inhalers. Eur J Respir Dis 63 (Supp. 119): 101-104, 1982.
- Pederson, S., Frost, L., and Arnfred, T.: Errors in inhalation technique and efficiency in inhaler use in asthmatic children. Allergy 41: 118-124, 1986.
- Willey, R. F., Milne, L. J. R., Crompton, G. K., and Grant, I. W. B.: Beclomethasone dipropionate aerosol and oropharyngeal candidiasis. Br.J.Dis. Chest 70: 32-38, 1976.
- Toogood, J. H., Jennings, B., Greenway, R. W., and Chuang, L.: Candidiasis and dysphonia complicating beclomethasone treatment of asthma. Journal of Allergy and Clinical Immunology 65: 145-153, 1980.
- Smith, M., and Hodson, M.: Effect of long term inhaled high dose beclomethasone dipropionate on adrenal function. Thorax 38: 676-681, 1983.
- Moren, F.: Drug deposition of pressurized inhalation aerosols I. influence of actuator tube design. Int. J. Pharm. 1:, 1978.
- Vidgren, M. T., Paronen, T. P., Kärkkäinen, A., and Karjalainen, P.: Effect of extension devices on the drug deposition from inhalation aerosol. Int. J. Pharm. 39: 107-112, 1987.
- Molina, M. J., and Rowland, F. S.: Stratospheric sink for chlorofluromethanes: chlorine atomc-atalysed destruction of ozone. Nature 249: 810-812, 1974.
- Leach, C.: Approaches and challenges to use of freon propellant replacements. Aerosol of Sci Technol 22: 328-334, 1995.
- Hetzel, M. R., and Clark, T. J. H.: Comparison of salbutamol rotahaler with pressurised aerosol. Clin Allergy 7: 563-568, 1977.
- Pitchard, J. N., Jefferies, S. J., and Black, A.: Sex differences in the regional deposition of inhaled particles in the 2.5-7.5 m size range. J. Aerosol Sci. 17: 385-389, 1986.
- Engel, T., Heinig, J. H., Madsen, F., and Nikander, K.: Peak inspiratory flow and inspiratory vital capacity of patients with asthma measured with and without a new dry-powder inhaler device (Turbuhaler). Eur Respir J 3: 1037-1041, 1990.
- Timsina, M., Martin, G., Marriott, C., Lee, K., Suen, K., and Yianneskis, M.: Studies on the measurement of peak inspiratory flow rate (PIF) with dry powder inhaler devices in healthy volunteers. Thorax 48: 433, 1993.
- Boer, A. H., Winter, H. M. I., and Lerk, C. F.: Inhalation characteristics and their effects on in vitro drug delivery from dry powder inhalers Part I Inhalation characteristics, work of breathing and volunteers preference in dependence of the inhaler resistance. Int. J. Pharm. 130: 231-244, 1996.
- Pedersen, S., Hansen, O. R., and Fuglsang, G.: Influence of inspiratory flow rate upon the effect of a Turbuhaler. Arch. Dis. Childhood 65: 308-309, 1990.
- Agertoft, L., and Pederson, S.: Effect of long-term treatment with an inhaled corticosteroid on growth and pulmonary function in asthmatic children. Respiratory Medicine 88: 373-381, 1994.
- Selroos, O., Pietinalho, A., Löfroos, A., and Riska, H.: Effect of early vs late intervention with inhaled corticosteroids in asthma. CHEST 108: 1228-1234, 1995.
- Carmichael, J., Duncan, D., and Crompton, G. K.: Beclomethasone dipropionate dry-powder inhaler inhalation compared with conventional aerosol in chronic asthma. British Medical Journal 2: 657-658, 1978.
- Bisgaard, H., and Andersen, J. B.: A clinical comparison of aerosol and powder administration of beclomethasone dipropionate in childhood asthma. Allergy 39: 365-369, 1984.
- Engle, T., Heining, J. H., and H.J, M.: Clinical comparison of inhaled budesonide delivered either via pressurised metered dose inhaler or Turbuhaler. Allergy 44: 220-225, 1989.
- Sinninghe Damste, H. E. J., Oostinga, P., and Heeringa, A.: A clinical comparison of inhaled budesonide administered either via a metered dose inhalers or via Turbuhaler. Eur Respir J 2: 832, 1989.
- Hetta, L., Larsson, L. G., and Nikander, K.: A comparative clinical study of inhaled budesonide delivered either via a pressurized metered dose inhalers or via Turbuhaler. Eur Respir J 2: 832, 1989.
- USP: The United States Pharmacopoeia. XXIII: 1765-1766, 1995.
- Steckel, H., and Muller, B. W.: Metered-dose inhaler formulation of fluticasone-17-propionate micorized with supercritical carbon dioxide using the alternative propellant HFA-227. Int. J. Pharm. 173: 25-33, 1998.
- Low, B. W., and Richards, F. M.: The use of gradient tube for the determination of crystal densities. Journal of the American Chemical Society 74: 1660-1666, 1952.
- Borgström, L., Bondessom, E., Morén, F., Trofast, E., and Newman, S. P.: Lung deposition of budesonide inhaled via turbuhaler a comparison with terbutaline suphate in normal subjects. Eur Respir J 7: 69-73, 1994.
- Ross, D. L., and Schultz, R. K.: Effect of inhalation flow rate on the dosing characteristics of dry powder inhaler (DPI) and metered dose inhaler (MDI) products. J Aerosol Med 9: 215-225, 1996.
- Kassem, N. M., Ho, K. K. L., and Ganderton, D.: The effect of air flow and carrier size on the characteristics of an inspirable cloud. J. Pharm. Pharmacol. 41(Suppl): 13P, 1989.
- Zanen, P., van Spiegel, P. I., van der Kolk, H., Tushuizen, E., and Enthoven, R.: The effect of the inhalation flow on the performance of dry powder inhalation system. Int. J. Pharm. 81: 199-203, 1992.
- Smith, K. J., Chan, H. K., and Brown, K. F.: Influence of flow rate on aerosol particle size distribution from pressurized and breath-actuated inhalers. J Aerosol Med 11: 231-245, 1998.
- Sumby, B. S., Cooper, S. M., and Smith, I. J.: A comparison of the inspiratory effort required to operate the Diskhaler inhaler and Turbuhaler inhaler in the administration of powder drug formulations. Br. J. Clin. Res. 3: 117-123, 1992.
- Brown, P. H., Creening, A. P., and Crompton, G. K.: Peak inspiratory flow rates in acute asthma: are they adequate for use of a Turbuhaler? Thorax 47: 2398, 1992.
Corresponding author: Dr. Neal Davies, Faculty of Pharmacy, The University of Sydney, Sydney, NSW, Australia 2006, ndavies@pharm.usyd.edu.au
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps
/N.Davies/Figure1.gif)
/N.Davies/Figure2.gif)
/N.Davies/Figure3.gif)
/N.Davies/Figure4.gif)
/N.Davies/Figure5.gif)