J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 4(3):207-216, 2001
Assessing the plasma pharmacokinetics, tissue distribution, excretion and effects on cholesterol pharmacokinetics of a novel hydrophilic compound, FM-VP4, following administration to rats.
Kishor M. Wasan1, Kathy D. Peteherych, Soheila Najafi
Division of Pharmaceutics and Biopharmaceutics, Faculty of Pharmaceutical SciencesCatalina Zamfir and P. Haydn Pritchard
Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, CanadaReceived June 29, 2001, Revised August 22, 2001, Accepted August 23, 2001
PDF Version
Abstract
PURPOSE. The purpose of this project was to 1) assess the disposition kinetics of [3H]-cholesterol following co-administration with a novel hydrophilic compound, FM-VP4, and 2) determine the pharmacokinetics, tissue distribution and excretion of [3H]FM-VP4 following single oral (150 mg/kg which includes 100 mCi of radiolabel) and intravenous (15 mg/kg which includes 10 mCi of radiolabel) doses.
METHODS. Following an overnight fast (12-16 h) and 48 h post-surgery, adult male Sprague Dawley rats were divided into six treatment groups (n=4/group). Groups received single oral doses of 25 mCi/ml [3H]cholesterol alone or with 5, 10, 20, 50 and 100 mg/kg FM-VP4 at 0700 h. Ten percent Intralipid was used to solubilize and co-administer [3H]-cholesterol and FM-VP4. LC-MS analysis confirmed minimal cholesterol and vegetable stanol content within 10% Intralipid. Thin layer chromatography was used to confirm that the majority of radioactivity measured in plasma was associated with either esterified or unesterified cholesterol. In a second study pharmacokinetics of [3H]FM-VP4 were studied following intravenous or orally gavaged doses (n=8). Tissues, urine and feces were also collected in FM-VP4 kinetics study to measure tissue distribution of radioactivity. Plasma [3H]-cholesterol and [3H]FM-VP4 were tested for radioactivity.
RESULTS. FM-VP4 co-administration significantly decreased [3H]-cholesterol AUC0-48h and Cmax, and increased CL/F and Vd/F of [3H]-cholesterol as compared to controls in a dose-dependent manner. Following oral administration of [3H]FM-VP4, the majority of radioactivity following was recovered in the feces and gastrointestinal (GI) tract. The compound exhibited an oral bioavailability of 6.5%. Following IV administration, a two-compartment pharmacokinetic model was observed and the majority of the radioactivity was recovered in the GI tract.
CONCLUSIONS. FM-VP4 reduces plasma concentration of [3H]-cholesterol in fasting rats. [3H]FM-VP4 has a very low oral bioavailability.
Introduction
Heart disease caused by atherosclerosis is one of the leading causes of death in North America. Although smoking and high blood pressure are considered major risk factors for this fatal disease, it is the elevation in plasma cholesterol concentrations that has been most widely accepted as playing an important role in the development of cardiovascular disease (1). Plant sterols and stanols also known as phytosterols and phytostanols are naturally occurring compounds that are found in vegetable oils, seeds, and nuts and in some fruits and vegetables. Dietary intake of phytosterols has been estimated to be approximately from 180 mg per day in a traditional North American diet (2) up to 400 mg per day in a Japanese diet (3). When ingested, preliminary findings have reported that phytosterols and phytostanols decrease low-density lipoprotein (LDL)-cholesterol levels without significant effects on high-density lipoprotein (HDL)-cholesterol levels in animal models and humans (4,5). They also prevent and delay the development of atherosclerotic lesions in animal models (5,6). Recent studies have shown that. apoE-deficient mice develop severe hypercholesterolemia and atherosclerotic lesions similar to that observed in humans (5,6). When these animals are fed a cholesterol-enriched diet containing a "tall-oil"-derived phytosterol mixture, significantly lower plasma total cholesterol levels and delays in the development of atherosclerosis were observed when compared to mice fed a cholesterol-enriched diet without phytosterols. Our laboratory has observed that when rats were co-administered a novel vegetable stanol mixture composed of sitostanol and campestanol with radiolabeled and cold cholesterol incorporated into a lipid emulsion (Intralipid), the area under [ 3 H]cholesterol concentration versus time curve and maximum plasma concentration of [ 3 H]cholesterol were decreased in a dose-dependent manner (7). An explanation for these findings may be due to the inhibition or displacement of cholesterol from cholesterol-containing micelles formed with bile acids in the gut by this vegetable stanol mixture resulting in less cholesterol being available for absorption. However, further investigations are required to elucidate the mode of action of these mixtures.
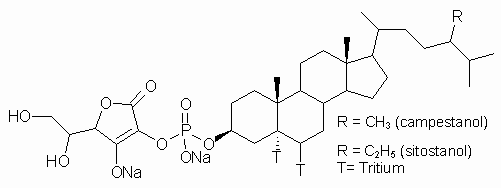
While numerous studies describing the effects of lipophilic phytosterols and phytostanols on total plasma and lipoprotein cholesterol levels have been reported (4,6-9), very little is known about the plasma pharmacokinetics of dietary cholesterol over a sustained period of time (24-48 h) following oral co-administration of a water soluble phytostanol, FM-VP4 (Figure 1), to fasting rats.
Figure 1: Chemical structure of FM-VP4, composed of sitostanol- and campestanol-ascorbyl-phosphate. Tritiated and unlabeled FM-VP4 were used in these studies.
Thus one of the objectives of this investigation was to determine the effect of FM-VP4 on the plasma pharmacokinetics of exogenous cholesterol in fasting rats. Furthermore, since no studies to date have been conducted to ascertain the bioavailability of FM-VP4, a second set of studies were completed to determine the plasma pharmacokinetics, tissue distribution and excretion of [ 3 H]FM-VP4 following oral and intravenous administration to rats
Materials and Methods
Plasma Pharmacokinetics of Cholesterol following FM-VP4 Administration:
Development of a FM-VP4-[3H]cholesterol oral gavage formulation- The formulations were composed of 25 mCi [ 3 H]-cholesterol (corresponding to 227.3 ng of cholesterol based on specific activity of 110 mCi/mg; Amersham, Mississauga, ON, Canada), 1 mg unlabeled cholesterol (7) and increasing amounts of FM-VP4 (1-20 mg). The formulations were mixed with 1 ml of Intralipid® (Clintec Nutritional Company; Deerfield, IL, USA) on the day of the study and gently vortexed. Intralipid® is a sterile non-pyrogenic fat emulsion prepared for administration as a source of calories and essential fatty acids (10), and was used as a vehicle to solubilize and co-administer exogenous [3H]-cholesterol and FM-VP4 in a palpable oral formulation. Liquid chromatography-mass spectrometry analysis revealed minimal total cholesterol and vegetable stanol content within 10% Intralipid® prior to the addition of exogenous cholesterol (labeled and unlabeled) and FM-VP4 as previously published (7).
Animal model- Adult male Sprague Dawley rats (340-410 g) were used in both the [ 3 H]-cholesterol and [ 3 H]FM-VP4 pharmacokinetics studies. The rat is an appropriate animal model to investigate the plasma pharmacokinetics of cholesterol following oral administration due to similarities in intestinal characteristics (i.e. anatomical, metabolic and biochemical characteristics) (11-14) and intestinal processing and absorption of dietary cholesterol (15) between rats and humans.
Analysis of radiolabeled cholesterol- [ 3 H]-Cholesterol concentrations in plasma were determined using external radioactivity calibration curves (corrected for quenching and luminescence). To confirm that the majority of radiolabel measured in plasma was associated with cholesterol (esterified and unesterified) thin layer chromatography was used as previously published (16).
Experimental design- All rats were cared for in accordance the Canadian Council on Animal Care and the University of British Columbia guidelines. Adult male Sprague Dawley rats were obtained from UBC animal care unit (Vancouver, B.C., Canada). The rats were maintained under a 12 h light (0700-1900)/dark cycle and supplied with a standard laboratory diet (PMI Feeds, Richmond, VA, USA) and water ad libitum . The right external jugular vein was cannulated with a two-part catheter consisting of PE 50 connected to a short length of 0.02-inch silastic tubing inserted 3.2 cm past the clavicle (17). The jugular cannula was tunneled beneath the skin and exteriorized through a small stab wound in the back of the neck.
Following an overnight fast (12-16 h) and 48 h post-surgery, rats were divided into six treatment groups and received a single-dose oral gavage (0.4 ml total volume) at 0700h of either: [3H]-cholesterol (227.3 ng/ml) plus unlabeled cholesterol (1 mg/ml) alone (control) or co-administered with FM-VP4 at concentrations of 5, 10, 20, 50 and 100 mg/kg. Plasma pharmacokinetics was initiated by sampling 0.4 ml of blood prior to and 0.25, 0.5 1.0, 2.0, 4.0, 6.0, 8.0, 10, 24, 28, 32 and 48 h post-oral gavage. An equal volume of normal saline (0.4 ml) was administered IV to the animal following each blood draw to prevent fluid depletion throughout the duration of the study. Since the majority of cholesterol within whole blood is recovered in the plasma (18), plasma was obtained by centrifugation and analyzed for [ 3 H]-cholesterol by radioactivity. [ 3 H]-cholesterol behaves in a similar fashion as unlabeled cholesterol as previously reported (18).
Pharmacokinetic data analysis- The pharmacokinetic parameters, total body clearance (CL/F; where F is the bioavailability constant) and volume of distribution (Vd/F) of [ 3 H]-cholesterol in individual animals were estimated by non-compartmental analysis using statistical moment theory (19). Concentrations of [ 3 H]-cholesterol in plasma were plotted against time on linear graph paper and terminal half-life (t 1/2β and oral absorption rate constant (k a ) were estimated by method of residuals (19). Area under the [ 3 H]-cholesterol concentration-time curve (AUC 0-48h ) was estimated by trapezoidal rule (19).
Statistical Analysis- Differences in the pharmacokinetic parameters of treatment and control groups were determined using an analysis of variance (PCANOVA; Human Dynamic Systems) (20). Statistical differences were determined using the Newman Keuls post-hoc test (20). Differences were considered significant if p<0.05. All data are expressed as mean +/- standard deviation.
Plasma Pharmacokinetics, Tissue Distribution and Excretion of FM-VP4
Radiolabeled FM-VP4-[3H]FM-VP4 was obtained from Chem Syn Laboratories (St. Louis, MO, USA) and has a specific radioactivity of 1.34 mCi/mg. Upon receipt, the material was solubilized in 20 mls of sterile water and stored at 4°C. Stability studies confirmed [ 3 H]FM-VP4 integrity for 6 months at 4°C (data not shown).
Experimental animals- Radiolabeled FM-VP4 was administered either IV through the jugular vein or by oral gavage to male Sprague-Dawley rats. Samples were collected as stated in Table 1.
Justification for Route, Duration, Frequency and Dose Level-The oral gavage route was selected for use since it is one of the routes intended for human clinical use. The intravenous route was selected in order to determine the absolute bioavailability of FM-VP4.
Table 1: Sample Collection Protocol
Number of Animals
Route of Administration
Radiolabeled Dose[3H]FM-VP4 mg/kg/mCi
Urine and Feces Collection
Hours post-administrationBlood Collection
Hours post-administrationTissue Collection
Hours post-administration4
intravenous
15/10
Urine: 0-10 h, 10-24 h, 24-48 h
Feces: 0-10 h, 10-24 h, 24-48 h0.5,1,2,4,8,10,24,28,32,48 h
400 ml blood sample at each time point48 h
4
oral
150/100
Urine: 0-10 h, 10-24 h, 24-48 h
Feces: 0-10 h, 10-24 h, 24-48 h0.5,1,2,4,8,10,24,28,32,48 h
400 ml blood sample at each time point48 h
Either a single oral or intravenous bolus of radiolabeled FM-VP4 was administered to each rat. The oral dose selected was based on the previous rodent pharmacology studies performed (7) and the projected human dose. The intravenous dose selected was based on allometric scaling and previous experience with bioavailability studies.
Preparation and Analysis of Dose Formulation-To prepare the radiolabeled dose formulation, appropriate amounts of radiolabeled and non-radiolabeled FM-VP4 (Table 1) were dissolved in distilled water to yield the desired concentration (150 mg/kg oral and 15 mg/kg intravenous) of FM-VP4 with the desired level of radioactivity. The mean concentration of radiolabeled FM-VP4 in the dosing formulation was determined by liquid scintillation counting of aliquots prior to and following administration. For these studies it was assumed that the cold FM-VP4 and the [ 3 H]FM-VP4 have the same physiochemical characteristics.
Administration of Dose Formulation-The oral dose formulation was administered using an oral-gavage-elongated needle and syringe of appropriate size at a dose volume of 2-ml/400g-body weight. The intravenous dose formulation was administered through a catheter inserted into the jugular vein of the animal at a dose volume of 0.5-ml/400g-body weight. Doses were based on the most recent body weights of the animal.
Sample Collection- Urine, feces and blood were collected at the designated times (Table 1) following FM-VP4/[ 3 H]FM-VP4 administration. Liver, lung, spleen, both kidneys, and heart were harvested from the animal 48-h post-administration of FM-VP4/[ 3 H]FM-VP4. The animals were humanely sacrificed under euthanol gas and their tissues were removed. The tissues were homogenized in sterile water and an aliquot of the homogenate was counted for radioactivity. Total tissue and feces weights and urine and blood volumes were determined.
Sample Processing and Analysis- [ 3 H]FM-VP4 concentrations in plasma, feces, urine and major tissues (liver, lung, spleen, kidney, heart) were determined against external calibration curves for each biological fluid and tissue (corrected for quenching and luminescence) using radioactivity.
Pharmacokinetic Analysis- Plasma concentration versus time data for [ 3 H]FM-VP4 in individual animals administered FM-VP4 by oral gavage was analyzed by model independent analysis using the WINNONLIN nonlinear estimation program. The linear trapezoidal rule was used to determine area under the concentration-time curve (AUC) from time 0 to 48 h postdosing for the oral gavage studies. Total clearance (CL) was calculated as the ratio of dose/AUC, where AUMC is the area under the first moment of the concentration versus time curve from time 0 to 48-h post-dosing.
The pharmacokinetic parameters CL and volume of distribution at steady state (V ss ) in individual animals administered FM-VP4 by IV were estimated by compartmental analysis using the WINNONLIN nonlinear estimation program (21). It was concluded that the [ 3 H]FM-VP4 plasma concentration data fit a two-compartment model based on "goodness-of-fit" and residual sum of square estimations using the WINNONLIN program. In addition, an independent criterion (the Akaike information ciriterion) for determination of the goodness-of-fit was used. Concentrations of [ 3 H]FM-VP4 in plasma were plotted against time on log-linear graph paper. Area under the [ 3 H]FM-VP4 concentration-time curve (AUC 0-48h ) was estimated by trapezoidal rule. Data collected from the intravenous study was used to determine the absolute bioavailability (F) of [ 3 H]FM-VP4.
Statistical Analysis- All analyses was performed using the SAS system (SAS Institute Inc.). [ 3 H]cholesterol pharmacokinetic parameters were statistically compared using analysis of variance (ANOVA). [ 3 H]FM-VP4 pharmacokinetic parameters following oral and IV dosing were statistically compared using an unpaired t-test. If the assumptions of normality or equal variances are violated, the analyses were performed on the normal-score ranks of the data. Normal-score ranks are obtained by first ranking the data (smallest to largest) across treatment groups, separately for each variable, and then applying the Blom transformation (20). The Tukey multiple comparisons procedure was used to determine significance among all possible pairs of treatments. Tukey's procedure controls the experiment-wise error at the p = 0.05 level.
Results and Discussion
Plasma Cholesterol Pharmacokinetic Studies
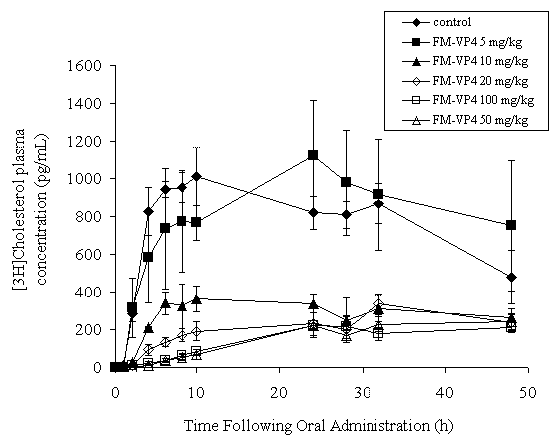
Co-administration of [ 3 H]-cholesterol with FM-VP4 at doses of 10 mg/kg and greater significantly decreased [ 3 H]-cholesterol AUC 0-48h and Cmax (Figure 2 and Table 2) in a dose-dependent manner. T max varied from 10-48 h (Table 2).
Figure 2: [3H]-Cholesterol plasma concentration-versus-time curve on a linear graph following a single oral dose of [3H]-cholesterol (227 ng/ml), unlabeled cholesterol (1 mg/ml) and FM-VP4 (0, 5, 10, 20, 50 and 100 mg/kg) co-administered in 10% Intralipid to fasting Sprague Dawley male rats. Data are shown as mean ± standard deviation. n for each treatment group the same as Table 2.
Table 2: Pharmacokinetic parameters of 3[H]cholesterol after a single oral gavage of 3[H]cholesterol, unlabeled cholesterol and different concentrations of FM-VP4 co-administered together in 10% Intralipid to Sprague Dawley male fasting rats.
Treatment Groups
AUC0-48h
(ng h / ml)Cmax (pg / ml)
Tmax(h)
CL/F (ml / h)
t1/2b (h)
Vd/F (L /kg)
Control (n=5)
37.1 +/- 8.5
1012 +/- 346
10
6.4 +/- 1.6
27.9 +/- 7.2
0.63 +/- 0.14
5 mg/kg (n=4)
41.8 +/- 15.7
1122 +/- 294
24
6.0 +/- 2.1
40.9 +/- 3.1*
6.0 +/- 5.0*
10 mg/kg (n=3)
13.9 +/- 4.2*
360 +/- 48*
10
17.3+/- 4.9*
71.6 +/- 41.8
21.2 +/- 12.7*
20 mg/kg (n=4)
10.4 +/- 2.1*
335 +/- 55*
32
22.5 +/- 3.9*
61.6 +/- 40.8 (n=3)
6.1 +/- 5.0* (n=3)
50 mg/kg (n=3)
8.2 +/- 0.8*
239 +/- 45*
48
27.9 +/- 2.9*
N/A Levels go up
N/A Levels go up
100 mg/kg (n=4)
7.2 +/- 3.1*
220 +/- 66*
24
38.1+/- 20.9*
44.9 +/- 30.5(n=2)
4.5 +/- 2.5 (n=2)
Data are presented as mean +/- standard deviation. Abbreviations; AUC0-48h, area under concentration-time curve for the radiolabel; Cmax, concentration at which peak radiolabel plasma concentrations were observed; Tmax, time at which peak radiolabel plasma concentrations were observed; CL/F, plasma clearance of the radiolabel, where F is the bioavailability constant; t1/2b; plasma elimination half-life of the radiolabel; Vd/F, volume of distribution. All formulations are dissolved in 10% Intralipid containing 0.1 mg/ml of total cholesterol and 0.04 mg/ml plant stanols determined by LC-MS.*p<0.05 vs. control.
N/A; not applicable; since plasma cholesterol levels are rising over time these parameters cannot be calculated.
Furthermore, co-administration with FM-VP4 at 10, 20, 50 and 100 mg/kg resulted in significant increases in CL/F and V d /F compared to controls (Table 2). Taken together these findings indicate that co-administration of cholesterol with FM-VP4 significantly modifies the plasma pharmacokinetics of [ 3 H]-cholesterol in fasting rats. Both oral and parenteral administration of phytosterols in humans and animal models resulted in reduced concentrations of plasma cholesterol (4,5,22-30,32-41). This reduction may be due not only to the inhibition of intestinal cholesterol absorption but also to other effects on hepatic and intestinal cholesterol metabolism (34,41,42). Evidence from human and animal studies indicates that hepatic cholesterol synthesis is influenced by intestinal cholesterol absorption: inhibition of intestinal cholesterol absorption by phytosterols stimulates de novo hepatic cholesterol synthesis, while increased cholesterol absorption suppresses it. Dietary phytosterols may cause an increase in hepatic cholesterol secretion (42). There are no convincing data on the effect of phytosterols on hepatic bile synthesis. Phytosterol therapy is also associated with a significant decrease in hepatic and lipoprotein lipase activities in apoE deficient mice (5) and a significant increase in serum lecithin: cholesterol acyltransferase activity in hypercholesterolemic subjects' (41).
In 1993 Aviram and Eias reported that the consumption of olive oil (50 g/day) was associated with an increase in the sitosterol content of LDL particles and a marked reduction of in vitro lipid preoxidation and in vitro uptake by macrophages (42). Thus, LDL that has been modified by the incorporation of phytosterols or by a shift in its fatty acid composition may have antiatherogenic properties owing to its resistance to peroxidation that results in reduced uptake by macrophages. Further work by Bhadra and Subbiah showed a significant reduction in cellular cholesterol content with a corresponding increase in sitosterol concentration in human skin fibroblasts and HepG2 cells incubated with liposomes containing sitosterol (43). Field and colleagues, in addition, reported that the incubation of Caco-2 cells with β −sitosterol decreased uptake of cholesterol from the incubation medium (44).
In this study increases in the FM-VP4 dose (10-100 mg/kg) were observed to decrease the rate of [ 3 H]-cholesterol absorption (Figure 2) and the overall [ 3 H]-cholesterol exposure to the body (AUC 0-48 h ) (Table 2). These findings suggest that increases in FM-VP4 dose may decrease the percentage of cholesterol available for absorption and the cholesterol that is available may be absorbed more slowly in the presence of FM-VP4. A possible explanation for the decrease in AUC may be the inhibition or displacement by FM-VP4 of cholesterol from cholesterol-containing micelles, formed with bile acids in the intestine, that are required for cholesterol absorption (8). The decrease in absorption rate may be due to decreased facilitated cholesterol uptake by enterocyte cholesterol transporters (45-47) to compensate for the limited amount of cholesterol available to be absorbed. These possible explanations warrant further investigation.
Plasma Pharmacokinetics, Tissue Distribution and Excretion of FM-VP4 Studies
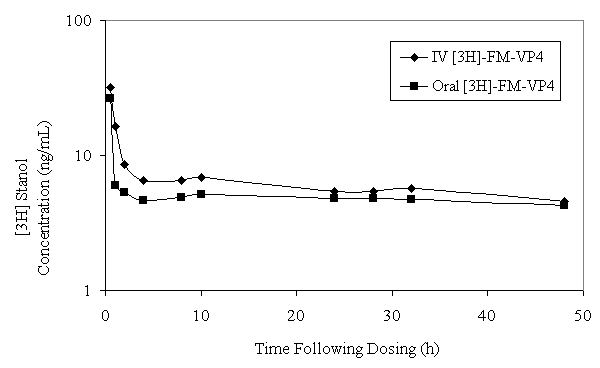
[ 3 H]FM-VP4 AUC and mean residence time (MRT) after a single IV dose of FM-VP4/[ 3 H]FM-VP4 in rats were significantly greater compared with the AUC and MRT following a single oral dose (Figure 3 and Table 3).
Figure 3: [3H]FM-VP4 plasma concentration-versus-time curve on a log-linear graph following a single intraenvous (15 mg/kg) or oral (150 mg/kg) dose of [3H]FM-VP4 and unlabeled FM-VP4 to fasting Sprague Dawley male rats. Data are shown as mean ± standard deviation; n=4 for each group.
Table 3: Pharmacokinetic parameters of [3H]FM-VP4 + unlabeled FM-VP4 following single intravenous (15 mg/kg containing 10 mCi of [3H]FM-VP4 ) and oral (150 mg/kg containing 100 mCi of [3H]FM-VP4) dose to fasting male Sprague Dawley rats (350-390 g).
Route of Administration
Cl
(ml/hr kg)AUC48h
(ng hr/ml)t1/2a
(h)t1/2b
(h)MRT
(h)Vss
(L/kg)Intravenous
80.3+/- 28.3
281.2 +/- 31.9
0.29+/-0.08
74.4+/-35.6
102.2+/-50.3
7.4+/-2.3
Oral
527.1+/-267*
183.1 +/- 56*
NA
139.7+/-49.4
19.3+/-4.0*
95.5+/-12.5*
Mean +/- standard deviation; n=4; *P<0.05 vs. intravenous. Bioavailability (F) of [3H]FM-VP4 based on intravenous and oral dosing and AUC is 0.065. NA, not applicable; CL, systemic clearance, AUC, area under concentration time curve; t1/2, distribution half-life; t1/2, terminal half-life; MRT, mean residence time; Vss, volume of distribution at steady state
These data were not corrected for dose. However, [ 3 H]FM-VP4 systemic CL and V ss were significantly greater in rats administered an oral dose of [ 3 H]FM-VP4 compared to rats administered a single IV dose (Table 3).
The plasma pharmacokinetics suggests that following oral administration of [ 3 H]-FM-VP4 the radioactivity recovered in the plasma does not represent FM-VP4, but some metabolites. This conclusion is supported by the differences in terminal half-life (t1/2b between the oral and intravenous administration. The longer half-life following oral administration suggests that a metabolite of FM-VP4 is being followed and is probably being pooled within the lipoprotein fraction of the plasma. Differences in both the Cl (rate at which a compound is removed from a unit volume of plasma) and Vss (extent of distribution within the body) of the oral compared to the intravenous administration is probably due to the fact that the oral dose is 10 times greater than the intravenous dose. When you correct for dose there appears to be no significant difference between the Cl and Vss of tritiated FM-VP4 following oral or intravenous administration.
The greatest recovery of radiolabel between heart, liver, lung, kidney and spleen was in the kidneys, regardless of [ 3 H]FM-VP4 administration route (Table 4). Lung recovery, although not statistically significant was greater in rats administered an IV dose than an oral dose of [ 3 H]FM-VP4 (Table 4). Radiolabel was recovered in heart and spleen of intravenously dosed rats only. No radiolabel was detectable in the liver of rats regardless of administration route. These studies suggest that following oral and intravenous administration most of the radioactivity is probably elsewhere (i.e. GI tract, water spacing between tissues and cells etc.) with minimal distribution into the major organs of the body. To further support this hypothesis an additional study to determine the total body recovery of radioactivity was done where the gastrointestinal tract tissues (stomach, colon, duodenum etc.) and contents were removed and analyzed for radioactivity.
Table 4: Tissue, Urine, Feces and Gatrointestinal (GI) Tract recovery of [3H]FM-VP4 48 hours following a single intravenous (15 mg/kg containing 10 mCi of [3H]FM-VP4) and oral (150 mg/kg containing 100 mCi of [3H]FM-VP4) dose of radiolabeled and unlabeled FM-VP4 to fasting male Sprague Dawley rats (350-390g).
Route of Administration
Heart
(ng/g tissue wt)Kidney
(ng/g tissue wt)Liver
(ng/g tissue wt)
Lung
(ng/g tissue wt)Spleen
(ng/g tissue wt)Intravenous (n=4)
1.2 +/- 0.9
78.9 +/- 25.7
ND
6.4 +/- 4.8
33.6 +/- 37.3
Oral (n=4)
ND
51.2 +/- 23.7
ND
0.5 ± 0.3
ND
Data presented as mean +/- standard deviation. *P<0.05 vs. Intravenous administration. ND, non-detectable (below the quantifiable limit of our assay)
The vast majority of [3H]FM-VP4 was recovered in the feces and gastrointestinal (GI) tract following oral administration (Table 5). In addition, a substantially larger proportion of [ 3 H]FM-VP4 was recovered in the GI tract and feces following oral administration compared to IV administration (Table 5). Calculating the bioavailability of [ 3 H]FM-VP4 using the oral and IV data results in about 6.5% of the original oral dose being available to the systemic circulation after first pass effect.
Table 5: Tissue, Urine, Feces and Gatrointestinal (GI) Tract recovery of [3H]FM-VP4 48 hours following a single intravenous (15 mg/kg containing 10 mCi of [3H]FM-VP4) and oral (150 mg/kg containing 100 mCi of [3H]FM-VP4) dose of radiolabeled and unlabeled FM-VP4 to fasting male Sprague Dawley rats (350-390g).
Route of Administration
% Tissuesa
% Urine
% Feces
% GI Tract
% of Dose Recovered
Intravenous (n=3)
2.0 +/- 0.5
1.9 +/- 0.7
6.8 +/- 4.5
43.7 +/- 12.0
54.3 +/- 8.5
Oral (n=3)
0.2 +/- 0.2*
2.2 +/- 0.5
34.5 +/- 31.0*
68.5 +/- 11.6*
105.5 +/- 20.6*
Data presented as mean +/- standard deviation. *P<0.05 vs. Intravenous administration. apercentage of original [3H]FM-VP4 dose administered.
The total body recovery studies suggest that following oral dosing that all of the radioactivity can be accounted for and that greater than 95% of it is found in the GI tract and/or GI tract contents and the feces. This radioactivity recovered in the GI tissues and content may represent unabsorbed drug, biliary excretion of absorbed drug, drug fecally excreted and/or re-ingested by way of coprophagia or drug in the GI tract tissue in the process of being absorbed.
However, following intravenous administration only 54% of the total radioactivity was recovered with the majority of it being in the GI tract/contents and feces (81% of radioactivity recovered). This high level of radioactivity in the GI tract tissues and contents suggests the possibility of coprophagia, which could occur in rats not fed for an extended period of time or biliary excretion. Take together, these findings further suggest that the radioactivity found in the GI tract and/or feces represent parent VP4.
The lack of recovery of radioactivity following intravenous administration may be due to loss of the tritium label into the interstitial spacing or aqueous fluids between tissues and cells. Furthermore, following intravenous administration it remains unknown why radioactivity appears in the gut but not in the liver. These findings from this preliminary study will be further evaluated in a follow-up ADME study.
Taken together these findings suggest that the vast majority of FM-VP4 is either retained in the GI tract or removed via the feces following oral administration. Only a small percentage of FM-VP4 is absorbed and/or available to the systemic circulation. Furthermore, due to the high concentration of FM-VP4 recovered in the GI tract following administration suggests that FM-VP4 may exert its effect on inhibiting cholesterol absorption within the gut. Additional studies to investigate these hypotheses are on going within our laboratory.
One of the limitations of these studies was ascertaining if the radiolabelled FM-VP4 is intact upon absorption into the bloodstream. We have assumed that the radioactivity represented the parent compound only. However, this does not take into account possibility of metabolites. Currently we are developing a LC-MS assay to ascertain if the radiolabelled compound does represent the parent drug and/or metabolites.
In conclusion, co-administration of FM-VP4 modifies the plasma pharmacokinetics [ 3 H]-cholesterol in fasting rats. In addition, FM-VP4 follows either a noncomparment (oral gavage) or two-compartment (IV) model, has a bioavailability of around 6.5% and is mainly found in the GI tract and feces following oral administration. Further studies to determine at where and how FM-VP4 prevents cholesterol GI absorption are warranted.
Acknowledgment
Canadian Institutes of Health Research-Forbes Medi Tech Inc. University/Industry Operating Grant (#UOP 48090) to KMW and HP.
References
P. Cullen and G. Assmann. High risk strategies for atherosclerosis. Clin. Chim. Acta. 286(1-2):31-45 (1999).
W. E. Connor. Dietary sterols: their relationships to atherosclerosis. J. Am. Diet Assoc. 73:39-47 (1968).
K. Hirai, C. Shimazu, R. Takezoe and Y. Ozeki. Cholesterol, phytosterol and polyunsaturated fatty acid levels in 1982 and 1957 Japanese diets. J. Nutr. Sci. Vitaminol. 32:363-372 (1986).
M. H. Moghadasian and J. J. Frohlich. Effects of dietary phytosterols on cholesterol metabolism and atherosclerosis: Clinical and experimental evidence. Am. J. Med. 107:588-594 (1999).
M.H. Moghadasian, B. M. McManus, D. V. Godin, B. Rodrigues and J. J. Frohlich. Proatherogenic and antiatherogenic effects of probucol and phytosterols in apoE-deficient mice: Possible mechanisms of action. Circulation 99:1733-1799 (1999).
M. H. Moghadasian, B. M. McManus, P. H. Pritchard and J. J. Frohlich. "Tail-Oil"-derived phytosterols reduce atherosclerosis in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 17:119-126 (1997).
K. M. Wasan, L. Holtorf, R. Subramanian, S.M. Cassidy, P. H. Pritchard, D. J. Stewart, E. Novak and M. H. Moghadasian. Assessing plasma pharmacokinetics of cholesterol following oral coadministration with a novel vegetable stanol mixture to fasting rats. J. Pharm. Sci. 90:23-28 (2001).
T. Heinemann, G. Axtmann and K. von Bergmann. Comparison of intestinal absorption of cholesterol with different plant sterols in man. Eur. J. Clin. Invest. 23:827-831 (1993).
I. Ikeda, K. Tanaka, M. Sugano, G. V. Vahouny and L. L. Gallo. Inhibition of cholesterol absorption in rats by plant sterols. J. Lipid Res. 29:1573-1582 (1988).
Kabi Pharmacia. 10% Intralipid Insert (1987).
U. Fagerholm, M. Johansson, and H. Lennernas. Comparison between permeability coefficients in rat and human jejunum. Pharm. Res. 13:1336-1342 (1996).
T. T. Kararli. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm. Drug Dispos. 16:351-380 (1995).
B. Levet-Trafit, M. S. Gruyer, M. Marjanovic, and R. C. Chou. Estimation of oral drug absorption in man based on intestine permeability in rats. Life Sci. 58:PL359-363 (1996).
I. Soria and C. L. Zimmerman. The validation of the intestinal permeability approach to predict oral fraction of dose absorbed in humans and rats. Biopharm. Drug Dispos. 17:817-818 (1996).
M. V. Pahl, F. Oveisi, G. Khamiseh and N. D. Vaziri. Intestinal absorption and biliary secretion of cholesterol in rats with nephrotic syndrome. Nephrology, Dialysis, Transplant. 13:1446-1451 (1998).
M. Dobiasova and J. J. Frohlich. Assays of Lecithin Cholesterol Acyltransferase (LCAT), p. 217-230. In J. M. Ordovas (ed.). Methods in Molecular Biology Volume 110: Lipoprotein Protocols. Humana Press Inc., Totowa, New Jersey, 1998, pp. 217-230.
D. J. Hauss, S. E. Fogal and J. V. Ficorilli. Chronic collection of mesenteric lymph from conscious tethered rats. Contemp. Topics Lab. Animal Sci. 37:56-58 (1998).
R. A. Davis and J. E. Vance. Structure, assembly and secretion of lipoproteins. In: D. E. Vance and J. E. Vance (eds.), Biochemistry of lipids, lipoproteins and membranes. Elsevier, New York, 1996, pp. 473-493.
M. L. Rocci, Jr. and W. J. Jusko. LAGRAN program for area and moments in pharmacokinetic analysis. Comp. Prog. Biomed. 16:203-211 (1983).
G. Blom. In: Statistical Estimates and Transformed Beta Variables. (eds. Blom G.); John Wiley and Sons Inc.; NY, 1958.
R. E. Ostlund Jr., C. A. Spilburg and W. F. Stenson. Sitostanol administered in lecithin micelles potently reduces cholesterol absorption in humans. Am. J. Clin. Nutr. 70:826-831 (1999).
M. Becker, D. Staab and K. Von Bergmann. Treatment of severe familial hypercholesterolemia in childhood with sitosterol and sitostanol. J. Pediatr. 122:292-296 (1993).
M. Becker, D. Staab and K. von Bergmann. Long-term treatment of severe familial hypercholesterolemia in children: effectsof sitosterol and bezafibrate. Pediatrics 89:138-142 (1992).
T. Gerson, F.B. Shorland and G.G. Dunckley. The effects of sitosterol on the metabolism of choleserol and lipids in rats on a diet low in fat. Biochem. J. 92:385-390 (1964).
H. Gylling, R. Radhakrishnan and T.A. Miettinen. Reduction of serum choleserol in postmenopausal women with previous myocardial infarction and cholesterol malabsorption induced by dietary sitostanol ester margarine: women and dietary sitostanol. Circulation 96:4266-4231 (1997).
H. Gylling and T.A. Miettinen. Effects of inhibiting cholesterol absorption and synthesis on cholesterol and lipoprotein metabolism in hypercholesterollemic non-insulin dependent diabetic men. J. Lipid Res. 37:1776-1785 (1996).
H. Gylling and T.A. Miettinen. Cholesterol reduction by different plant stanol mixtures and with variable fat intake. Metabolism 48:575-580 (1999).
H. Gylling, M.A. Siimes and T.A. Miettinen. Sitostanol ester margarine in dietary treatment of children with familial hypercholesterolemia. J. Lipid Res. 36:1807-1812 (1995).
H. Gylling and T.A. Miettinen. Serum choleterol and cholesterol and lipoprotein metabolism in hypercholesterolemic NIDDM patients before and during sitostanol ester-margarine treatment. Diabetologia. 37:773-780 (1994).
M.A. Hallikainsen and M.I. Uusitupa. Effects of 2 low-fat stanol ester-containing margarines on serum cholesterol concentrations as part of a low-fat diet in hypercholesterolemic subjects. Am. J. Clin. Nutr. 69:403-410 (1999).
T. A. Miettinen, P. Puska, H. Gylling, et al. Reduction of serum cholesterol with sitostanol-ester margarine is a mildly hypercholesterolemic population. New England J. Med. 333:1308-1312 (1995).
M. H. Moghadasian, L. B. Nguyen, S. Shefer, et al. Histologic, hematologic, and biochemical characteristics of apo E-deficient mice: effects of dietary cholesterol and phytosterols. Lab Invest. 79: 355-364 (1999).
M. H. Moghadasian, D.V. Godin, B.M. McManus and J.J. Frohlich. Lack of regression of atherosclerotic leisions in phytosterol-treated apo E-deficient mice. Life Sci. 64:1029-1036 (1999).
M. H. Moghadasian. Effects of a "tall-oil"-derived phytosterol mixture on the development of atherosclerotic leisons in apo E-deficient mice. Ph.D. thesis. Vancouver, B.C., University of British Columbia; Faculty of Medicine, Department of Pathology and Laboratory Medicine (1998).
H. F. J. Hendriks, J. A. Weststrate, T. van Vliet and G.W. Meijer. Spreads enriched with three different levels of vegetable oil sterols and the degree of cholesterol lowering in normocholesterolemic and mildly hypercholesterolemic subjects. Eur. J. Clin. Nutr. 53:319-327 (1999).
P. J. H. Jones, F. Y. Ntanios, M. Raeini-Sarjaz and C.A. Vanstone. Cholesterol-lowering efficacy of a sitostanol-containing phytosterol mixture with a prudent diet in hyperlipidemic man. Am. J. Clin. Nutr. 69:1144-1150 (1999).
J. E. Konlande and H. Fisher. Evidence for a nonabsorptive antihypercholesterolemic action of phytosterols in the chicken. J. Nutr. 98:435-442 (1969).
D. W. Peterson. Effect of soybean sterols in the diet on plasma and liver cholesterol in chicks. Proc. Soc. Biol. Med. 78:143-147 (1951).
H. T. Vanhanen, J. Kajander, H. Lehtovirta and T.A. Miettinen. Serum levels, absorption efficacy, fecal elimination and synthesis of cholesterol during increasing dosing of dietary sitostanol esters in hypercholesterolemic subjects. Clin. Sci. 87:61-67 (1994).
H. T. Vanhanen, S. Blomqvist, C. Ehnholm, et al. Serum cholesterol, cholesterol precursors, and plant sterols in hypercholesterolemic subjects with different apo E phenotypes during dietary sitostanol ester treatment. J. Lipid Res. 34:1535-1544 (1993).
P. Weisweller, V. Heinemann, and P. Schwandt. Serum lipoproteins and lecithin:cholesterol acyltransferase (LCAT) activity in hypercholesterolemic subjects given b-sitosterol. Int. J. Clin. Pharmacol. 22:204-206, (1984).
M. Aviram and K. Eias. Dietary olive oil reduces low-density lipoprotein uptake by macrophages and decreases the susceptibility of the lipoprotein to undergo lipid peroxidation. Ann.Nutr. Metab. 37:75-84 (1993).
S. Bhadra and M.T. Subbiah. Incorporation of liposomal phytosterols into human cells in culture: a potential in vitro model for investigating pathological effects of phytosterolemia. Biochem. Med. Met. Biol. 46:119-124 (1991).
F. J. Field, E. Born and N.M. Satya. Effects of micellar sitosterol on cholesterol metabolism in Caco-2 cells. J. Lipid Res. 38:348-360 (1997).
K. E. Berge, H. Tian, G.A. Graf, L. Yu, N.V. Grishin, J. Schultz, P. Kwiterovich, B. Shan, R., Barnes and H. H. Hobbs. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science 290:1771-1775 (2000).
J. J. Repa, S. D. Turley, J-M. A. Lobaccaro, J. Medina, L. Li, K. Lustig, B. Shan, R. A. Heyman, J. M. Dietschy and D. J. Mangelsdorf. Regulation of Absorption and ABC1-mediated efflux of cholesterol of RXR Heterodimers. Science 289:1524-1529 (2000).
K. Simons and E. Ikonen. How cells handle cholesterol. Science 290:1721-1725 (2000).
Corresponding Author: Kishor M. Wasan, Division of Pharmaceutics and Biopharmaceutics, Faculty of Pharmaceutical Sciences, University of British Columbia, 2146 East Mall Avenue, Vancouver,British Columbia, Canada, V6T 1Z3. kwasan@interchange.ubc.ca
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps