J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 4(2):201-206, 2001
A Novel Extractionless HPLC Fluorescence Method for the Determination of Glyburide in the Human Plasma: Application to a Bioequivalence Study
Jayantilal Khatri1, Sami Qassim, Omar Abed, Boby Abraham, Ali Al-Lami, Syed Masood,
Tabuk Pharmaceutical Manufacturing Co., Tabuk, Kingdom of Saudi Arabia.Received June 12, 2001, Revised July 19, 2001, Accepted July 20, 2001
PDF Version
Abstract
Purpose. To develop a simple, sensitive and rapid HPLC fluorescence method with single step sample preparation for the determination of glyburide in the human plasma.
Methods. Glyburide and ketoconazole (internal standard) were extracted from the 0.5 mL plasma by addition of 0.5 mL acetonitrile and 50 mL CuSO4 solution (5% w/v in water). The separation was achieved on the Kingsorb 3 mm, C8 reverse phase column at ambient temperature with a mobile phase consisted of 45% buffer solution (0.05 M NH4H2P4 ), 40% acetonitrile and 15% methanol adjusted to pH 5.7 by diluted ammonia solution. A fluorescence detector was set at 235 nm excitation wavelength and 354 nm emission wavelengths to monitor eluted components.
Results. The internal standard and glyburide eluted at about 6.7 and 9.6 min, respectively at the flow rate of 1 mL/min. The regression equation was established for every calibration curves (5 ng/mL to 400 ng/mL), which resulted in the correlation coefficient of 0.99 or greater. The absolute recovery ranged from 94.32 to 98.12% and the relative recovery ranged from 91.12 to 97.15%. The intraday coefficient of variation (CV) ranged from of 6.52 to 12.35% and interday varied from 6.21 to 16.07%. The limit of quantitation (LOQ) of glyburide was set to five ng/mL.
Conclusion. This simple, rapid and sensitive method is suitable for pharmacokinetic, bioavailability and biequivalence studies.
Introduction
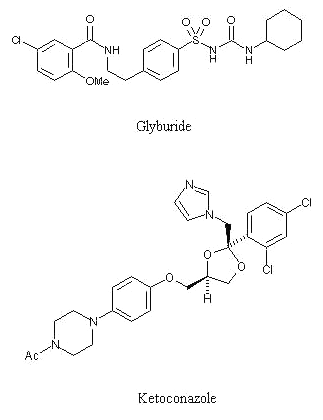
The sulphonylurea hypoglycemic agent glyburide (Figure 1) also referred as glibenclamide in British pharmacopoeia is widely used in the treatment of type 2 diabetes mellitus. The usual initial dose for this highly potent drug is 2.5 to 5 mg daily given as a single dose just before the breakfast (1). Because of relatively prolonged duration of action and episodes of hypoglycemia associated with the treatment, it is essential to monitor the bioavailability of glyburide (2). Several high performance liquid chromatographic methods with UV or fluorescence detector have been developed for the determination of glyburide in body fluids (3-13). Most of the methods are utilizing liquid-liquid extraction or solid phase extraction of drug. All these methods require lengthy sample processing and are time consuming.
Figure 1. Chemical structure of glyburide and ketoconazole (IS)
Materials and Methods
Materials
The master reference standard of glyburide and ketoconazole was purchased from USP (USP, 12601 Twinbrook Parkway, Rockwille, MD 20852, USA). Subsequently, pharmaceutical grade raw material of glyburide and ketoconazole was evaluated against the master reference standard and considered as reference standards. All other chemicals and reagents were of analytical grade (E.Merck, Darmstadt, Germany). The solvents used were of HPLC grade purchased from Carlo-Erba, Milan, Italy. The deionized water was prepared using Milli-Q system (Millipore, Molsheim, France)
HPLC conditions
The chromatographic system was composed of a solvent delivery pump (model 600), an auto injector (model 717), a scanning fluorescence detector (model 474) with excitation and emission wavelengths set at 235 nm and 354 nm, respectively; all from Waters associates (Milford, MA, U.S.A). A 15 cm x 4.6 mm i.d. Kingsorb C8 analytical column packed with 3 mm particle size and a precolumn insert packed with C8 (Phenomenex, Torrance, CA, U.S.A.) were used for separation. The mobile phase consisted of 45% buffer solution (0.05 M NH4H2PO4 ), 40% acetonitrile and 15% methanol adjusted to pH 5.7 by diluted ammonia solution was pumped at 1 mL/min flow rate. The mobile phase was prepared daily, filtered through 0.45 mm nylon membrane filter (Whatman, Maidstone, England) and degassed before use. The chromatograms were acquired and analyzed using EZ-Chrom chromatography data system (Shimadzu, Columbia, MD, U.S.A.). All work was carried out at 22°C laboratory temperature.
Stock solutions
Accurately weighed 50 mg glyburide reference standard was dissolved and diluted to 200 mL in acetonitrile to give primary standard at the concentration of 250 mg/mL. Subsequent dilutions were made to give 0.1, 0.2, 0.5, 1, 2, 4 and 8 mg/mL stock solutions for calibration curve and 0.3, 1.5 and 6 mg/mL stock solutions for validation samples. The primary standard of ketoconazole (IS) was prepared at 1 mg/mL by dissolving accurately weighed 100 mg ketoconazole reference standard to 100 mL in acetonitrile. Further dilution was made to obtain stock solution of internal standard at the concentration of six mg/mL. Primary standards and stock solutions were prepared once weekly. All the solutions were stored at (8 to 12°C).
Calibration samples
In a series of 12 mL, PTFE sealed glass test tubes containing 0.5 mL blank plasma and 25 mL of 6 mg/mL ketoconazole (internal standard) were added with 25 mL stock solution of appropriate concentration to provide calibration samples of 5, 10, 25, 50, 100, 200 and 400 ng/mL. After brief vortex mixing 50 mL of CuSO4 (5% solution in water) and 500 mL of acetonitrile were added to precipitate proteins. Each calibration sample was then vortex-mixed and centrifuged for 15 min. The supernatant solution was transferred to an auto sampler vial (1 mL capacity) and 25 mL aliquot was injected in to the HPLC column.
Plasma samples
Samples obtained from the subject were processed similar to calibration sample as mention under calibration samples except that 25 mL acetonitrile was added in place of 25 mL stock solution.
Pharmacokinetics
The study protocol was approved by the Internal Review Board at the Prince Fahd Bin Sultan Hospital, Tabuk, Saudi Arabia. Twenty six healthy male subjects participated in a single dose fasting crossover bioequivalence study. Following informed consent each subject received 5 mg glyburide tablet either of test formulation (Gliburan, batch no. PD27, manufactured by Tabuk Pharmaceutical Mfg. Co., Saudi Arabia) or reference formulation (Daonil, batch no. 41C224, manufactured by Hoechst AG, Germany). Blood samples (6 mL) were collected in heparinized tubes at predose and 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, 6.0, 7.0, 8.0, 9.0, 10.0, 12.0, 16.0, and 24 h following oral administration of the drug. Plasma was immediately separated by centrifugation and stored at -50°C in 5 mL polypropylene tubes until analyzed.
Results and Discussions
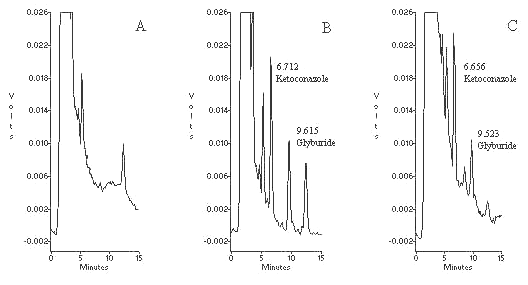
The mobile phase used for the assay provided a well defined separation between the drug, internal standard and endogenous components (Figure 2). The optimum flow rate for the mobile phase (1 mL/ min) resulted in an analysis time of less than 15 min/sample. Possible interference from other drugs was investigated by examining their peak separation from glyburide using the same HPLC conditions. All the investigated OTC drugs including paracetamol, caffeine, aspirin and ibuprofen showed no interference in the assay. The retention times for ketoconazole (internal standard) and glyburide were 6.7 and 9.6 minutes, respectively. The zero hour (predose) samples of all subjects showed no interference at retention time of both the drugs. The possible interference from the metabolites (cis-3-hydroxyglyburide and trans-4-hydroxyglyburide) was assessed by running a set of samples from a subject without addition of internal standard that gave clear base line around the retention time of internal standard. Theoretically, hydroxy metabolites are relatively more polar than parent drug and they should elute before the peak of glyburide in reversed phase chromatography system.
Figure 2. Representative chromatograms of: A) blank pasma, B) plasma spiked with 150 ng/mL ketoconazole (IS) and 100 ng/mL glyburide and, C) plasma sample from a subject 2 h after administration of 5 mg glyburide tablet, the concentration of glyburide is 51.7 ng/mL.
The quantification of the chromatogram was performed using peak area ratios of the drug to internal standard. The calibration curves were constructed routinely for spiked plasma containing 5 ng/mL, 10 ng/mL, 25 ng/mL, 50 ng/mL, 100 ng/mL, 200 ng/mL and 400 ng/mL of glyburide during the process of prestudy validation and in-study validation. Least squares linear regression analysis of the calibration curves resulted in the following equations: Y = 0.0063X + 0.0374, R 2 = 0.998. Standard curves of glyburide in plasma were constructed on eleven different days over a four weeks period to determine the variability of the slopes and intercepts. The results indicated little interday variability of slopes and intercepts, as well as good linearity (r 2 >0.99) over the concentration range studied. The coefficient of variations for the slopes was 6.58%, which indicates good precision of the method. The lowest standard on the calibration curve was five ng/mL and that was accepted as LOQ on the basis of less than 20% CV as acceptance criteria.
The intraday precision was evaluated by analysis of plasma samples containing glyburide at three different concentrations i.e. 5 ng/mL, 75 ng/mL and 300 ng/mL using six replicate determinations for three occasions. The intraday precision showed a coefficient of variation (CV) of 6.52 to 12.35% (Table 1). The interday precision was similarly evaluated over two-week period and showed a CV varying from 6.21 to 16.07% (Table 1).
Table 1: Precision and accuracy of quality control samples
Added
ConcentrationWithin Day
Concentration foundBetween days
Concentration found5 ng/mL
glyburide5.51 (12.35)
Bias% +10.205.37 (16.07)
Bias% +7.4015 ng/mL
glyburide15.32 (10.31)
Bias% +2.3114.12 (9.22)
Bias% -5.8775 ng/mL
glyburide78.38 (7.88)
Bias% 4.5171.22 (6.21)
Bias% -5.04300 ng/mL
glyburide291.73 (6.52)
Bias% -2.76305.74 (7.32)
Bias% +1.91150 ng/mL
ketoconazole143.42 (6.17)
Bias% -4.39144.50 (7.88)
Bias% -3.66Values in () are %CV
Bias% = {(concentration added – concentration found)/concentration added} x 100
The absolute recovery of glyburide was determined by comparing the peak area of the drug obtained from the plasma with peak area obtained by the direct injection of pure aqueous drug standard at three different concentrations on three occasions. The relative recovery of the drug was calculated by comparing the concentration obtained from the drug supplemented plasma to the actually added concentration. As shown in the (Table 2), absolute recoveries of glyburide ranged from 94.32 to 98.12% and the relative recovery ranged from 91.12 to 97.15%.
Table 2: Recovery of glyburide and ketoconazole (IS) in human plasma
Concentration
Peak area in plasma
Peak area in mobile phase
Recovery
%Relative recovery %
15 ng/mL
glyburide38344
(9.58)40653
(2.32)94.32
93.94± 6.82
75 ng/mL
glyburide162803
(8.49)166926
(0.97)97.53
91.12±7.12
300 ng/mL
glyburide602227
(5.54)613766
(1.88)98.12
97.15±4.31
150 ng/mL
ketoconazole327065
(6.55)336556
(0.92)97.18
95.27±6.13
Values in parentheses are % CV
The stability studies of plasma samples spiked with glyburide (5 ng/mL, 75 ng/mL and 300 ng/mL) were performed over four weeks (Table 3). In addition, stability studies of plasma samples spiked with glyburide were performed for 24 hour period at room temperature storage and for three freeze-thaw cycles. The plasma samples were stored in the freezer at -50°C until the time of analysis. The results show that glyburide can be stored frozen in the plasma for one month without degradation. The results of short term storage at room temperature, solution stability and freeze-thaw cycles indicated no degradation of glyburide in plasma as well as in solution therefore samples could be handled without special precautions.
Table 3: Stability of glyburide in human plasma during storage and sample handling
Concentration in plasma
15 ng/mL
75 ng/mL
300 ng/mL
Freeze and thaw:
Cycle 1
Cycle 2
Cycle 315.5
15.1
14.276.72
67.62
68.01296.79
285.70
291.58Short term (plasma sample at room temperature):
After 2 h
After 8 hr
After 24 hr15.11
15.73
15.0573.36
71.61
69.52294.54
303.41
314.72Long term (plasma sample at –50° C):
After 1 week
After 2 week
After 3 week
After 4 week16.30
15.18
16.64
15.9372.27
69.50
79.90
73.52281.12
309.30
303.66
292.39Auto sampler (extracted sample at room temperature):
After 2 h
After 8 hr
After 24 h15.14
15.38
15.2774.81
72.49
75.05301.69
297.77
301.08Stock solution (µg/mL)
After 6 h at RT
After one week in Freeze0.3
1.5
6.0
6.0 (IS)
0.289
0.2861.428
1.4616.166
6.2486.010
6.027
The bioequivalency of two glyburide products, reference and generic formulation was compared in 26 healthy male volunteers who received a single 5 mg oral dose in crossover design. As shown in (Figure 3) the generic lot that exhibited a wide difference in bioavailability compared to the reference product. The peak plasma levels were significantly higher for test product than reference.
Figure 3. Mean concentration-time profiles of 5 mg glyburide following administration of test and reference tablet to 26 healthy subjects, error bars represent + SD.
The 90% confidence intervals of T/R ratio of the Cmax, AUC0-24 , AUC0-¥, were out side the bioequivalence acceptable range of 80-125% and therefore two products were concluded bioinequivalent (Table 4). The pharmacokinetic parameters and ANOVA was calculated using WinNonlin Professional program (Pharsight Corporation, 299 California Avenue, Palo Alto, CA 94306.). The mean value of Cmax, AUC0-24, AUC0-¥, Tmax, Ke, T1/2 for test product was 175.9 ng/mL, 782.6 ng.h/mL, 870.4 ng.h/mL, 3.25 h, 0.4387 h-1 , 2.49 h and that for reference was 107.9 ng/mL, 543.3 ng.h/mL, 622.3 ng.h/mL, 0.4098 h-1 , 2.93 h, respectively.
Table 4: Bioequivalence statistics (Two, One-Sided T tests)
Dependent|
variableLeast Squares Mean (LSM)
LSM
ratio90%|Cl
Prob.
(<80%)Prob.
(>125%)Power
Gliburan
Daonil
Ln (Cmax)
5.0667
4.6179
156.6
134.88-181.91
0.0000
0.9972
0.6854
Ln (AUC0-24)
6.5003
6.2066
134.1
114.71-156.87
0.0000
0.8825
0.6446
Ln (AUC0-¥)
6.6267
6.3559
131.1
114.91-149.52
0.0000
0.8694
0.7952
Conclusions
This simple, rapid and validated high performance liquid chromatographic method with single step sample preparation is permitting to process at least 60 samples a day. The method is successfully applied to about 700 samples collected for a bioequivalence study. The stability results indicated that glyburide and IS are quite stable in plasma and in the solutions therefore no special precautions are needed during sample treatment and storage. Because of high sensitivity and 0.5 ml sample requirement, this method can be used in pharmacokinetics, bioavailability and bioequivalence studies.
Acknowledgments
We wish to thank Dr. Zaid Salhab of Prince Fahd Bin Sultan Hospital for his guidance in instituting norms of Good Clinical Practice, particularly, the ethical issues and documentation involved in conducting bioequivalence studies.
References
Kathleen P., Martindale. The pharmaceutical press, London, UK, 1999.
Ferner RE, Neil HAW. Sulphonylureas and hypoglycemia. Br Med J 1988; 296: 949-950.
Al-Dhawailie AA, Abdulaziz MA, Tekle A, Matar KM. A simple, specific, and rapid high-performance liquid chromatographic assay for glibenclamide in plasma. J Liq Chromatogr 1995; 18: 3981-3990.
Arcelloni C, Fermo I, Calderara A, Pacchioni M, Pontiroli AE, Paroni R. Glibenclamide and tolbutamide in human serum: Rapid measurement of free fraction. J Liq Chromatogr 1990; 13: 175-189.
Shenfield GM, Boutagy JS, Webb CA. Screening test for detecting sulfonylureas in plasma. Ther Drug Monit 1990; 12: 393-397.
Starkey BJ, Mould GP, Teale JD. The determination of sulphonylurea drugs by HPLC and its clinical application. J Liq Chromatogr 1989; 12: 1889-1896.
Othman S, Shaheen O, Jalal I, Awidi A, Al-Turk W. Liquid chromatographic determination of glibenclamide in human plasma. J Assoc Off Anal Chem 1988; 71: 942-944.
Zecca L, Trivulzio S, Pinelli A, Colombo R, Tofanetti O. Determination of glinenclamide, chlorpropamide and tolbutamide in plasma by high performance liquid chromatography with ultraviolet detection. J Chromatogr 1985; 339: 203-209.
Rydberg T, Wahlin-Boll E, Melander A. Determination of glibenclamide and its two major metabolites in human serum and urine by column liquid chromatography. J Chromatogr 1991; 564: 223-233.
Emilsson H, Sjoberg S, Svedner M, Christenson I. High-performance liquid chromatographic determination of glibenclamide in human plasma and urine. J Chromatogr 1986; 383: 93-102.
Abdel-Hamid ME, Suleiman MS, El-Sayed YM, Najib NM, Hasan MM. A rapid high-performance liquid chromatography assay of glibenclamide in serum. J Clin Pharm Ther 1989; 14: 181-188.
Gupta RN. Determination of glyburide in human plasma by liquid chromatography with fluorescence detection. J Liq Chromatogr 1989; 12: 1741-1758.
Adams WJ, Skinner GS, Bombardt PA, Courtney M, Brewer JE. Determination of Glyburide in human serum by liquid chromatography with fluorescence detection. Anal Chem 1982; 54: 1287-1291.
Corresponding Author: Jayantilal Khatri, Tabuk Pharmaceutical Manufacturing Co., P.O.Box 3633, Tabuk, Kingdom of Saudi Arabia. khatrijm@hotmail.com
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps