J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 4(2):138-158, 2001
Potential of polysaccharide anchored liposomes in drug delivery, targeting and immunization
V. Sihorkar, S.P. Vyas1
Drug Delivery Research Laboratory, Department of Pharmaceutical Sciences, Dr. H.S. Gour University, Sagar, M.P., IndiaReceived October 25, 2000, Revised July 6, 2001, Accepted July 7, 2001
PDF version
Abstract
Purpose: Recently the emphasis has been laid upon the carbohydrate mediated liposomal interactions with the target cells. Among the various carbohydrate ligands, such as glycoproteins, glycolipids, viral proteins, polysaccharides, lipo-polysaccharides and other oligosaccharides, this review deals with the polysaccharide anchored liposomal system for their potential in drug delivery, targeting and immunization. Over the years, various strategies have been developed which include coating of the liposomal surface with natural or hydrophobized polysaccharides, namely mannan, pullulan, amylopectin, dextran etc., or their palmitoyl or cholesteroyl derivatives. The polysaccharide(s) coat tends vesicular constructs physicochemically stable in bio-environments and site-specific. The aim of improving the physical and biochemical stability of liposomes and the ability to target liposomes to specific organs and cells, were the major attributes of the polysaccharide anchored liposomes. In this review the authors attempted to overview various applications of polysaccharide bearing liposomes, including lung therapeutics, targeted chemotherapy, cellular targeting, cellular or mucosal immunity and macrophage activation. Future prospects of the delivery module are also discussed. The review in general explores the concepts, options and opportunities of polysaccharide anchored liposomes with newer perspectives.
Drug delivery with liposomes as carrier systems provide options and opportunities for designing bio-stable and/or site specific drug therapy. Liposomal systems have been optimistically considered as "magic bullets" for more than 3 decades. The engineered or tailored versions of liposomes offer potentials of exquisite levels of specificity and drug targetability (1). The structural versatility of liposomal systems in terms of vesicle size, shape, surface morphology, composition, surface charge and bilayer fluidity; their ability to incorporate a wide spectrum of drugs; or to carry cell-specific ligands render them clinically and therapeutically viable and clinically versatile.
The incorporation of various explicit and site directing bio-molecules (ligands) on colloidal carriers make them suitable either for stability in bio-fluids or site specificity towards receptors or antigenic determinants expressed on target sites. Paul Ehrlich (6) in his pioneering `magic bullet' concept proposed and realized that the drug could be targeted with the help of groups/ligands having well defined affinity for specific cells (or receptors/antigenic determinants expressed on target cells). The ligands can either covalently or non-covalently be attached to the surface of liposomes and could direct liposomes and encapsulated contents en route to predefined accessible cells. Liposomes as a drug carrier system has been utilized as circulating units for therapeutic delivery of various bio-sensitive and bio-active ligands including antibodies (2), glycopeptides (3), oligo-saccharides (4), viral proteins and fusogenic residues (5). The ligands confer target specificity and recognition ability to the drug-carrier system. However, in some cases, ligands only confer stability and better structure integrity against harsh bio-environments encountered after oral or parenteral administration.
Liposomes based target recognition is a critical prerequisite for ligand mediated targeting and customarily selected ligands should have a well defined propensity, avidity and specificity towards receptor portals expressed selectively on the selected target cell(s). Various ligands have been investigated for their bio-signaling and bio-sensing potential. Some of them are, anti-target monoclonal antibodies or haptens (7), sialic acid (3), lectins (8), polysaccharides (9), glyco-conjugates like glycoproteins (10), glycolipids (11) and sialo-glycoconjugates (12).
The objective of this article is to provide an overview on possibilities and potentials of polysaccharide(s) as ligands for liposomes. The major focus of the previous reviews being the stability and physicochemical characterization of the polysaccharide coated liposomes and their utility to serve as models for cell-cell adhesion or interaction studies. However, in the present review their potential, as drug delivery systems and drug targeting systems, will be revealed.
Polysaccharides As Delivery Portals
The role of cell surface oligo-saccharide(s) and their functional attributes are pivotal in order to rationalize and realize their significance in targeted drug delivery. It is a well-known fact that the surface of the mammalian or microbial cells contains carbohydrate moieties in abundance mainly oligosaccharides associated with membrane lipids, proteins or peptide glycans. This membrane associated carbohydrate-rich material referred to as "glycocalyx" and is the focal and prime locus around which research over last few years has been revolving and proliferating. The glycocalyx is specially involved in cell processes such as cell-cell recognition and adhesion, the binding of pathogens, bacteria and virus to their target tissue, sperm-egg binding and lymphocyte-endothelium recognition. These oligosaccharides (saccharide determinants) of the cell surface glycocalyx play a central role in cellular adhesions and biological recognition processes and constitute potential recognition sites for carbohydrate-mediated interactions between cells and drug carriers bearing suitable site directing molecules (13). The glycocalyx of cells usually contains high proportions of polysaccharides therefore, it has been thought appropriate to explore and investigate the utility of polysaccharides in targeted drug delivery. Some of the naturally occurring polysaccharides anchored to artificial cell walls can potentially become a target cell-sensing device imparting specificity, avidity and targetability to the carrier. Moreover, the polysaccharide appended drug carriers can circumvent bio-environment-derived stresses and could effectively deter bio-sensitive contents from biodegradation (14-16).
Polysaccharides from higher plants and algae have already been used intensively on the technical scale for a long time. Recently, more attention is being paid towards polysaccharides from microorganisms and yeast. In addition to the well-established polysaccharides containing pharmaceutical materials, considerable interest has been generated in a number of polysaccharides with intrinsic pharmacological activities. These include immuno-modulation, anti-tumor, anti-inflammatory, anti-coagulant, hypoglycemic and antiviral activities. However more recently, these polysaccharide modules have been exploited to navigate the carrier to its destined site of action (14-23).
Among the macromolecular polysaccharides reported as molecular carriers are, mannan, amylopectin, pullulan and dextran, either in their native form or as carrier-conjugates. Almost all naturally occurring polysaccharides mentioned here are known to protect cell plasma membranes against physicochemical stimuli, such as osmotic pressure and ionic stress. However, on adsorption to lipid carriers, the peptization or coagulation of the system may occur, and probably due to this reason partially hydrophobized polysaccharides are recommended and used in drug delivery system (scheme 1).
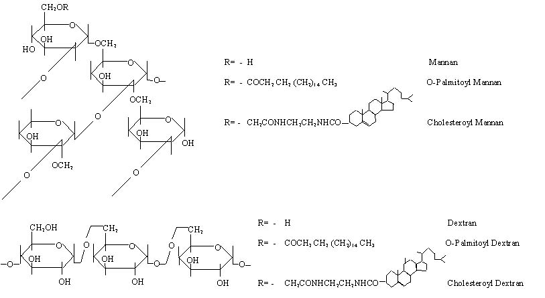
Scheme 1: Naturally occurring polysaccharides used for the surface anchoring of liposomes with their hydrophobized derivatives
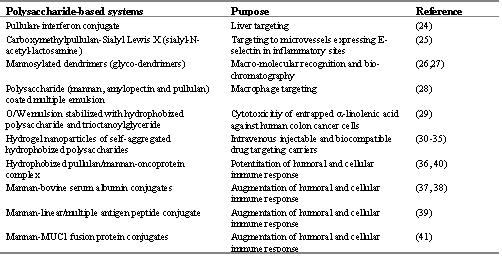
Some of them have intrinsic biological responses however others show their targetability when they are administered in conjugated macromolecular form and some are used when they are anchored on an appropriate delivery system (24-42) (Table 1)
Table 1: Various polysaccharide-based systems and their therapeutic significance reported in the literature
Polysaccharide Anchored Liposomes
In the development of polysaccharide anchored liposomes for therapeutic purposes, it is important to consider the mechanisms and methodologies of the polysaccharide association with the bilayer membrane and resultant effect on the bilayer permeability, fluidity, and integrity. The affinity and selectivity of the anchored polysaccharide towards its complementary ligand(s) is a desirable prerequisite that makes the system site specific and target oriented. A great deal of work has been reported on liposomal systems involving polysaccharide-mediated interactions however, the realistic use of polysaccharide(s) for the drug delivery to the desired site(s) has not been realized clinically. Nevertheless, the significance of carbohydrate and polysaccharide specific recognition domains on the cell surface has stimulated the research quantitatively towards exploitation of technology to develop systems for drug(s) and/or antigen(s) (43-45).
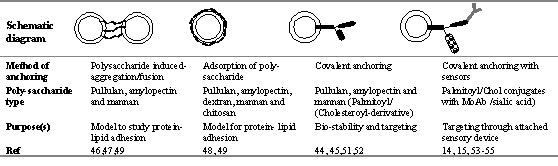
Polysaccharide-anchored liposomes are well documented and mostly studied as a model to study cell-cell adhesion (reviewed by Jones, 13) but recently they are also studied for varied therapeutic potentials. Considering the potential of natural or hydrophobized polysaccharides, methods have been developed to link polysaccharides to the surface of liposomes (43). Earlier methods were attempted to anchor polysaccharides on the surface of the liposomes through adsorption, however recently spacer activated covalent coupling or hydrophobic anchoring have been appreciated as methods of anchoring (Table 2).
Earlier methods of anchoring exploited possible interaction of liposomes and polysaccharides. Sunamoto and co-workers (46,47,49) investigated interactions of simple polysaccharides and liposomal membranes. These workers revealed that simple and naturally occurring polysaccharides, such as dextran, chitosan, pullulan, mannan, or amylopectin, strongly adhere on to the liposomal surface mostly via hydrophobic interactions inducing subsequent aggregation and fusion of liposomes. Under specific conditions however, which do not allow for aggregation or fusion, the adsorption of polysaccharides over liposomal membranes may be due to diffusion controlled mechanism of constitutive components and coat, followed by lateral diffusion and subsequent inter-digitization of adsorbed polysaccharide molecules into bilayers (48). This hypothesis was later confirmed and substantiated by fluorescence depolarization technique using FITC-dextran as marker probe (49).
Table 2: Various polysaccharides capped liposomes with their formulation strategies and aims
Polysaccharide anchoring by adsorption was found to be thermodynamically unstable and pharmaceutically unacceptable due to following reasons:
- The polysaccharides adsorbed on the liposomal surfaces easily desorb/delodge on dilution or on mechanical agitation.
- Peptization or coagulation of the polysaccharides could lead to subsequent destabilization of the liposomal bilayer
- Stoichiometric ligand density is often non-reproducible.
In order to obviate adsorptive coating related limitations, Sunamoto and Iwamoto (50) employed chemically modified polysaccharides, i.e., palmitoylated polysaccharides, to coat the liposomes. These partially hydrophobized polysaccharides were allowed to react covalently and subsequently integrate with the lipid constituents of liposomal membranes.
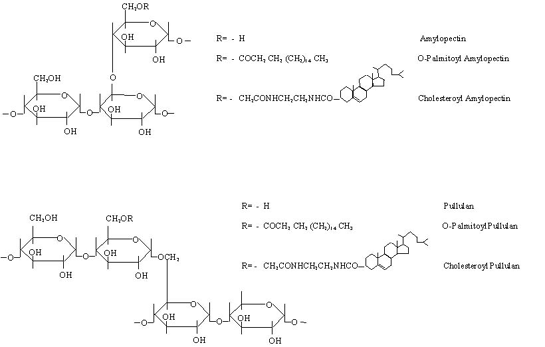
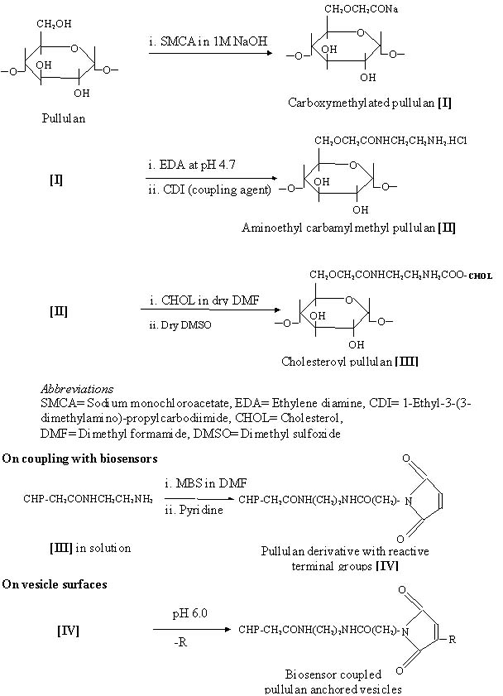
The surface modification of liposomes is mediated through the hydrophobic legs (chemically conjugated or palmitoylated) of pullulan or amylopectin derivatives that digitize into the liposomal bilayer. Palmitoyl conjugates of pullulan, pullulan phosphate, amylopectin, amylopectin phosphate, mannan and dextrans have been employed intensively to coat the liposomes (10). When added to liposomes the hydrophobic anchors interact with the outer half of the bilayer orienting and projecting hydrophilic portion towards the aqueous bulk. This architect a polysaccharide based artificial cell wall on the outermost surface of the liposomes (Scheme 2).
Scheme 2: Preparation of O-palmitoyl poly-saccharides (pullulan is being used as an example) anchored vesicles
An improved methodology in order to prepare both bio-stable and targetable drug carrier systems, has been developed (15). In place of palmitoyl anchor, cholesteroyl anchor has been employed for polysaccharide inter-digitization. Cholesterol moiety can be introduced in to the (Scheme 3). In place of palmitoyl anchor, cholesteroyl anchor has been employed for polysaccharide inter-digitization.
/S.Vyas/SCHEME3(B)-JPPS-995656.jpg)
Scheme 3: Preparation of cholesteroyl polysaccharide-anchored vesicles with the possibilities of anchoring of biosensors
Coating of liposomes with these hydrophobized polysaccharides can be performed by incubation of aqueous solutions of polysaccharide derivatives with preformed liposomal dispersion. In some cases, Chol substituted polysaccharide was used to conjugate sensory devices like sialic acid derivative (51) or an IgM fragment (52). The sialic acid conjugated Chol substituted polysaccharide or immuno-polysaccharide derivatives were subsequently anchored over liposomes by dispersion-incubation technique under optimized standard conditions. The method has been used in the preparation of newly designed immunoliposomes where PC based large oligolamellar vesicles were anchored to the polysaccharide pullulan (54). The system has been modified to carry both, i.e., cholesterol as a hydrophobic anchor, and monoclonal antibody fragment (anti-sialosyl Lewis x IgMs) as a sensory device (Scheme 3). Various studies moreover reflect that polysaccharides anchored on liposomes using above-mentioned methods retained their ligand affinity and specificity (29,60).
Stability Of Polysaccharide Anchored Liposomes And Their Oral Delivery Potential
Polysaccharide capped liposomes have been appreciated to be physically and chemically stable systems against biochemical and physicochemical stresses encountered in bio-fluids specially after oral administration. Sunamoto and Iwamaoto (50) categorically reviewed some characteristics of polysaccharide capped liposomes which rationalize their use in drug delivery:
- Reduced permeability to water-soluble encapsulated materials in the presence of blood plasma/serum or its components.
- Increased stability against enzymatic attack and protection of phospholipids from lipases and lipoxygenases.
- Mechanical and biochemical stability towards bio-stimuli such as pH, osmotic pressure, ionic strength, temperature and dynamic challenges of bio-fluids.
Unfortunately, most of the studies to date cover in vitro stability aspects of these liposomes and lack parallel in vivo studies to correlate the results.
Structural stability of polysaccharide-anchored liposomes is examined by Moellerfeld and co-workers (53) employing both hydrophilic and lipophilic markers. O-palmitoyl amylopectin anchored liposomes labeled with [3H] inulin in the internal aqueous phase and [14C] coenzyme Q10 in the lipid bilayer were fairly stable in the blood circulation as well as in tissues as evident from radio-isotope analysis. Increased long-term stability and membrane integrity was also recorded in black lipid membranes anchored with polysaccharide derivatives bearing hydrophobic (mainly palmitoyl or cholesteroyl) anchor groups (53). In another study, Sunamoto and co-workers (15) developed site-specific and target oriented liposomes anchored with immuno-polysaccharide conjugates and demonstrated their structural stability in the presence of 18% (v/v) human serum in vitro .
Coating using hydrophobized polysaccharide not only stabilizes liposomes but also stabilizes proteo-liposomes in bio-environment (54). The stability of proteo-liposomes prepared from Escherichia coli phospholipids and anchored with hydrophobized dextran was evaluated (54). A high concentration of hydrophobized dextran protected the liposomes against detergent degradation, decreased the fluidity of the membrane, prevented fusion of the liposomes and enhanced their biochemical stability. Reduced fusion, protection against the loss of membrane by freezing/thawing, and reduced permeability of the water soluble marker 6- carboxyfluorescein (6-CF) were the substantial evidences towards better stability (mechanical and chemical) of the proteo-liposomes. In the latest developments, Sehgal and Rogers (55) reported polymer-anchored liposomes containing cytosine-arabinoside (Ara-C) anchored with OPP. These liposomes were challenged with sodium cholate (SC) concentrations at varied pH conditions. Stability of OPP anchored liposomes in sodium cholate (SC) concentrations up to 16 mM at pH 5.6 or in SC solutions at pH 7.4 was appreciably improved. Further, at pH 2.0 and 37∞C, the ratio of Ara-C released from uncoated versus coated liposomes (kuo/kco) was found to be 1.9 and 5.7 for the liposomes constructed of DMPC and DPPC respectively. At pH 7.4 and 37 ° C in the presence of 10 mM SC, the ratio kuo/kco was 5.1 and 1.4 respectively. These studies suggest that polysaccharide anchored liposomes are relatively stable systems and hence could be exploited for the delivery of drugs to harsh bio-environments such as those encountered after oral administration.
Moreira et al. (56) prepared fluorescent probe bearing liposomes anchored with partially hydrophobized O-palmitoyl pullulan at an OPP/PC weight ratio of 3. The improved stability probably imparted by decreased permeability and the fluidity of the outer region of the liposomal membranes. Furthermore, the same group encapsulated carboplatin in OPP anchored liposomes and studied platinum latency in liposomes to relate liposome stability on shelf and long term storage (57). The polysaccharide/lipid weight ratio has been reported to be critical and necessitates optimization. The higher ratio presumably causes dislocations or defects in the bilayer probably due to interference of the derivatized or palmitoyl anchored lipid molecules in membrane packing characteristics. The derivatized lipids create locus of defects allowing free volume with passage of encapsulated content.
In different studies however, the oral delivery potential of polysaccharide and polymer coated liposomes have been addressed. These studies suggest the role of transport via paracellular and transcellular routes from normal epithelial tissue or Peyer's patches, leading to different outcomes of drug delivery (Rogers and Anderson, 58) and immunization (Aramaki et al., 59).
Recently, Mumper and Hoffman (61) reported stabilization of hirudin (a potent and specific inhibitor of thrombin) entrapped liposomes upon coating with palmitoyl dextran. These workers correlated the effect of hirudin stability provided by the coated liposomes with the sustained release and resultant higher inhibition of thrombin formation, using an in vitro thrombin chromogenic substrate assay. Palmitoyl dextran− coated-liposomes showed a burst of 30% hirudin released in 5 hours with an additional 10% to 35% released over the next 600 hours. The released hirudin retained only 33% of its ability to inhibit thrombin when released from uncoated liposomes. However, hirudin retained 95% of its thrombin inhibitory activity when released from palmitoyl dextran− coated liposomes. Coated liposomes were found to stabilize hirudin and result in greater retention of hirudin's ability to inhibit thrombin's enzymatic activity.
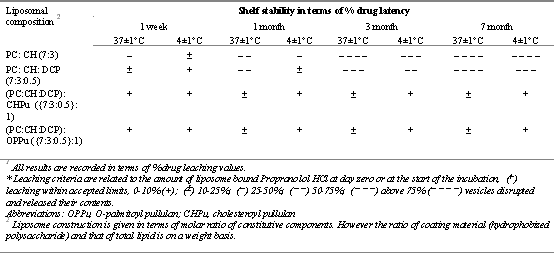
Sihorkar and Vyas (62) investigated the oral delivery potential of palmitoyl pullulan (OPPu) and cholesteroyl pullulan (CHPu) coated liposomes against the challenges of detergent and bile (bile salts and fresh-pooled rat bile), freeze-thaw cycling and long-term storage. The stability of liposomes was tested by incubating them with different molar concentrations of bile salt, taurocholate (below, above and near CMC) at 37 ° C for a period of 2 hours. No significant changes in the vesicle size, integrity and drug content were observed and both OPPu and CHPu coated liposomes exhibited exceptional stability as compared against plain formulations. (Fig 1).
Figure 1: Interaction of different polysaccharide anchored and plain liposomal formulations with sodium taurocholate. The experiment was conducted with sodium taurocholate dissolved in phosphate buffered saline (pH 7.4) to concentrations below, at and above critical micelle concentrations. At an incubation time of 2 h at 37°C aliquots of plain liposomes (
), OPPu anchored liposomes (
), and CHPu anchored liposomes (
), were removed, centrifuged and supernatant analyzed for the drug content and from the data so obtained % of initial contents were calculated. Reproduced from (62), with permission.
Double diffusion barrier encountered by diffusing drug(s) in case of anchored liposome could be a determinant in offering exceptional stability profile. Similarly, freeze-thaw cycling could not bring any fusion or collapse of the liposomal membrane (unlike unanchored ones). Furthermore, an appreciable shelf stability of the anchored vesicles both at 37 ± 1 ° C and at 4 ± 1 ° C (Table 3) was recorded. These results establish the potential of polysaccharide coated liposomes as a stabilized delivery system for oral administration of water-soluble agents. However, these in vitro stability studies need a better correlation with the in vivo performances of these stabilized systems.
Table 3: Stability studies of various anchored and plain liposomal systems1. Adapted and reproduced from (62) with permission.
Therapeutic And Clinical Applications
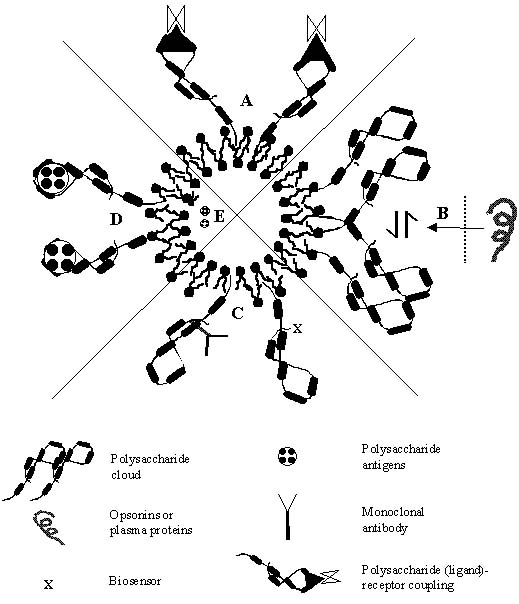
Polysaccharides have been utilized in recent years for various delivery and targeting strategies, either for they provided stabilization and formed a skeleton on which suitable sensory molecules were appended or otherwise they themselves behave as sensory devices to bring out the resultant targeted therapeutic effects (Figure 2). Some of the therapeutic benefits that polysaccharide anchored vesicles offer is discussed.
Figure 2: Various drug delivery, targeting and biotechnological areas where polysaccharide -bearing liposomes could be exploited as tools for future therapy. (A) Macrophage targeting based upon the ligand-receptor interaction. (B) Stealth behavior of the polysaccharide cloud, which further provides a hydrophilic environment to deter them from the opsonins and plasma proteins. (C) Polysaccharides as bio-sensors (targeting ligands) and offering bio-protection. (D) Vaccination potential of anchored bacterial polysaccharides or lipo-polysaccharides. (E) Encapsulating polysaccharide antigens for vaccination.
Lung Therapeutics
Increased lung accumulation of polysaccharides anchored liposome promises for their selectivity and potential as drug delivery system for the therapy of lung diseases (63). The alveolar macrophages selectively sequester the O-palmitoyl amylopectin anchored liposomes (64). The macrophagic uptake has been confirmed with the help of fluorescent probe marker, as marker was traced mainly in macrophages after IV injection (65). In experiments based on fluorescent probe-loaded and 14C-labelled liposomes, it has been observed that both human monocytes and alveolar macrophages of guinea pig can internalize the O-palmitoyl amylopectin anchored liposomes at higher accumulation levels than conventional liposomes. Moreover, OPA anchored liposomes are reported to be sequestered and retained selectively in lungs by anionic-scavenging receptors. Liposomes appended with O-palmitoyl pullulan (OPP) and O-palmitoyl amylopectin (OPA) are rapidly cleared from the blood as compared to `naked' liposomes. Though they have a relatively wide tissue distribution including liver and spleen, it is found that OPA anchored liposomes are selectively intercepted sequestered and internalized by the lung macrophages and monocytes. Subsequent to this observation, investigations were made on OPA anchored liposomes to explore their potential as a delivery system for sisomycin treatment of lung diseases in guinea pigs infected with Legionella pneumophila (49,66). The therapeutically beneficial results of these studies subsequently promoted further investigations, where OPA anchored liposomes were tested for targeted delivery of antimicrobial agents against intracytoplasmic pathogens and fungus (67). Specifically, amylopectin anchored liposomes were found to be effective for the delivery of ceftazidine to L pneumophila infected guinea pigs where relative to treatment with free drug the survival rate achieved following the liposome treatment was 30%.
The liposomally encapsulated drug accumulation in the lung was two-fold higher compared to lung drug concentration following free drug administration. In the treatment of Listeria monocytogenes infection in mice with ampicillin or minocycline bearing surface modified liposomes (anchored with cholesteroylated amylopectin), the survival rate recorded was 100% as compared against 70% recorded for free ampicillin, and 80% as compared against 20% for free minocycline. The toxicity of amphotericin B was found to be substantially reduced in the case of amylopectin anchored liposomes when administered in mice infected with pulmonary candidiasis. The LD50 for free amphotericin B in mice was 1.2 mg kg-1. It was increased to 12 mg kg-1 when administered encapsulated in polysaccharide-anchored liposomes, suggesting increased therapeutic benefits with greater safety index. Miyazaki and coworkers (68) revealed that coating liposomes with amylopectin negotiates targeting of the incorporated amphotericin B to the lungs. The LD50 of amylopectin-anchored liposomal amphotericin B in normal mice was more than 10.0 mg/kg, whilst for conventional amphotericin B, LD50 recorded to be 1.2 mg/kg. Amylopectin-anchored liposomes showed two-fold higher accumulation in the lungs as compared to conventional liposomes. These workers further studied in vivo efficacy of the system using murine model of pulmonary candidiasis. Candida albicans was inoculated intratracheally into BALB/C mice and the number of Candida in the lungs of mice treated with amylopectin-anchored liposomes and conventional liposomes were compared. The amylopectin-anchored liposomes improved the survival rate of inoculated mice.
Recently, Poiani and co-workers (69) encapsulated the copolymer of cis-4-hydroxy-L-proline (an anti-fibrotic agent) and PEG in liposomes. These liposomes were anchored with amylopectin and their efficacy was compared with liposomes conjugated with PEG in terms of improved lung uptake after intravenous infusion. The proline analogue cis-4-hydroxy-L-proline (cHyp) inhibits collagen accumulation and diffuses out of tissues. The encapsulation in amylopectin anchored liposomes prolonged the antifibrotic effect of the polymer. Amylopectin anchored liposomes had approximately 3-fold greater uptake in cultured endothelial cells compared with PEG-liposomes with greater lung retention as estimated 1 week after infusion (5.2 ± 0.8% vs. 2.7± 0.2%, p < 0.05). Sustained antifibrotic activity was assessed by recording the inhibition of collagen accumulation in pulmonary arteries of hypoxic (10% O2) rats and by growth inhibition of cultured endothelial cells and fibroblasts. The activity was greater for amylopectin anchored liposomes/copolymer system than PEG-liposomes/copolymer system. Amylopectin-liposomes/copolymer attenuated increased right ventricular pressure by approximately 50% and it was inferred that antifibrotic polymer could totally prevent collagen accumulation for nearly 1 week, which in fact is desirable for vascular remodeling in pulmonary arteries. Very recently, Cansell and co-workers (70,71) reported phosphatidylcholine (PC), phosphatidylethanolamine (PE), and cholesterol (70:10:20 mol%) liposomes anchored with dextran (Dx) or functionalized dextran (FDx), both hydrophobized using a cholesterol anchor (CholDx or CholFDx) that penetrates the lipid bilayer during the vesicle formation. The study was performed using radiolabeled markers and fluorescent probes and revealed that coating of liposomes with FDx enables specific interactions with human endothelial cells in culture. Conclusively, bioactive polymer anchored liposomes hold promise as an attractive approach for vascular cell targeting.
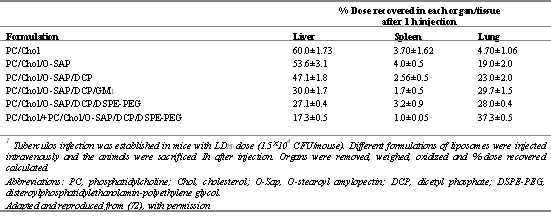
Deol and Khullar (72) who developed polysaccharide-anchored liposomes for long circulation and/or lung localization experimented with in vitro and in vivo models and suggestively reported similar findings. The anchored modules were reported effective in chemotherapy against tuberculosis. Modification of surface of stealth (pegylated) liposomes by tagging O-stearoylamylopectin (O-SAP) resulted in an increased affinity and hence a higher accumulation in lungs than RES predominant organs in normal and tubercular mice (Table 4).
Table 4: Distribution of different labeled liposome formulations after 1 h of intravenous injection in tuberculous mice1. Adapted and reproduced from (72) with permission
Isoniazid and Rifampicin encapsulated in stealth liposomes designed with predominant lung specificity demonstrated reduced in vivo toxicity (72). These observations suggest that lung specific stealth liposomes will certainly improve the chemotherapy against human pulmonary tuberculosis and related pulmonary disorders.
Thus it may be realized that alveolar macrophages constitute ligand responsive cellular species which could operate bio-mechanically for drug-carrier uptake, especially those which are anchored with hydrophobized polysaccharides. The uptake may be either by a receptor mediated or receptor independent affinity mechanisms. Furthermore, the colloidal carriers with neutral surface charge are taken up more slowly by macrophages as compared to those, which bear charged surfaces (74,75). The negative charged surfaces are rapidly and excessively taken up probably via charged scavenger receptors. The role of anionic ligands and their subsequent receptor mediated uptake was further evidenced by coating amphotericin bearing liposomes using O-palmitoylated mannan (OPM) and p-aminophenyl-mannopyranoside (PAM) as specific ligand modules and assessing their selective role in receptor-mediated endocytosis (73). Comparative in vivo distributions and targeting profiles of O-palmitoylated mannan and P-aminophenyl-mannopyranoside anchored liposomes as compared to plain liposomes were studied in terms of% drug localization indices (Figure 3).
Figure 3: Drug localization indices for different organs recorded with different formulations, i.e., OSAP anchored liposomes (OPM-PC3) and their plain version (PC3) and PAM anchored liposomes (PAM-CE3) and their plain versions (CE3). Drug localization indices for liver (
), spleen (
), lung (
) and kidney (
) were calculated using the data from organ distribution studies after 1 hr. Data points are means ± S.D., n=3.
The comparison of bio-distribution patterns of ligand anchored MLVs revealed that PAM linked liposomes are subjected to higher hepato-splenic accumulation. The drug accumulation in lungs was maximum in the case of OPM anchored liposomes. Thus, mannopyranoside is a specific ligand for targeting bioactives to the macrophages of liver and spleen while OPM could preferentially negotiate selective uptake of bioactives by alveolar macrophages. In an attempt to combine the concept of nebulization and ligand mediated targeting to alveolar macrophages, Vyas and co-workers (unpublished data) used macrophages specific ligands as sensing module appended to liposomes. The study deals with the oral nebulization of rifampicin entrapped ligand appended liposomes as a possible means for direct targeting to the infected alveolar macrophages. The ligands chosen for the study were maleylated bovine serum albumin (MBSA) and O-stearyl amylopectin (O-SAP). The quantitative in vitro phagocytic activity and therapeutic index were determined and the degree of (alveolar) macrophage uptake of negatively charged and ligand anchored liposomes, which are comparable to plain liposomes at 1 h interval, nearly doubled at 6-h interval, suggesting that these formulations were avidly phagocytosed. The developed system being a ligand-anchored system is selective for alveolar macrophages because the abundance and exclusive expression of the specific (scavenger) or non-specific (receptors for amylopectin) receptors on the surface of the mature alveolar macrophages.
Targeted chemotherapy
The polysaccharide-anchored liposomes have been studied as stable and targetable drug carriers adaptable in effective chemotherapy, particularly for introducing chemo-therapeutics into target cells or tumor cell lines. These systems possess a unique targetability to specific tissues such as alveolar macrophages and other macrophages of RES. Polysaccharide-anchored liposomes could be employed as carrier constructs on to which an site-specific sensing molecule(s) like MoAb against the tumor surface antigens could be physically or chemically attached (15). The system being site-specific can transport a sufficient quantity of an anti-tumor drug releasing it within or in the vicinity of the target. Polysaccharide-anchored liposomes directed by monoclonal antibody fragment (anti-sialosyl Lewisx, IgMs) as the sensory device were investigated for their tumor cell binding specificity against human stomach cancer cell line (KATO -III), human lung cancer cell line (PC-9) and the mouse Lewis lung carcinoma cell line (3LL). These ligand directed liposomes showed relatively high specificity towards tumor cell lines in-vitro as estimated from radioisotope and fluorescence microscopic techniques. Adriamycin bearing liposomes anchored with immuno-polysaccharide derivative demonstrated in-vivo targetability against human lung cancer (PC-9 grafted) in experimental athymic mice (15, 76). Yagi and co-workers (77,78) in different studies have developed and engineered liposomal constructs for brain targeting at human glioma. They employed sulfatides and MoAb as site directing devices to endow targetability to the liposomes. Targeted chemotherapy of brain tumor using polysaccharide-anchored liposomes loaded with antitumor drug cisplatin has been attempted by Ochi et al. (79). Survival of 9L-glioma implanted rats with CHP based liposomes loaded with cis-platinum diamino-dichloride (cisplatin) was significantly higher as compared to average survival recorded for untreated groups. Targeted chemotherapy to colon cancer cell lines was performed using polysaccharide-anchored immunoliposomes bearing anti-CEA Fab'(IgG) fragments. The system was able to induce specific binding to carcinoembryonic antigen producing BM314 cell lines, indicating that anti-CEA IgG is an excellent site-specific ligand for CEA producing cells, where encapsulated chemotherapeutic agents in polysaccharide-anchored liposomes could be delivered selectively to the site (80).
Recently, the polysaccharide-anchored liposomes have been exploited as carrier cargo equipped with a targeting ligand, where the polysaccharide coating stabilizes the system both in vivo and in vitro while the anchored recognition ligand confer the system a target selectivity. Shinkai and co-workers (81) prepared magnetoliposomes for hyperthermia based treatment of cancer. The liposomes were anchored with hydrazide pullulan to stabilize the phospholipid capsules and to provide an anchor for the immobilization of antibodies. By this method, 90-180 molecules of a specific monoclonal antibody were immobilized on to magnetoliposomes. When the antibody-conjugated and polysaccharide stabilized magnetoliposomes were incubated with cancer cells, they bound to the cell surface and were taken up by cells in about 12 times higher magnitude than the control formulations.
Ichinose and co-workers (82,83) evaluated anti-tumor effects of polysaccharide-stabilized and ligand anchored liposomal Adriamycin on A66 hepatoma transplanted in nude mice. Tumor recognition ligand, 1-amino lactose (1-AL) was appended on the surface of cholesteroyl amylopectin (CHP)-anchored liposomes. The study was aimed at evaluating a role of polysaccharide coating on stability and tumor recognition ligand as a target site ligand. The uptake of these liposomes by AH66 rat hepatoma cells was estimated to be higher than liposomes without 1-aminolactose in vitro . Furthermore, 1-AL/CHP liposomal Adriamycin showed a stronger antitumor effect compared to other types of liposomal Adriamycin in vitro . When in vivo tumor-targeting efficacy was investigated in AH66 tumor transplanted mice using 3H-liposome, the tumor/serum radioactivity ratio in mice injected with 1-AL/CHP liposome was higher than with mice injected with other liposomes. These observations suggest that on anchoring cell recognition elements along with polysaccharide anchored liposomes, anticancer drug carriers can be engineered for the active targeting to tumor cells.
Systemic and mucosal vaccination
Polysaccharides from the natural or bacterial origin exhibit excellent immune responses in association with protein carriers. However, their conjugation with liposomes for provoking desired systemic or mucosal immune responses has not been widely investigated. Immunization potential of the bacterial polysaccharides encapsulated within liposomes is frequently suggested in the literature (85-87). Recently, the potential of natural polysaccharide-anchored liposomes as an adjuvant for cell mediated immunity has also been explored (88-90). Wachsmann and co-workers (85) reported serum and salivary antibody responses in rats orally immunized with Streptococous mutans carbohydrate protein conjugate associated with liposomes as vaccine adjuvant. The purified polysaccharide antigen of S. mutans was coupled through reductive amination to a cell wall protein of molecular weight 74000. The liposomes bearing the conjugate on intra-gastric administration to rats produced a local immunoglbulin A response (secretory IgA). The conjugate of cell wall protein and polysaccharide incorporated in liposomal system may thus be presented as a potential adjuvant for oral vaccination against S. mutans vis a vis dental caries.
The intracellular components or antigens are processed through MHC class-I thus restricted to CD8+ T cells, whereas MHC class-II and CD4+ T lymphocytes are involved in the processing of exogenous components, pathogens or antigens (Scheme 4).
Scheme 4: Proposed mechanisms for systemic or mucosal immunization by polysaccharide-anchored liposomes
It has also been demonstrated in several cases that MHC class I restricted CD8+ cytotoxic T lymphocytes are responsible for providing immuno-protection (or rejection) against grafted tumors and viral infections. For instance, cytotoxic T lymphocytes (CTL) were induced in WKA/H rats against syngeneic human T-lymphotropic virus-I positive (HTLV-I) cell lines
Cellular immunity was examined after immunizing rats with a truncated hybrid protein (228 amino acids) of gag and env of HTLV-1 produced by Escherichia coli . It was found that CTL recognizes gag-env coded antigens more explicitly than env -coded antigen (86). On the basis of these findings, in vivo immunization of WKA/H rats was attempted by Noguchi and co-workers (87) with the use of cholesteroyl-mannan (CHM) anchored liposomes bearing a HTLV-I related antigen ( gag-env hybrid protein). The gag-env hybrid protein-reconstituted liposomes (gag-env-lipo) with or without polysaccharide coat were used to immunize WKA/H rats subcutaneously twice, with one- week intervals. One week after the last immunization, HTLV-I+ tumor cells (TARS-I) were implanted intradermally and subsequently splenic cells were isolated for assessing CTL responses. Isolated splenic cells were sensitized in vivo with mitomycin-C treated TARS-cells. Killer cell activity against TARS-I was observed only in the case of immunization with CHM anchored gag env -reconstituted liposomes. Rats immunized with gag-env-lipo displayed accelerated rejection of TARS-1 but not of other HTLV-1-negative tumor lines. Injection of carrageenan into animals strongly inhibited generation of killer cells, which indicates for the need of macrophages for priming of CD8+ T cells with gag-env-lipo. No killer activity was recorded when spleen cells were obtained from animals immunized with the hybrid protein alone, the liposome alone, or the hybrid protein reconstituted into conventional liposomes without any polysaccharide coating. (87). The induced killer cells were MHC class-I restricted CD8+/CD3+ CTL (not CD4+ cells) and were completely specific to syngeneic HTLV-I+ cells. The effective tumor rejection was assumed to involve a macrophagic phagocytic process for CHM anchored liposomes. The generation of MHC class-I restricted CD8+CTL could be due to the cytosolic processing of gag-env hybrid protein after it releases from the endosomal apparatus.
The antigen presenting cells taking part in the immunological consequences in case of viral infections express mannose receptors on the cell surface and hence mannose-terminated polysaccharide ligand could be appreciated as a targeting ligand in viral infections. Ohishi and co-workers (91) developed a peptide-based vaccine that induced cell-mediated immunity. 20-mer synthetic peptide, spanning the 98-117 amino acids of bovine leukaemia virus (BLV) envelope glycoprotein (Env) gp51 was encapsulated in mannan-anchored liposomes. The liposomes induced specific delayed-type hypersensitivity, lymphocyte proliferative responses with a weak cytotoxic lymphocyte response in mice. The spleen cells from the immunized mice secreted a large amount of IFN-gamma and IL-2, indicating the induction of Th-1 type immunity in mice elicited through T-cell epitope on synthetic peptide-liposomes.
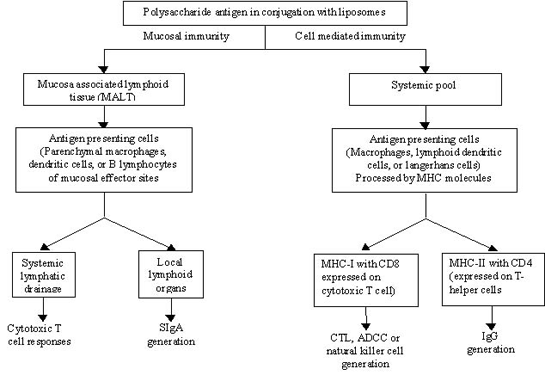
Promising results were recorded when cDNA of HIV-1 was incorporated into mannan-anchored liposomes (90,92). These workers studied the adjuvanticity of mannan-anchored liposomes for human immunodeficiency virus type-1 (HIV-1) DNA vaccine and the mechanism involved in the immunogenicity enhancement. Coating of cationic liposomes with mannan significantly potentiated the vaccine and induced an HIV-specific delayed-type hypersensitivity (DTH) response. HIV-specific cytotoxic T-cell (CTL) activity elicited by DNA vaccination was also significantly potentiated on co-administration with mannan-liposome in the form of a therapeutic cocktail. This mannan-liposome-mediated activity was inhibited noticeably by pre-injection of anti-interferon (IFN)- γ antibody suggesting an important role of IFN- γ in HIV-specific immune response. The results of both isotype-specific antibody and cytokine analysis revealed that mannan-anchored liposome-based DNA vaccination could prove to be a valuable tool for the enhancement of HIV-1 specific cell-mediated immune response (Table 5).
Table 5: Cytokine profile of supernatants of lymphoid cell cultures of mice immunized with IIIB/REV plus mannan and/or liposomes1. Reproduced from (90) with permission
A predominance of Th1 cells through use of mannan-anchored liposomes was evident from cytokine assay data, wherein high IFN- γ production and moderate IL-4 levels were estimated on administration of liposomal adjuvant based DNA vaccine.
The finding that mannan abundant in mannose residues is critical in eliciting cytotoxic T lymphocyte (CTL) response substantiates and supports the findings of Fukusawa and co-workers (89,93).
The coating of liposomes with various oligosaccharides and yeast derived mannan drastically enhanced the induction of ovalbumin-specific delayed-type footpad swelling response in Balb/c mice with a peak at 24 to 48 host-challenge (93). The mannan- coated liposome was included in the study as a reference to compare the effects of various neoglycolipids for their augmentation of a delayed type of response. It is possible that the receptor-mannose interaction of oligomannose- or mannan-anchored liposomes might have augmented the processing of OVA reconstituted in these liposomes. In addition, the mannose residues may possess some other activity such as stimulation of IL-12 release culminating the activation of T-lymphocytes. Only those neoglycolipids (oligosaccharides) with mannose residues at non-reducing termini were found effective. However, these workers suggested that instead of using mannan, which can elicit antibody and B-cell mitosis (immunogenic) and toxic effects on iv administration, safer neoglycolipids like oligomannose, which are ubiquitously found in the body, should be used as an adjuvant for the induction of cell mediated immunity.
Recently, Venketesan and Vyas (94) rationalized the role of polysaccharide anchored liposomes in the enhancement of the immunogenicity of the model antigen after oral administration. The results were compared in terms of serum IgG and IgA titers against bovine serum albumin (BSA) administered as control, plain and anchored formulations (Figure 4).
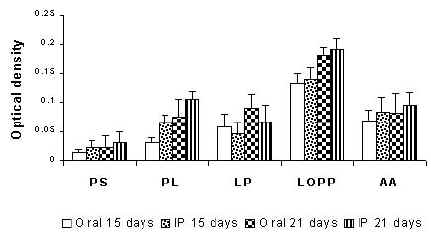
Serum IgA (Figure 4A) and IgG (Figure 4B) level in rats following oral and intra-peritoneal administration of various formulations in albino rats. PS, plain BSA solution; PL, plain BSA-liposome; LP, liposomes anchored with pullulan; LOPP, liposomes anchored with O-palmitoyl pullulan (OPP); AA, alum adsorbed. On day 1, groups of rats were administered orally with preparations containing BSA equivalent to 100mg. A similar group of mice was injected intraperitoneally. Secondary immunization was done on day 15 with plain and polysaccharide anchored liposomes containing BSA equivalent to 100 mg. Blood was collected from the orbital plexus on day 15 and 21. Specific anti-IgG and IgA antibody levels in the serum were determined. Adapted from 94 with permission.
The antibody titre (IgA) value recorded using ELISA with a microplate reader as optical density at 405 nm, was found to be 0.134 following oral administration and 0.139 after intraperitoneal administration for OPP-anchored liposomes. The results showed insignificant effect of the route of administration on secretion of IgA, whilst IgG levels were significantly different. Serum IgG levels on day 15, in the case of orally administered OPP-anchored liposomes were found to be 0.394 while those administered through intraperitoneal route recorded an optical density of 0.500. Antibody levels on administration of liposomes anchored with pullulan alone did elicit an immune response better compared to plain liposomes. This seemingly attributes to the intrinsic immuno-genicity provoked by pullulan. The pullulan borne antigenicity may contribute to an increase in serum IgG levels. However, the serum IgA level recorded was lower as compared to its palmitoyl derivative. The increased IgA level recorded following oral administration of OPP anchored liposomes as compared to plain liposomes or pullulan anchored liposomes suggests that the OPP anchored liposomes were stable in harsh bio-environment of stomach and might be taken up by the Peyer's patches. However, the role of polysaccharide anchored liposomes for mucosal immunization especially through the Peyer's patches and gut associated lymphoid tissue (GALT) is yet to be explored.
Macrophage activation
Immunomodulators are biological response modifiers and defined as substances, which activate macrophages, e.g., muramyldipeptide and its derivative (95), polyanionic polymer (96) and polynucleotides (97). These immuno-modulators by themselves, however, do not usually have any specificity or affinity to activate macrophages. Liposomes interact efficiently with macrophages, thus macrophages may serve as antigen presenting cells for liposomal antigens/immuno-modulators. In order to modulate the in-vivo activity of several immunomodulators, they are generally administered encapsulated in polysaccharide-anchored liposomes. Polyanion polymers and synthetic polynucleotides were encapsulated into macrophage specific liposomes such as those anchored with mannan-Chol dervatives (97-99). Ottenbrite and coworkers (100) reported improvement in immuno-potentiation activity of polyanionic polymers following encapsulation in polysaccharide-anchored liposomes. The effective activation of mouse peritoneal macrophages was observed by poly (maleic acid -alt-2-cyclohexyl-1,3-doxap-5ene) (MA-CDA), when administered encapsulated in CHM anchored liposomes. Macrophage activation was mediated through superoxide anion production and as a result tumoricidal activity was increased nearly 5 times over plain MA-CDP at three-day post administration interval. Akashi and co-workers (101) evaluated potential immuno-modulator activities of polysaccharide-anchored liposome bearing synthetic polynucleotide, polyvinyladenine and vinyladenine-alt-maleic acid (poly VA-MA). CHM anchored liposomes bearing poly VA-MA were more effective as compared against the free poly (VA-MA), when evaluated for superoxide anion production by mouse peritoneal macrophages. These studies signify measurable macrophage activation and increased immunopotentiation of liposome encapsulated contents on surface anchoring of polysaccharides.
Gastric mucoadhesion
The ability of polysaccharides to interact with liposomes has been reported to have three possible implications: The stabilization of the liposomes, the targetability of the appended polysaccharide ligand, and the possibility of targeting the vesicles to a specific site due to its bioadhesive and mucoadhesive properties. Among the polysaccharides used in drug delivery, chitosan has been widely employed for its bioadhesive properties. This is due in part to its characters like high molecular weight and degree of de-acetylation, its gel forming at low pH and also due to its poly-cationic character, which imparts it ability to bind strongly to mammalian cells.
Takeuchi and co-workers (102) studied polymer anchored muco-adhesive multilamellar liposomes consisting of dipalmitoyl phosphatidylcholine (DPPC) and dicetyl phosphate (DCP) (8:2 molar ratio) and anchored with three different types of polymers: chitosan, polyvinyl alcohol having a long alkyl chain, and poly (acrylic acid) bearing cholesterol. The muco-adhesive function of the polymer-anchored liposomes was evaluated in vitro using rat intestine and % adhesion was estimated by a particle counting method. Chitosan anchored liposomes showed the highest% adhesion among the polymer-anchored liposomes tested while non-anchored liposomes exhibited negligible to no adhesion. The adhesion of chitosan-anchored liposomes to the intestine wall was further confirmed by fluorescence microscopy using pyrene-loaded liposomes. The muco-adhesiveness of chitosan-anchored liposomes was subsequently evaluated to develop a novel drug carrier system for oral administration of poorly absorbed drugs such as peptides (103). Takeuchi and co-workers (103) prepared muco-adhesive chitosan-anchored liposomes to improve oral absorption of insulin. After in vivo administration of the chitosan-anchored liposomes to male wister rats, the hypoglycemic response was prolonged over a period of up to 12 h. This sustained effect was attributed to the muco-adhesiveness of the system leading to an increased duration of contact with intestinal mucosa and hence an increased probability of insulin absorption. These studies explore the possibilities of polysaccharide anchored liposomes for the administration and muco-adhesion of poorly adsorbed drugs and macromolecules.
Conclusion And Future Prospects
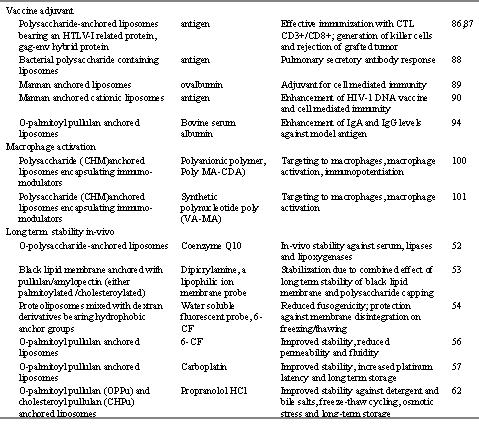
Ligand mediated bio-disposition and cellular interaction of liposomes especially at the target sites would be a focal paradigm of the future research in the field of drug and antigen delivery. Polysaccharide anchored liposomes have paved the way for the bio-stable, site-specific and ligand directed delivery systems with desired therapeutic and immunological characteristics. Encapsulation of cytotoxic drugs, antimicrobial agents, immunomodulators and macrophage activators, and natural or bacterial polysaccharides into polysaccharide anchored liposome generates better pharmacological and immunological activity at the desired limits (Table 6).
Table 6: Therapeutic applications of polysaccharide-anchored liposomes

The delivery module appears conceivably promising for delivery of pharmacological and biologically active molecules to the specific cells or tissues. Furthermore, by keying a relevant ligand as polysaccharide-Chol derivatives, the system can be utilized either for cellular targeting or as a long circulatory stealth system, depending upon the nature of the conjugated biosensor molecules. Reports are quite vague and controversial as far as the priming of liposomes with polysaccharides for systemic long circulation is concerned (50,84). Amongst the abounding reports in the literature, the majority of the cases refer to the intrinsic or natural distribution pattern of the polysaccharide-anchored liposomes towards the alveolar macrophages and monocytes. Though they themselves seem not to offer any long circulation, the increased surface hydrophilicity due to inclusion of negatively charged sialic acid reported to provide a much better stealth character to the polysaccharide-anchored liposomes. Polysaccharide-anchored liposomes bearing a sialic acid moiety [sialosyl µ(2→6) glycopyranose] have been reported to evade phagocytosis as a consequence of decreased RES interception (54). Conjugation of sialic acid derivatives to pullulan or amylopectin demonstrated their effective rejection by phagocytosis (and hence in accumulation/uptake in MPS) when compared for their internalization efficiencies in human blood monocytes in vitro. Hence, liposomes anchored with such modified polysaccharides promise enormous potential as long circulatory drug carrying vehicle. The role of the glycocalyx in regulating access of particles in to the apical plasma membranes of intestinal epithelial cells is mainly clearing up, the microbial attachment and holds for oral vaccination potential of polysaccharide bearing systems, which may become a future therapeutic tool. With the emergence of mannan binding lectins known to participate in complement activation, a new field of drug carrier investigation seems to open up (36-40). Recently, anti-tumor activity of mannan binding protein has been established hence mannan as epitope on liposomal surface can prove to be an ideal carrier for expressing cell specific cytotoxicity. Mannan-anchored liposomes were found to deliver the paramagnetic contrast agent (Gadolinium-diethylene-triamine-penta-acetic acid, Gd-DTPA), which is used in the magnetic resonance imaging (MRI) primarily of liver and lungs at a significantly lower dose than required by conventional means thus increasing MRI contrast precision (104). The transformation of cells in disease(s) results in to subsequent specific changes in carbohydrate recognition domains (lectins) of the cell surfaces thus offers a means of specific targeting to the diseased cells. Mannan-binding lectin deficiency was found to be associated with recurring cutaneous abscesses, prurigo and possibly atopic dermatitis and a plethora of pathological disorders and immuno-defects. The findings suggest that liposomes appended with mannan could serve as handles to selectively deliver the drugs to the complementary lectin receptors, which are down regulated in these diseased states. The role of polysaccharide anchored liposomes in topical and cosmetic applications, non-viral gene vectorization, oligonucleotide delivery, oral, intragastric, nasal or other mucosal immunization and bio-film targeting is yet to be explored and realized. It is conceivably convincing that the polysaccharide(s) imparts specific functional characteristics to the liposomes and a simultaneous improvement in vitro and in vivo stability. They therefore have a distinctive role and potential in site-specific specialized drug delivery.
References
- Vyas, S.P. and Sihorkar, V., Endogenous Ligands and carriers in non-immunogenic site-specific drug delivery. Adv Drug Deliv Rev, 43:101-164, 2000.
- Wright, S., and Huang, L., Antibody-directed liposomes as drug-delivery vehicles. Adv Drug Deliv Rev, 3: 343-389, 1989.
- Yamauchi, H., Yano, T., Kato, T., Tanaka, I., Nakabayashi, S., Higashi, K., Miyoshi, S., and Yamada, H., Effects of sialic acid derivative on long circulation time and tumor concentration of liposomes. Int J Pharm, 113: 141-148, 1995.
- Pinnaduwage, P., and Huang, L., The role of protein-linked oligosaccharides in the bilayer stabilization activity of glycophorin A for dioleoylphosphatidylethanolamine liposomes. Biochim Biophys Acta, 986: 106-114, 1989.
- Amselem, S., Barenholz, Y., Loyter, A., Nir, S., and Lichtenberg, D., Fusion of sendai virus with negatively charged liposomes as studied by pyrene-labelled phospholipid liposomes. Biochim Biophys Acta, 860: 301-313, 1986.
- Ehrlich, P., A general review of the recent work in immunity (Collected papers of Paul Eahrlich). In "Immunology and Cancer Research", Pergamon Press, London, Vol. 2, p. 456-461, 1956.
- Maruyama, K., Holmberg, E., Kennel, S.J., Klibnov, A., Torchilin, V.P., and Huamg, L., Characterization of in vivo immunoliposomes targeting to pulmonary endothelium. J Pharm Sci, 74: 978-984, 1990.
- Hutchinson, F.J., Francis, S.E., and Jones, M.N., The integrity of proteoliposomes targeted to a model bio-surface. Biochim Soc Trans, 17: 558-559, 1989.
- Sunamoto, J., Sato, T., Taguchi, T., and Hamazaki, H., Naturally occurring polysaccharide derivatives, which behave as an artificial cell wall on an artificial cell liposome. Macromolecules, 25: 5665-5670, 1992.
- Sarkar, D.P., and Blumenthal, R., The role of target membranes in fusion with Sendai virus. Membr Biochem, 7: 231-247, 1987.
- Podder, S.K., Chakraborti, A., Vijayalakshmi, K., and Singh, P.L.K., Liposome-bearing glycosphingolipids: Model membrane system for studying molecular mechanism of cell surface carbohydrate-mediated processes. Ind J Biochem Biophys, 25: 156-165, 1988.
- Saito, K., Ando, J., Yoshida, M., Haga, M., and Kato, Y., Tissue distribution of sialo-glycopeptide bearing liposomes in rats. Chem Pharm Bull, 36: 4187-4191, 1988.
- Jones, M., Carbohydrate mediated liposomal targeting and drug delivery. Adv Drug Deliv Rev, 13: 215-250, 1994.
- Sunamoto, J., Sato, T., Horota, M., Fukushima, K., Hiratani,K., and Hara, K., A newly developed immunoliposomes-an egg phosphatidylcholine liposome anchored with pullulan bearing both a cholesterol moiety and an IgM fragment. Biochim Biophys Acta, 898: 323-330, 1987.
- Sato, T., and Sunamoto, J., Recent aspects in the use of liposomes in biotechnology and medicine. Prog Lipid Res, 31: 345-372, 1992.
- Ueda, H., Coating of POPC giant liposomes with hydrophobized polysaccharide. Chem Lett, 5: 417-418, 1998.
- Catley, B.J., and Whealn, W.J., Structure of pullulan. Arch Biochem Biophys, 143: 138-142, 1971.
- Larsen, C., Dextran prodrugs-structure and stability in relation to therapeutic activity. Adv Drug Deliv Rev, 3: 103-154, 1989.
- Sezaki, H., Takakura, Y., and Hashida, M., Soluble macromolecular carriers for the delivery of anti-tumor drugs. Adv Drug Deliv Rev, 3: 247-266, 1989.
- Nishida, K., Mihara, K., Takino, T., Nakane, S., Takakura, Y., Hashida, M., Sezaki, S., Hepatic disposition characteristics of electrically charged macromolecules in rat in vivo and in the perfused liver. Pharm Res, 8: 437-444, 1991.
- Mehvar, R., Robinson, M.A., and Reynolds, J.M., Molecular weight dependent tissue accumulation of dextrans in rats. J Pharm Sci, 81: 908-912, 1994.
- Charpentier, C., Lhoste, P., Nguyen, V.L.T., and Fontanges, R., Comparative study of physicochemical properties of mannan from nine yeasts. Mycopathologica, 59: 11-23, 1976.
- Roberts, G.A.F., Chitin Chemistry. McMillan Press, Houndmills, USA, pp. 1-16, 1992.
- Xi, K., Tabata, Y., Uno, K., Yoshimoto, M., Kishida, T., Sokawa, Y.and Ikada, Y., Liver targeting through pullulan conjugation. Pharm Res, 13: 1846-1850, 1996.
- Horie, K., Sakagami, M., Kuramochi, K., Hanasaki, K., Haman, H. and Ito, T., Enhanced accumulation of sialyl lewis X-carboxymethyl pullulan conjugate in acute inflammatory lesion. Pharm Res, 16: 314-320, 1999.
- Page, D., Zanini, D., and Roy, R., Macromolecular recognition: effect of multivalency in the inhibition of binding of yeast mannan to concanavalin A and pea lectins by mannosylated dendrimers. Bioorg Med Chem, 4: 1949-1961, 1996.
- Page, D., and Roy, R., Synthesis and biological properties of mannosylated starburst poly(amidoamine) dendrimers. Bioconjug Chem, 8:714-723, 1997.
- Iwamoto, K., Kato, T., Kawahara, M., Koyama, N., Watanabe, S., Miyake, Y., and Sunamoto, J., Polysaccharide-anchored oil droplets in oil-in-water emulsions as targetable carriers for lipophilic drugs. J Pharm Sci, 80: 219-224, 1991.
- Fukui, H., Akiyoshi, K., Sunamoto, J., O/w-emulsion of alpha-linolenic acid stabilized with hydrophobized polysaccharide. Its effect on the growth of human colon cancer cells. J Biomater Sci Polym Ed, 7: 829-838, 1996.
- Akiyoshi, K., Kobayashi, S., Shichibe, S., Mix, D., Baudys, M., Kim, S.W., and Sunamoto, J., Self-assembled hydrogel nanoparticle of cholesterol-bearing pullulan as a carrier of protein drugs: complexation and stabilization of insulin. J Control Rel, 54: 313-320, 1998.
- Akiyoshi K, Sasaki, Y., and Sunamoto, J., Molecular chaperone-like activity of hydrogel nanoparticles of hydrophobized pullulan: thermal stabilization with refolding of carbonic anhydrase B. Bioconjug Chem, 10: 321-324, 1999.
- Jeong, Y.I., Nah, J.W., Na, H.K., Na, K., Kim, I.S., Cho, C.S., and Kim, S.H., Self-assembling nanospheres of hydrophobized pullulans in water. Drug Dev Ind Pharm, 25: 917-927, 1999.
- Sun L, Durrani, C.M., Donald, A.M., Fillery-Travis, A.J., and Leney, J., Diffusion of mixed micelles of bile salt-lecithin in amylopectin gels: a Fourier transform infrared microspectroscopy approach. Biophys Chem, 61: 143-150, 1996.
- Sun L, and Donald, A.M., The effects of ageing of amylopectin gels on the diffusion of BSA. Int J Biol Macromol, 20: 205-207, 1997.
- Tabata, Y., Matsui, Y., and Ikada, Y., Growth factor release from amylopectin hydrogel based on copper coordination. J Control Rel, 56: 135-148, 1998.
- Gu, X.G., Schmitt, M., Hiasa, A., Nagata, Y., Ikeda, H., Sasaki, Y., Akiyoshi, K., Sunamoto, J., Nakamura, H., Kuribayashi, K., and Shiku, H., A novel hydrophobized polysaccharide/oncoprotein complex vaccine induces in vitro and in vivo cellular and humoral immune responses against HER2-expressing murine sarcomas. Cancer Res, 58: 3385-3390, 1998.
- Savolainen, J., Rantala, A., Nermes, M., Lehtonen, L., and Viander, M., Enhanced IgE response to Candida albicans in postoperative invasive candidiasis. Clin Exp Allergy, 26: 452-460, 1996.
- Han, Y., Ulrich, M.A., and Cutler, J.E., Candida albicans mannan extract-protein conjugates induce a protective immune response against experimental candidiasis. J Infect Dis, 179: 1477-1484, 1999.
- Lett, E., Klopfenstein, C., Klein, J.P., Scholler, M., and Wachsmann, D., Mucosal immunogenicity of polysaccharides conjugated to a peptide or multiple-antigen peptide containing T- and B-cell epitopes. Infect Immun, 63: 2645-2651, 1995.
- Wang, L., Ikeda, H., Ikuta, Y., Schmitt, M., Miyahara, Y., Takahashi, Y., Gu, X., Nagata, Y., Sasaki, Y., Akiyoshi, K., Sunamoto, J., Nakamura, H., Kuribayashi, K., and Shiku, H., Bone marrow-derived dendritic cells incorporate and process hydrophobized polysaccharide/oncoprotein complex as antigen presenting cells. Int J Oncol, 14: 695-701, 1999.
- Apostolopoulos, V., Pietersz, G.A., and McKenzie, I.F., Cell-mediated immune responses to MUC1 fusion protein coupled to mannan. Vaccine, 14: 930-938, 1996.
- Felt, O., Buri, P., and Gumy, R., Chitosan: A unique polysaccharide for drug delivery. Drug Dev Ind Pharm, 24: 979-993, 1998.
- Sunamoto, J., Application of polysaccharide-anchored liposomes in chemotherapy and immuno-therapy, in Yagi, Y (ed), Medical applications of liposomes. Japan Scientific Society Press, Tokyo, Karger Basal, pp. 121-131, 1986.
- Sunamoto, J., Iwamoto, K., Takada, M., Yujuriha, T., and Katayama, K., Polymer anchored liposomes for drug delivery to target specific organs, in Anderson, JH: Kim, SW (eds), Recent Advances in Drug Delivery Systems. Plenum Press, NY, pp. 153-162, 1984a.
- Sunamoto, J., Iwamoto, K., Takada, M., Yuzuriha, T., and Katayama, K., Improved drug delivery to target specific organs using liposomes anchored with polysaccharides, in Chiellini, E: Giusti, P (eds), Polymers in Medicine. Plenum Press, NY, pp. 157-168, 1984b.
- Sunamoto, J., Iwamoto, K., and Kondo, H., Liposome Membranes. VIII. Fusion and aggregation of egg lecithin liposomes as promoted by polysaccharides. Biochim Biophys Res Commun, 94: 1367-1373, 1980a.
- Sunamoto, J., Iwamoto, K., Kondo, H., and Shinkai, S., Liposome Membranes. VI. Polysaccharide induced aggregation of multilamellar liposomes of egg lecithin. J Biochem, 88: 1219-1227, 1980b.
- Mobed, M., and Chang, T.M., Kinetic aspects of polyelectrolyte adsorption: adsorption of chitin derivatives onto liposomes as a model system. Artif Cells Blood Substit Immobil Biotechnol, 25: 367-377, 1997.
- Iwamoto, K., and Sunamoto, J., Liposomal membranes. XII. Adsorption of polysaccharides on liposome membrane as monitored by fluorescence depolarization. J Biochem, 91: 975-981, 1982.
- Sunamoto, J., and Iwamoto, K., Protein anchored and polysaccharide-anchored liposomes as drug carriers. CRC Crit Rev Ther Drug Carrier Syst, 2: 117-136, 1986.
- Akiyoshi, K., Takanabe, H., Sato, T., Kondo, H., and Sunamoto, J., Cell specificity of polysaccharide derivatives on liposomal surface. Chem Lett, 473: 1990-1992, 1990.
- Sunamoto, J., Sakai, K., Sato, T., and Kondo, H., Molecular recognition of polysaccharide-anchored liposomes. Importance of sialic acid moiety on liposomal surface. Chem Lett, 1781-1784, 1988.
- Moellerfeld, J., Prass, W., Ringsdorf, H., Hamazaki, H., and Sunamoto, J., Improved stability of black lipid membranes by coating them with polysaccharide derivatives bearing hydrophobic anchor groups. Biochim Biophys Acta, 857: 265-270, 1986.
- Elferink, M.G.L., Wit, J.G., Veld, G., Reichert, A., Driessen, A., Ringsdorf, H., and Konings, W., The stability and functional properties of proteoliposomes mixed with dextran derivatives bearing hydrophobic anchor groups. Biochim Biophys Acta, 1106: 23-30, 1992.
- Sehgal, S., and Rogers, J.A., Polymer-anchored liposomes: improved liposome stability and release of cytosine arabinoside (Ara-C). J Microencap, 12: 37-47, 1995.
- Moreira, J.N., Almeida, L.M., Geraldes, C.F., and Costa, M.L., Evaluation of in vitro stability of large unilamellar lipsomes anchored with a modified polysaccharide (O-palmitoyl Pullulan). J Mat. Sci Mat Med, 7: 301-303, 1996.
- Moreira, J.N., Almeida, L.M., Geraldes, C.F., Madeira, V.M.C., and Costa, M.L. Carboplatin liposomes anchored with O-Palmitoyl Pullulan: In vitro Characterization. Int J Pharm 147: 153-164, 1997.
- Rogers, J.A. and Anderson, K.E., The potential of liposomes in oral drug delivery. Crit Rev Ther Drug Carrier Syst 15:421-480, 1998.
- Aramaki, Y., Tomizawa, H., Hara, T., Yachi, K., Kikuchi. H. and Tsuchiya, S., Stability of liposomes in vitro and their uptake by rat Peyer's patches following oral administration. Pharm Res, 10:1228-1231, 1993.
- Velve, O.D., Assembly of protein structures on liposomes by non-specific and specifc interactions. Adv Biophys, 34: 139-157, 1997.
- Mumper, R.J. and Hoffman, A.S., The stabilization and release of hirudin from liposomes or lipid-assemblies coated with hydrophobically modified dextran, AAPS Pharm Sci Tech, 1: article 3, 2000.
- Sihorkar, V., and Vyas, S.P., Polysaccharide coated liposomes for oral drug delivery: Development and characterization, in Vyas, SP: Dixit, VK (eds), Advances in liposomal Therapeutics. CBS Publishers, New Delhi, India, pp. 231-239, 2001.
- Takada, M., Yuzuriha, T., Katayama, K., Iwamoto, K., and Sunamoto, J., Increased lung uptake of liposomes anchored with polysaccharides. Biochim Biophys Acta, 802: 237-244, 1984.
- Sunamoto, J., Iwamoto, K., Takada, M., Yuzuriha, T., and Katayama, K., Improved drug delivery to target specific organs using liposomes anchored with polysaccharides. Polym Sci Technol, 23: 157-168, 1983.
- Sunamoto, J., and Sato, T., Improved drug delivery directed to specific tissue using polysaccharide-anchored liposomes, in Tsuruta, T: Nakajima, A (eds), Multiphase Biomedical Materials. The Netherlands, pp. 169-171, 1989a.
- Sunamoto, J., Goto, M., Iida, T., Hara, K., Saito, A., and Tomonaga, A., Unexpected tissue distribution of liposomes anchored with amylopectin derivative and successful use in the treatment of experimental Legionnaires' disease, in Poste, G: Senior, J: Trouet, A (eds), Receptor Mediated Targeting of drugs. NATO ASI Ser. Ser. A 82, Plenum Press, London, pp. 359-371, 1984c.
- Kohno, S., Miyazaki, T., Yamaguchi, K., Tanaka, H., Hayashi, T., Hirota, M., Saito, A., Hara, K., Sato, T., and Sunamoto, J., Polysaccharide-anchored liposomes with antimicrobial agents against inracytoplasmic pathogens and fungus. J Bioact Compat Polym, 3: 137-147, 1988.
- Miyazaki, T., Kohno, S., Sasayama, K., Inoue, Y., Hara, K., Ogasawara, M., Sato, T., and Sunamoto, J., Polysaccharide-anchored liposomal amphotericin B for the treatment of murine pulmonary candidiasis. Tohoku J Exp Med, 168:483-490, 1992.
- Poiani, G.J., Kemnitzer, J.E., Fox, J.D., Tozzi, C.A., Kohn, J., Riley, and D.J., Polymeric carrier of proline analogue with antifibrotic effect in pulmonary vascular remodelling. Am J Respair Crit Care Med, 155: 1384-1390, 1997.
- Cansell, M., Parisel, C., Jozefonvicz, J., and Letourneur, D., Liposomes anchored with chemically modified dextran interact with human endothelial cells. J Biomed Mater Res, 44: 140-148, 1999.
- Letourneur, D., Parisel, C., Prigent-Richard, S., and Cansell, M., Interactions of functionalized dextran-anchored liposomes with vascular smooth muscle cells. J Control Rel, 65: 83-91, 2000.
- Deol, P., and Khuller, G.K., Lung specific stealth liposomes: Stability, biodistribution and toxicity of liposomal antitubercular drugs in mice. Biochim Biophys Acta, 1334: 161-172, 1997.
- Vyas, S.P., Katare, Y., Mishra, V. and Sihorkar, V., Ligand directed macrophage targeting of amphotericin B loaded liposomes: development and characterization. Int J Pharm, 210: 1-14, 2000.
- Mukhopadhyay, A., Chaudhuri, G., Arora, S., Sehgal, S., and Basu, S., Receptor mediated drug delivery to macrophages in chemotherapy of leishmaniasis. Science, 244: 705-707, 1989.
- Rigotti, A., Acton, S.L., and Krieger, M., The class B scavenger receptor SR-B1 and CD36 are receptors for anionic phospholipids. J Biol Chem, 270: 16221-16224, 1995.
- Hirota, M., Fukushima, K., Hiratani, K., Kawano, K., Oka, M., Tomonaga, A., Saitoh, A., Hara, K., Sato, T., and Sunamoto, J., Targetting therapy of human tumor cell line xenografts in nude mice using pullulan-anchored liposome encapsulated with adriamycin. Gan To Kagaku Ryoho, 13: 2875-2878, 1986.
- Yagi, N., Naoi, M., Sasaki, H., Abe, H., Konishi, H., and Arichi, S., Incorporation of enzyme into the brain by means of liposomes of novel composition. J Appl Biochem, 4: 121-125, 1982.
- Yagi, K., and Naoi, M., Glycolipid insertion into liposomes for their targeting to specific organs, in Yagi, Y (ed), Medical Application of Liposomes. Japan Scientific Societies Press, Tokyo, pp. 91-95, 1986.
- Ochi, A., Shibata, S., Mori, K., Sato, T., and Sunamoto, J., Targeting chemotherapy of brain tumor using liposome encapsulated cisplatin. Part 2. Pullulan anchored liposomes to target brain tumor. Drug Delivery Syst, 5: 261-271, 1990.
- Sato, T., Sunamoto, J., Ishii, N., and Koji, T., Polysaccharide-anchored immunoliposomes bearing anti-CEA Fab' fragment and their internalization by CEA-producing tumor cells. J Bioactive Compatible Polym, 3: 196-204, 1988.
- Shinkai, M., Suzuki, M., Iijima, S., and Kobayashi, T., Antibody-conjugated magnetoliposomes for targeting cancer cells and their application in hyperthermia. Biotechnol Appl Biochem, 21:125-137, 1995.
- Ichinose, K., Yamamoto, M., Khoji, T., Ishii, N., Sunamoto, J., and Kanematsu, T., Antitumor effect of polysaccharide-anchored liposomal adriamycin on AH 66 hepatoma in nude mice. Anticancer Res, 18 (1A): 401-404, 1998.
- Yamamoto, M., Ichinose, K., Ishii, N., Khoji, T., Akiyoshi, K., Moriguchi, N., Sunamoto, J., and Kanematsu, T., Utility of liposomes anchored with polysaccharide bearing 1-amino-lactose as targeting chemotherapy for AH66 hepatoma cells. Oncol Rep, 7: 107-111, 2000.
- Pain, D.M., Das, P.K., Ghosh, P.C., and Bachawat, B.K., Increased circulatory half-life of liposomes after conjugation with dextran. J Biosci, 6: 810-816, 1984.
- Wachmann, D., Klein, J.P., Schollar, M., Ogier, J., Ackerman, F., and Frank, R.M., Serum and salivary antibody responses in rats orally immunized with Streptococcus mutans carbohydrate protein conjugate associated with liposomes. Infect Immun, 52: 408-413, 1986.
- Noguchi, Y., Tateno, M., Kondo, N., Yoshiki, T., Shida, H., Nakayama, E., and Shiku, H., Rat T lymphocytes against human T-lymphotropic virus type 1-infected cells recognize gag gene and env gene encoded antigens. J Immunol, 143: 3737-3742, 1989.
- Noguchi, Y., Noguchi, T., Sato, T., Yokoo, Y., Itoh, S., Yoshida, M., Yoshiki, T., Akiyoshi, K., Sunamoto, J., Nakayama, E., Priming for in vitro and in vivo anti-human T lymphotropic virus type 1 cellular immunity by virus-related protein reconstituted into liposome. J Immunol, 146: 3599-3603, 1991.
- Abraham, E., Intranasal immunization with bacterial polysaccharide containing liposomes enhances antigen-specific pulmonary secretory antibody response. Vaccine, 10: 461-468, 1992.
- Sugimoto, M., Ohishi, K., Fukasawa, M., Shikata, K., Kawai, H., Itakura, H., Hatanaka, M., Sakakibara, R., Ishiguro, M., Nakata, M., Mizuochi, T., Oligomannose anchored liposomes as an adjuvant for the induction of cell-mediated immunity. FEBS Lett, 363: 53-56, 1995.
- Toda, S., Ishii, N., Okada, E., Kusakabe, K.I., Arai, H., Hamajima, K., Gorai, I., Nishioka, K., and Okuda, K., HIV-1-specific cell-mediated immune responses induced by DNA vaccination were enhanced by mannan-anchored liposomes and inhibited by anti-interferon-gamma antibody. Immunology, 92: 111-117, 1997.
- Ohishi, K., Kabeya, H., Amanuma, H., and Onuma, M., Induction of bovine leukaemia virus Env-specific Th-1 type immunity in mice by vaccination with short synthesized peptide-liposome. Vaccine, 14: 1143-1148, 1996.
- Sasaki, S., Fukushima, J., Arai, H., Kusakabe, K.I., Hamajima, K., and Ishii, N., Hirahara, F., Okuda, K., Kawamoto, S., Ruysschaert, J.M., Vandenbranden, M., Wahren,B., and Okuda, K., Human immunodeficiency virus type-1-specific immune responses induced by DNA vaccination are greatly enhanced by mannan-anchored diC14-amidine. Eur J Immunol, 27: 3121-3129, 1997.
- Fukusawa, M., Shimizu, Y., Shikata, K., Nakata, M., Sakakibara, R., Yamamoto, N., Hatanaka, M., and Mizouchi, T., Liposome oligomannose-anchored with neoglycolipid, a new candidate for a safe adjuvant for induction of CD8+ cytotoxic T lymphocytes. FEBS Lett, 441: 353-356, 1998.
- Venketesan, N., and Vyas, S.P., Polysaccharide coated liposomes for oral immunization: development and characterization. Int J Pharm, 203: 169-177, 2000.
- Lederer, E., Synthetic immunostimulants derived from the bacterial cell wall. J Med Chem, 23: 819-823, 1980.
- Inaki, Y., and Takemoto, K., Functionality and applicability of synthetic nucleic acid anlogues, in Ottentribe RM: Utracki, LA: Inoue, S (eds), Current topics in polymer science., Vol 1, Hanser Publishers, New York, pp. 79, 1987.
- Sato, T., Kojima, K., Iida, T., Sunamoto, J., and Ottenbrite, R.M., Macrophage activation by poly (maleic acid-alt-2-cyclohexyl-1,3-doxap-5-ene) encapsulated in polysaccharide-anchored liposomes. J Bioactive Compatible Polym, 1: 448-458, 1986.
- Oka, M., Enhancement of antitumor activity in mouse alveolar macrophages by immuno-activators encapsulated within polysaccharide-anchored liposomes. Acta Med Nagasaki, 34: 88-90, 1989.
- Sunamoto, J., and Sato, T., Development of cell-specific liposomes and its application in biotechnology. J Chem Soc Jpn, 161: 24-32, 1989b.
- Ottenbrite, R.M., Sunamoto, J., Sato, T., Kojima, K., Sahara, K., Hara, K., and Oka, M., Improvement of immuno-potentiator activity of polyanionic polymers by encapsulation into polysaccharide-anchored liposomes. J Bioactive Compatible Polym, 3: 184-191, 1988.
- Akashi, M., Iwasaki, H., Miyauchi, N., Sato, T., Sunamoto, J., and Takemoto, K., Potent immunomodulating activity of (polyvinyladenine and vinyladenine-alt-maleic acid) copolymer. J Bioactive Compatible Polym, 4: 124-137, 1989.
- Takeuchi, H., Yamamoto, H., Niwa, T., Hino, T., and Kawashima, Y., Mucoadhesion of polymer-anchored liposomes to rat intestine in vitro. Chem Pharm Bull (Tokyo), 42: 1954-1956, 1994.
- Takeuchi, H., Yamamoto, H., Niwa, T., Hino, T., and Kawashima, Y., Enteral absorption of insulin in rats from mucoadhesive chitosan-anchored liposomes. Pharm Res, 13: 896-901, 1996.
- Kunimasa, J., Inui, K., Hori, R., Kawamura, Y., and Endo, K., Mannan-anchored liposome delivery of Gadolinium-Diethylenetriaminepentaacetic acid, a contrast agent for use in magnetic resonance imaging. Chem Pharm Bull, 40: 2565-2567, 1992.
Corresponding Author: S.P. Vyas, Drug Delivery Research Laboratory, Department of Pharmaceutical Sciences, Dr. H.S. Gour University, Sagar, M.P., India. spvyas@bom6.vsnl.net.in
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps
/S.Vyas/SCHEME2.jpg)
/S.Vyas/FIG.1.jpg)
/S.Vyas/FIG.3.jpg)
/S.Vyas/FIG.4A.jpg)
