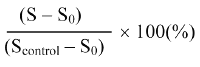J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 5(1):19-23, 2002
An In Vitro Evaluation of Human DNA Topoisomerase I Inhibition by Peganum harmala L. Seeds Extract and Its
b-Carboline AlkaloidsArmin Madadkar Sobhani, Sultan-Ahmad Ebrahimi, Massoud Mahmoudian1
Department of Pharmacology, Iran University of Medical Sciences, Tehran, IranReceived September 13, 2001, Revised January 22, 2002, Accepted March 8, 2002
PDF version
Abstract
Purpose: Peganum harmala L. (Zygophyllaceae) seeds extract is one of the main components of an ethnobotanical preparation used in the treatment of neoplasms in Iran. Cytotoxic effects of P. harmala extract on cancerous cell-lines have been reported before. b-carbolines like harmaline and harmine are the major alkaloids present in the seeds of the P. harmala. Considering reports concerning DNA topoisomerase inhibition by other b-carbolines like harmane, we have used DNA relaxation assays to investigate topoisomerase I inhibitory activity of P. harmala seeds extract and its b-carboline alkaloids to further inspecting the mechanism of its cytotoxic activity.
Methods: Harmine and harmaline contents of the extract were determined using an HPTLC method. DNA topoisomerase I enzyme needed for investigating inhibitory effect of the compounds using DNA relaxation assay, was partially purified from the human placenta. DNA relaxation assay is based on the conversion of a supercoiled plasmid substrate to its relaxed form by the catalytic activity of the enzyme. The supercoiled substrate and its relaxed product can be easily distinguished using agarose gel electrophoresis, since the relaxed topological isomers of DNA migrate more slowly than supercoiled species.
Results: Using HPTLC method, it was found that each gram of dried extract contained 55.5 and 79.0 mg of harmine and harmaline respectively. In the DNA relaxation assay, order of potency was harmine > harmane > harmaline > extract. The most active compound was harmine with IC50 value of 13.5 ± 1.7 mg/ml.
Conclusions: Our in vitro findings demonstrate that P. harmala seeds extract do inhibit human DNA topoisomerase I and based on the results of HPTLC analysis, it appears that the biological activity of the extract can be explained by its b-carboline content.
Introduction
One of the current strategies for drug discovery involves the study of plants and plant materials based on the ethnobotanical usage. In the search for anticancer drugs, use of a plant or plant material for the treatment of certain cancer-related diseases can provide a guide for further studies. This includes: cancer treatment, immune disorders, infectious diseases, parasitic diseases and viral diseases (1). Urgent collection, recording and confirmation of ethnobotanical knowledge has been emphasized, since many local healers are elderly and lack apprentices (2). As they die, much of their knowledge of local plants dies too.
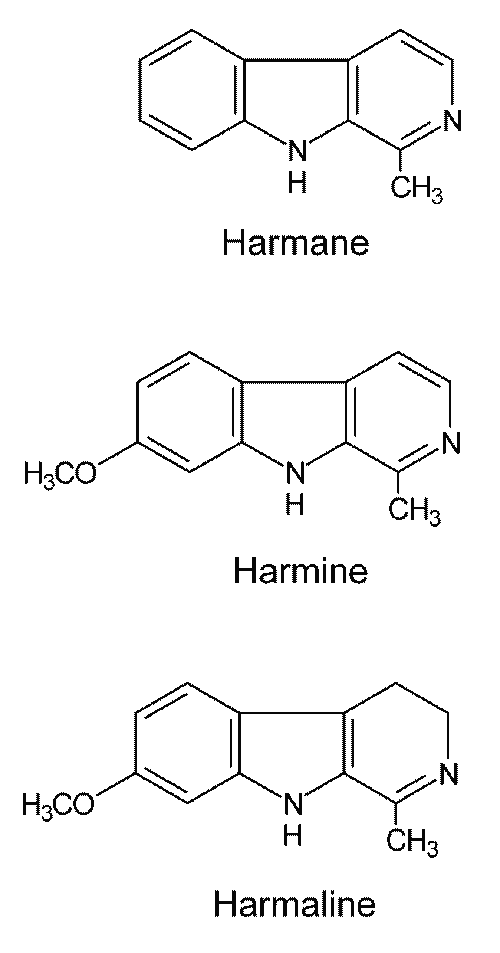
An ethnobotanical preparation used in the treatment of cancer by the late Hakim Analoui, a local healer in Iran, is a mixture of the extracts of Peganum harmala L. (Zygophyllaceae) and Dracocephalum kotshyi Boiss. (Labiatae). Eighty percent of this local remedy is P. harmala seeds extract, which its cytotoxic effects on cancerous cell-lines have been demonstrated in previous studies (3-5). P. harmala is a poisonous plant that grows in Central Asia, North Africa and Middle East and b-carboline alkaloids like harmine (7-methoxy-1-methyl-9H-pyrido[3,4-b]indole) and harmaline (4,9-dihydro-7-methoxy-1-methyl-3H-pyrido[3,4-b]indole) constitute more than 60% of its seeds extract total alkaloids (6). In addition to their effects on CNS (7) and cardiovascular system (8), there are reports of antimicrobial (9) and cytotoxic activities (5, 10) of b-carbolines.
DNA topoisomerases are enzymes that catalyze the passage of individual DNA strands (type I) or double helices (type II) through one another, which is manifested in the interconversions between topological isomers of DNA (11). These enzymes have important roles in replication, recombination, transcription, chromosome condensation and the maintenance of genome stability (12) and hence are good targets for antibacterial agents and antineoplastic drugs (13). Unlike topoisomerase II, topoisomerase I is not a cell cycle-dependent enzyme, i.e. its activity does not change with different phases of cell growth and, therefore, it is a more desirable cellular target for anticancer drug development than topoisomerase II (14). Recently, it has been reported that harmane (1-methyl-9H-pyrido[3,4-b]indole) another b-carboline present in cooked foods and cigarette smoke, has inhibitory effect on human DNA topoisomerases (15).
Considering the above-mentioned facts, we have used DNA relaxation assays to investigate topoisomerase I inhibitory activity of P. harmala seeds extract and its b-carboline alkaloids to further inspecting the mechanism of its cytotoxic activity. DNA relaxation assay is based on the conversion of a supercoiled plasmid substrate to its relaxed form by the catalytic activity of the enzyme (16). The supercoiled substrate and its relaxed product can be easily distinguished using agarose gel electrophoresis, since the relaxed topological isomers of DNA migrate more slowly than supercoiled species.
Materials and methods
Chemicals
Camptothecin, quinidine sulfate, harmane.HCl, harmine.HCl and harmaline.HCl were obtained from Sigma Chemical Co. (St. Louis, MO, USA) and were dissolved in dimethylsulfoxide (DMSO) (Merck, Germany) at 10 mg/ml for camptothecin and 50 mg/ml for the others. They were stored at -20°C until use. Teniposide was obtained from Vehem-Sandoz. Polyethyleneimine (PEI) (5% w/v, i.e.10% v/v solution) was prepared from 50% (w/v) stock solution (Sigma Chemical Co.) as described before (17). pBC KS plasmid was provided by Dr. B. Goliaei, Institute of Biochemistry and Biophysics, Tehran, Iran.
Plant material
The aerial parts of P. harmala were collected around Tehran province. The plant was verified by Dr Gh. Amin, and a voucher specimen (6520 TEH) has been deposited in the Herbarium of Department of Pharmacognosy, Faculty of Pharmacy, University of Tehran.
Preparation of extract
The crude extract was obtained by maceration of 100 g dried seeds in 200 ml solvent A (55 ml double distilled water, 45 ml ethyl acetate and 100 ml 95% ethanol) for 48 h at 50°C. The fresh extract was filtered and concentrated in vacuo below 60°C to an extract with dry matter of about 700 mg/ml. Finally; resulting concentrated extract was dried below 70°C in an oven. All the concentrations of the extract are based on the dry weight of the extract.
High performance TLC (HPTLC)
The content of b-carboline alkaloids in P. harmala extract were determined by HPTLC on 20x20 cm silica gel 60, F254 (Merck, Germany) plate, using methanol/concentrated ammonia solution (100:1.5) as the mobile phase and quinidine sulfate as the internal standard. Application of samples was carried out using Desaga AS30 applicator (10 ml application volume) and a Desaga CD60 densitometer was used to analyze the plate (fluorescence mode, lex = 320 nm, lf = 420 nm).
Purification of human DNA topoisomerase I
Topoisomerase I was partially purified from human placenta by the modified procedure of Holden at al (18). All purification steps were performed at 4°C unless otherwise stated. Fresh human placentas were obtained after delivery from maternity department of Firoozabadi hospital (IUMS, Tehran) and umbilical cord and fetal membranes were immediately removed. The placenta was washed eight times in 500 ml aliquots of physiological serum. About 200 g of tissue was minced in 500 ml buffer A (30 mM Tris-HCl, pH 7.5; 150 mM NaCl, 0.3 M sucrose, 0.2 mM EDTA, 15 mM 2-mercaptoethanol and 1 mM phenylmethylsulfonyl fluoride [PMSF]) by a hand operated meat grinder. The minced tissue was gently squeezed in cheesecloth to remove excess buffer, and resuspended in 400 ml of buffer B (30 mM Tris-HCl, pH 7.5; 0.3 M sucrose, 4 mM CaCl 2 , 15 mM 2-mercaptoethanol and 1 mM PMSF) and homogenized in 50 ml aliquots with a Polytron PT2000 homogenizer (4 pulses, 15 sec each at maximum speed). The homogenate was filtered once through cheesecloth and centrifuged at 2,000 g for 15 min to pellet nuclei. The nuclei were resuspended in 100 ml buffer C (10 mM Tris-HCl, pH 7.5; 10 mM NaCl, 7 mM 2-mercaptoethanol, 1 mM PMSF) and lysed by adding an equal volume of buffer D (50 mM Tris-HCl, pH 7.5; 2M NaCl, 7 mM 2-mercaptoethanol, 1 mM PMSF). The suspension was stirred for 15 min and the resulting viscose solution centrifuged at 5,000 g for 5 min. For each ml of supernatant, 10 ml of PEI solution was added with continuous stirring and after 15 min, centrifuged at 5,000 g for 20 min. The volume of the supernatant was measured and solid ammonium sulfate was slowly added to a concentration of 45% (w/v) with stirring. Stirring was continued for 15 min and the suspension centrifuged at 5,000 g for 20 min. Solid ammonium sulfate was added to the supernatant to a concentration of 65% (w/v) and after centrifugation (5,000 g for 20 min), further amount of ammonium sulfate was added to a final concentration of 85% (w/v). The suspension was centrifuged (5,000 g for 20 min), and the pellet was resuspended in minimum amount of buffer E (50 mM Tris-HCl, pH 7.8; 10 mM Na2S2O3, 7 mM 2-mercaptoethanol, 10% w/v glycerol). During ammonium sulfate precipitation, pH was adjusted to 7.5 using 2 M Tris base. Protein concentration of the partially purified topoisomerase I preparation was determined by measuring UV absorbance at 280 nm (19) and the preparation was aliquoted in small batches and stored at -80°C for later use. The protein content and specific activity at this stage were 7.8 mg/ml and 1.3 × 105 U/mg respectively.
Topoisomerase I assay
Topoisomerase I activity was measured by in vitro relaxation of supercoiled pBC KS plasmid DNA to the relaxed topoisomer. DNA relaxation assay has been used by many investigators to study the catalytic activity of both type I and type II topoisomerases, but relaxation mediated by eukaryotic topoisomerase II requires both Mg 2+ and ATP (16).
The 20 ml reaction mixture contained 10 mM Tris-HCl, pH 7.9; 150 mM NaCl, 1 mM EDTA, 1 mM 2-mercaptoethanol, 1mM PMSF, 0.1 mM spermidine, 100 mg/ml bovine serum albumin and 50% w/v glycerol, 25 mg/ml pBC KS DNA and various amounts of enzyme preparation. Mixtures were incubated at 37°C for 15 min in an Eppendorf 5436 thermomixer. The reactions stopped by addition of 5 ml stop buffer (5% sodium dodecyl sulfate, 0.0025% bromophenol blue; 30% w/v glycerol). DNA was resolved on 1% w/v agarose submarine gels in the electrophoresis buffer (TBE 0.5c: 45 mM Tris-borate, pH 8.0; 1 mM EDTA) for 3 hours at 45 V (3 V/cm). The gels were subsequently stained with 0.5 mg/ml ethidium bromide for 15 min, destained in H2O for 30 min, photographed under UV illumination and the relative amounts of relaxed and supercoiled DNA was determined by scanning with an ImageMaster 1D digital imaging system (Pharmacia Biotech).
One unit of topoisomerase I activity was defined as the minimum amount of enzyme required to achieve complete relaxation of 0.5 mg superhelical pBC KS DNA in 15 min at 37° C (about 100-fold dilution of stock enzyme preparation).
Inhibition of topoisomerase I relaxation activity was investigated by the same procedure using one unit of enzyme and different concentrations of test compounds. Three independent experiments have been carried out for each compound. The percent of relaxed DNA was calculated by the following formula:
Where Scontrol is the percent of supercoiled DNA in the control lane (without enzyme and test compounds) and S0 is the percent of supercoiled DNA in the lane without test compounds. The IC50 was defined as the concentration of the test compound resulting in a 50% reduction of relaxed DNA. Compounds dissolved in DMSO gave final DMSO concentrations of 2.5%, which at these concentrations, DMSO had no effect on drug-free controls.
Results
HPTLC analysis
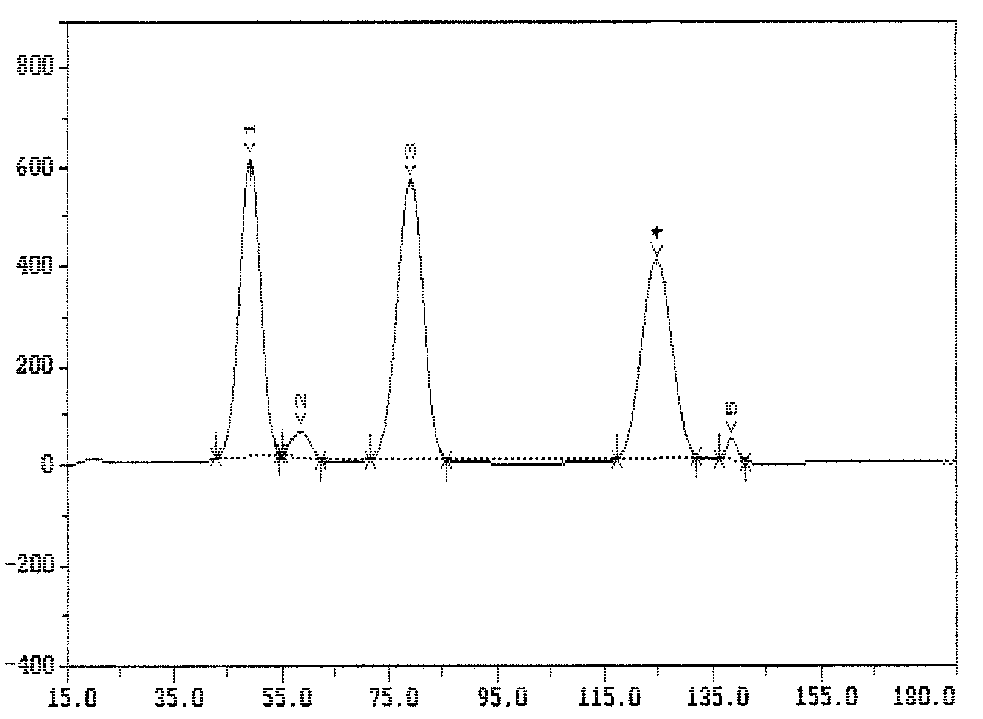
In HPTLC analysis, harmine and harmaline were separated from each other with Rf values of 0.66 and 0.21 respectively (Figure 1). Harmane (Rf 0.70) was not detected in the extract. The plots of the ratio of peak area of the alkaloids to quinidine sulfate (Rf 0.40), which was used as an internal standard, versus concentration, were straight lines with slopes of 0.0274 (r2 = 0.9897) for harmine and 0.0211 (r2 = 0.9925) for harmaline. Using this method, it was found that each gram of dried extract contained 55.5 and 79.0 mg of harmine and harmaline respectively.
Figure 1: Chromatogram of HPTLC analysis of P. harmala seeds extract.
Peak No. 1: harmaline, Peak No. 3: quinidine sulfate (internal standard), Peak No. 4: harmine. Horizontal axis is distance in millimeter. Vertical axis is fluorescence intensity expressed in an arbitrary unit.
Topoisomerase I assay
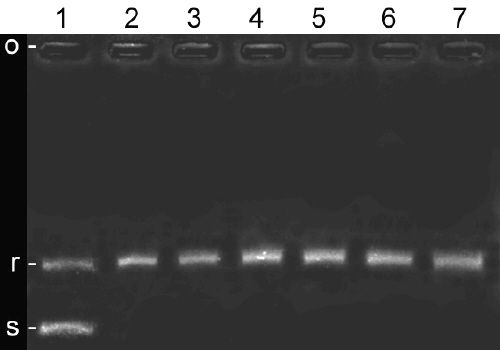
Partially purified topoisomerase I obtained in this study had enzymatic properties similar to other mammalian type I topoisomerases. It readily relaxed supercoiled plasmid DNA, and the relaxation activity was stimulated by MgCl2 and inhibited in the presence of ATP as described by other workers (18). MgCl2 was omitted from reaction mixture in order to reduce nuclease activity and spermidine was added to stimulate relaxation activity of topoisomerase I (20). Teniposide, a known topoisomerase II inhibitor (21), did not inhibit relaxation activity even at 250 mg/ml concentration (Figure 2).
Figure 2: Effect of teniposide on DNA relaxation by placental preparation. Positions of origin, relaxed and supercoiled plasmids have been shown on the left side by o, r and s letters respectively.
Lane 1: supercoiled pBC KS DNA, lane 2: no drug, lanes 3-7: 50, 100, 150, 200 and 250 mg/ml of teniposide.
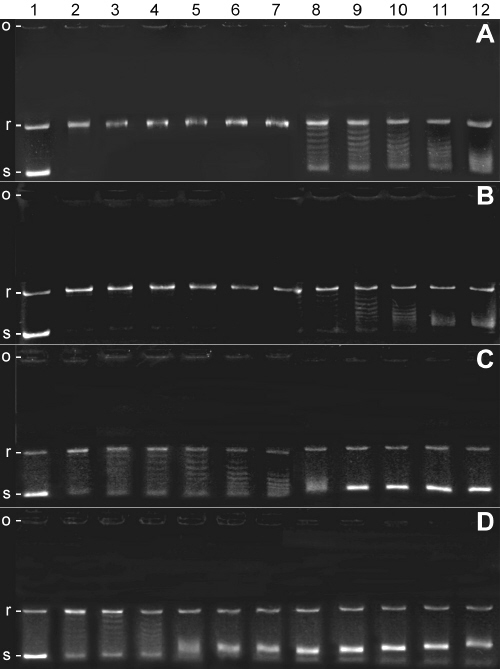
Effects of P. harmala extract, harmane, harmine and harmaline on topoisomerase I was determined by measuring relaxation of pBC KS plasmid DNA, using camptothecin as positive control (maximal inhibition was observed at 1 mg/ml or 2.87 mM). As shown in Figure 3, both harmine and harmaline like harmane, inhibited formation of the relaxed form of DNA in a concentration-dependent manner.
Figure 3: Inhibitory effects of investigated compounds on topoisomerase I mediated DNA relaxation.
A: P. harmala seeds extract; B: harmaline; C: harmane; D: harmine. Positions of origin, relaxed and supercoiled plasmids have been shown on the left side by o, r and s letters respectively. Lane 1: supercoiled pBC KS DNA, lane 2: no drug, lanes 3-12: 5, 10, 20, 30, 40, 50, 100, 150, 200 and 250 mg/ml of corresponding drug, respectively.
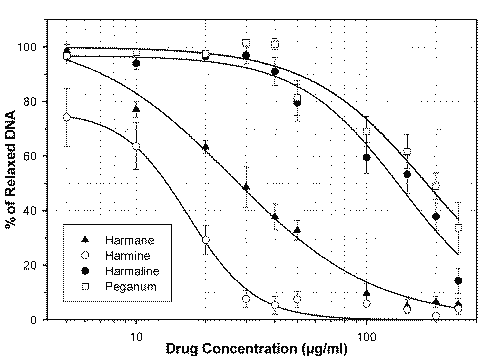
Fifty percent decrease in formation of relaxed DNA (IC50 ) occurred at 13.5 ± 1.7, 28.5 ± 2.8, 140.5 ± 8.2 and 186.4 ± 35.8 mg/ml of harmine, harmane, harmaline and P. harmala extract respectively (Figure 4).
Figure 4: Dose response curves for effects of harmane (▲), harmine (o), harmaline (●) and P. harmala seeds extract (o) on topoisomerase I mediated DNA relaxation: The proportion of relaxed DNA to total DNA was measured by scanning with an ImageMaster 1D digital imaging system (Pharmacia Biotech). Data points represent the mean ± S.E.M. of three independent experiments.
Discussion
Mechanism of the cytotoxic activity of P. harmala seeds extract was the main motivation of this investigation. Previous work (22) has shown that b-carbolines could intercalate into DNA. In theory, this effect may cause inhibition of DNA topoisomerases and results in cytotoxicity. In this study, DNA relaxation assay was used to determine the extent of DNA topoisomerase I inhibition by P. harmala extract and its b-carboline alkaloids. A preparation of human placenta topoisomerase with type I activity prepared in our laboratory, was used in the assay. Our results show that harmine and harmaline like harmane do inhibit topoisomerase I. Order of potency of topoisomerase I inhibition by tested b-carbolines is: harmine > harmane > harmaline which is the same order of potency observed in the cytotoxicity assays reported previously (5, 10).
In order to correlate topoisomerase I inhibitory effect of P. harmala extract with its b-carboline content, HPTLC method was used. The results of HPTLC analysis show that harmine and harmaline constitute 13.5% of dry weight of the extract. Harmane content of the extract, if present, was below the detection limit of the HPTLC analysis that was used. Results from DNA relaxation assay on P. harmala extract and the pure b-carboline alkaloids suggest that there is a good correlation between the observed biological activity of the extract and its b-carboline content.
Intercalation and binding to the DNA minor groove or cleavable DNA-topoisomerase complex are among the methods that can be utilized by both topoisomerase poisons and inhibitors as a mechanism of inhibition (23). As described before (22, 24), absorption spectrophotometry and fluorescence quenching analysis revealed intercalating activity of b-carbolines with the same order of potency (harmine > harmane > harmaline). This is in good agreement with our finding that harmine is a considerably more potent topoisomerase I inhibitor than the others. In that case, the planar ring system in harmine and harmane, which is absent in harmaline (Figure 5), may explain their much greater topoisomerase I inhibitory effects. Other structural analogues should be investigated to enhance our understanding of structure activity relationship of b-carbolines as topoisomerase I inhibitors and potential antineoplastic agents.
Figure 5: Structures of harmane, harmine and harmaline
Conclusion
Our in vitro findings demonstrate that P. harmala seeds extract do inhibit human DNA topoisomerase I and based on the results of HPTLC analysis, it appears that the biological activity of the extract can be explained by its b-carboline content.
Acknowledgements
We thank Dr B. Goliaei (Institute of Biochemistry and Biophysics, Tehran, Iran) for supplying pBC KS plasmid, staff of maternity ward of Firoozabadi hospital, IUMS, Tehran for their help with human placenta collection, Dr Gh. Amin for establishing the authenticity of the plant used in this study, Dr M.E. Zolfaghari of Temad Chemical Co. for his assistance in preparation of the extract and Cellular and Molecular Sciences Research Center of IUMS.
References
1. Cordell G. A., Beecher C. W. W., Pezzuto J. M. Can ethnopharmacology contribute to the development of new anticancer drugs? J Ethnopharmacol. 32:117-133, 1991.
2. Cox P. A., Balick M. J. The ethnobotanical approach to drug discovery. Sci Am. 270:82-87, 1994.
3. Lamchouri F., Settaf A., Cherrah A., Zemzami Y., Lyoussi M., Zaid B., Attif N., Hassar M. Antitumour principles from Peganum harmala. Therapie. Paris. 54:753-758, 1999.
4. Lamchouri F., Settaf A., Cherrah Y., Hassar M., Zemzami M., Atif N., Nadori E. B., Zaid A., Lyoussi B. In vitro cell-toxicity of Peganum harmala alkaloids on cancerous cell-lines. Fitoterapia. 71:50-54, 2000.
5. Sobhani A. M., Ebrahimi S. A., Hoormand M., Rahbar N., Mahmoudian M. Cytotoxicity of Peganum harmala L. seeds extract and its relationship with contents of b-carboline alkaloids. J Iran Uni Med Sci. 8:432-438, 2002.
6. El-Rifaie ME-S. Peganum harmala: its use in certain dermatoses. Int J Dermatol. 19:221-222, 1980.
7. Airaksinen M. M., Kari I. beta-Carbolines, psychoactive compounds in the mammalian body. Part II: Effects. Med Biol. 59:190-211, 1981.
8. Aarons D. H., Rossi G. V., Orzechowski R. F. Cardiovascular actions of three harmala alkaloids: harmine, harmaline, and harmalol. J Pharm Sci. 66:1244-1248, 1977.
9. Ahmad A., Khan K. A., Sultana S., Siddiqui B. S., Begun S., Faizi S., Siddiqui S. Study of the in vitro antimicrobial activity of harmine, harmaline and their derivatives. J Ethnopharmacol. 35:289-294, 1992.
10. Al-Allaf T. A., Khuzaie R. F., Rashan L. J., Halaseh W. F. Cytotoxic activity against a series of tumour cell lines of dimethyltin dichloride complexes with various donor ligands. Boll Chim Farm. 138:267-271, 1999.
11. Wang J. C. DNA topoisomerases. Annu Rev Biochem. 65:635-692, 1996.
12. Liu L. F. DNA topoisomerase poisons as antitumor drugs. Annu Rev Biochem. 58:351-375, 1989.
13. Wang, J. C., DNA topoisomerases as targets of therapeutics: An overview, in Liu LF (ed), Advances in Pharmacology. vol 29A, Academic Press, San Diego, pp 1-19, 1994.
14. Sinha B. K. Topoisomerase inhibitors. A review of their therapeutic potential in cancer. Drugs. 49:11-19, 1995.
15. Funayama Y., Nishio K., Wakabayashi K., Nagao N., Shimoi K., Ohira T., Hasegawa S., Saijo N. Effects of b- and g-carboline derivatives on DNA topoisomerase activities. Mutat Res. 349:183-191, 1996.
16. Barrett J. F., Sutcliffe J. A., Gootz T. D. In Vitro assays used to measure the activity of topoiomerases. Antimicrob Agents Chemother. 34:1-7, 1990.
17. Sauer, R. T., Protein-DNA Interactions, in Abelson JN; Simon MI (eds), Methods Enzymol. Academic Press, San Diego, pp 3-10, 1991.
18. Holden J. A., Rolfson D. H., Low R. L. DNA topoisomerase I from human placenta. Biochim Biophys Acta. 1049:303-310, 1990.
19. Stoscheck, M. C., Quantitation of protein, in Deutscher MP (ed), Methods Enzymol. Academic Press, San Diego, pp 50-68, 1990.
20. Srivenugopal K. S., Morris D. R.. Defferential modulation by spermidine of reactions catalyzed by type I prokaryotic and eukaryotic topoisomerases. Biochemistry. 24:4766-4771, 1985.
21. Minocha A., Long B. H. Inhibition of the DNA catenation activity of type II topoisomease by VP-16-213 and VM26. Biochem Biophys Res Commun. 122:165-170, 1984.
22. Taira Z., Kanzawa S., Dohara C., Ishida S., Matsumoto M., Sakiya Y. Intercalation of six b-carboline derivatives into DNA. Jap J Toxicolo Environ Health. 43:83-91, 1997.
23. Capranico G., Binaschi M., Borgnetto M. E., Zunino F., Palumbo M. A protein-mediated mechanism for the DNA sequence-specific action of topoisomerase II poisons. Trends Pharmacol Sci. 18:323-329, 1997.
24. Duportail G., Lami H. Studies of the interaction of the fluorophores harmine and harmaline with calf thymus DNA. Biochim Biophys Acta. 402:20-30, 1975.
Corresponding Author: Massoud Mahmoudian, Department of Pharmacology, Iran University of Medical Sciences, Tehran, Iran, P.O. Box 14155-6183, Tehran, Iran. masmah99@iums.ac.ir
JPPS Contents
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps