J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 5(2):131-134, 2002
A quantitative thin layer chromatography method for determination of theophylline in plasma
A. Mirfazaelian
Department of Pharmaceutics, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, IranM. Goudarzi
Kowsar Pharmaceutical company, Tehran, IranM. Tabatabaiefar
Qazvin University of Medical Sciences, Qazvin, IranM. Mahmoudian1
Department of Pharmacology, School of Medicine, Iran University of Medical Sciences, Tehran, IranReceived February 11th, 2002, Revised April 27th, 2002, Accepted May 14th, 2002
PDF version
Abstract
A simple assay method for theophylline in plasma using thin layer chromatography (TLC) was developed. The method involves extraction of the drug and internal standard (acetaminophen) by chloroform-isopropanol (75:25) followed by separation on TLC silica plates using a mixture of acetic acid, isopropanol, toluene (1: 12: 6), as the eluting solvent. Both peak height ratios and peak are ratios showed high correlation coefficient (r>0.98, p<0.001). However we used peak heights for the determinations. Within-day and between-day coefficients of variation were less than 4.4% and 7.8% respectively. The assay proved inexpensive, accurate and reproducible with a limit of detection of 100ng/ml that makes it suitable for bioavailibility studies.
Introduction
Theophylline is a mehtylxantine derivative proven efficacy as bronchodilator in asthma (1).
For determination of theophylline in biological fluids many methods such as UV (2), HPLC (3-5), HPTLC (6), GC (7), GC-MS (8), Fluorescence Immunoassay, Fluorescence Polarized Immunoassay, EMIT, Radio Immunoassay (9-12), capillary electrophoresis (13, 14) and reflectance photometry assay (15) are reported. Most of these methods are insensitive to concentrations obtained after single dose administration of this drug or otherwise time consuming and/or expensive. We therefore developed a sensitive, inexpensive, easy to handle and reliable normal-phase TLC method for quantification of this drug in human plasma. The results indicate the suitability of this method in bioavailability studies.
Materials and methods
Chemicals
Theophylline standard was supplied by Daroupakhsh pharmaceutical company (Tehran, Iran). Methanol was of HPLC grade (Riedel-de Haen). Other chemicals were of analytical grade and were supplied by Merck (Germany).
Apparatus and Chromatographic Conditions
Samples were spotted on pre-coated TLC plates (silica gel 60 F254, 10x20cm, 0.25mm layer thickness, Merck). Mobile phase consisted of acetic acid, isopropanol, and toluene (1: 12: 6). A Camag Linomat IV (Switzerland) was used for spotting the plates. The sample volume was 50mL, which was spotted with a speed of 10ml/sec on a band of 4mm width. A Camag TLC Scanner II (Switzerland) equipped with a deuterium lamp set at 277nm in the reflection mode was used for scanning the plates. The peak heights and areas of chromatograms were determined using Camag version 3.11 software.
Preparation of Standard Solutions
A stock solution containing 10mg/ml theophylline or acetaminophen was prepared in methanol. The working standard solutions were prepared by diluting 1.0 mL stock solution up to 10ml water resulting in a final concentration of 1 mg/ml. These working solutions were then used for making spiked plasma samples.
Sample Preparation
To 2mL of the plasma sample in a stoppered tube was added 20 mL Acetaminophen (1mg/ml) as internal standard and 1mL saturated ammonium sulfate. The sample was then extracted using 5ml chloroform-isopropanol (75:25) after being vortex mixed. The tube was shaked for 10 minutes on a rotary mixer (Ikawerk, Germany) and centrifuged for 10 min at a speed of 8000 rev min-1 (Demred, Iran). The organic phase was then separated and evaporated to dryness under gentle stream of nitrogen. The dry residue was re-dissolved in 0.2 ml methanol, which was then spotted on TLC plates. All separations were carried out at room temperature.
RESULTS
The chromatograms obtained from blank plasma (a); plasma spiked theophylline and acetaminophen (b) and plasma of a volunteer 6h after taking a 200mg dose of Theophylline SR (TheoDur 200mg) are shown in Figure 1.
Figure 1: Plasma chromatograms of blank plasma (A), plasma spiked with 5000 ng/mL theophylline (B) and plasma of a healthy subject collected 6h after taking a 200-mg single oral dose of TheoDur (C); [a, Theophylline b, Acetaminophen (internal standard)]
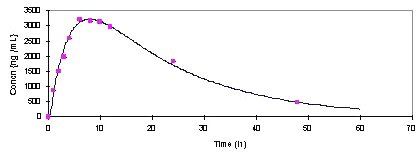
Theophylline metabolites had no interference with chromatograms. More than 30 blank samples were analyzed at different times, which showed no interference of endogenous substances with the method. The Rf values for theophylline and acetaminophen were 0.70 and 0.85 respectively. The migration time was 60 minutes and migration distance was 11 centimeters. Mean plasma concentration-time profile of 12 healthy overnight fasting subjects after taking a 200-mg single oral dose of theophylline is provided in Figure 2. The subjects were sampled before and at 1, 2, 3, 4, 6, 8, 10, 12, 24, 48 hours after drug administration. The curve fitted to the mean points using Drug-Knt (16). The highest theophylline plasma concentrations of the subjects were found to be about 650 ng/mL in our study.
Figure 2: Plasma profile of theophylline in healthy subjects taking single oral dose of TheoDur 200mg (Mean of 12 subjects).
Calibration curves
The linearity of plasma calibration curves were studied at the concentration of 500-10,000ng/mL based upon ratio of peak areas and peak heights (theophylline/acetaminophen). The suitability of peak height ratios and peak area ratios were also studied. Calibration curve based on peak height ratios (correlation coefficient (r) =0.993, p<0.001) was quite comparable to the one resulting from peak area ratios (correlation coefficient (r) =0.988, p<0.001). i.e. Each standard curve showed good linearity over the range of concentrations examined (Figure 3.).
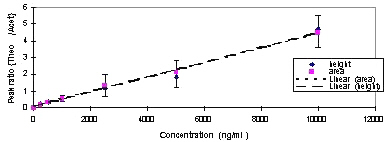
Figure 3: Calibration curve of theophylline in plasma. (Mean ± SD)
However we used peak height ratios for determination of plasma samples and suitability tests of the method.
Limit of Detection & Quantitation
The limit of detection with considering signal to noise ratio of 2 was 100ng/mL (considering area or height ratio of peaks). Limit of quantitation was about 200ng/mL.
Reproducibility and Accuracy and Recovery
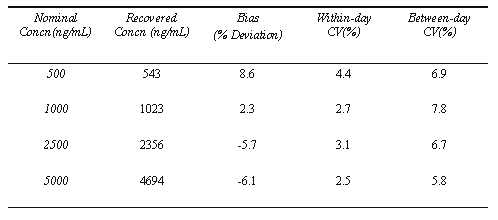
Within-day and between-day assay coefficients of variation and accuracy test results are summarized in Table-1. Within-day and between-day coefficients of variation were less than 4.4% and 7.8% respectively. Bias of the assays was between -6.1 and 8.6. Efficiency of the extraction procedure was about 85% for theophylline.
Table 1: Analysis validation of theophylline assay in plasma
Discussion
A number of assay methods have been developed for quantification of theophylline in biological fluids, but many of them suffer from time consuming (2) and/or expensive assay procedures (6,8). Several automated analytical systems have also been developed for therapeutic drug monitoring (TDM) which mostly lack sufficient detection for single dose analyses and because of the need to special instrumentation are uneconomical for bioavailability laboratories (10,12,15,17-19).
As this method concentrates the samples in sample preparation (x10) its detection limit is very low and as more than 10 samples are analyzed in one run -i.e., it is relatively fast-, this method turns to be inexpensive and suitable for bioavailability studies.
In summary, we have reported an analytical method for the determination of theophylline, which is to be suitable for human bioavailability studies and possesses important advantages, notably speed, inexpense and easy handling, over currently published methods.
ACKNOWLEDGEMENTS
The authors wish to express grateful thanks to R. Shokri and N. Asgharifard for analytical assistance. They wish to acknowledge M. Joneidi and M. Sahraei for their sincere help in preparation of the manuscript. The authors also thank Dr. A. Karbasi the head of Daroupakhsh Research Center for his support of the study.
References
- Serafin W.E., Drugs used in the treatment of asthma. In: P.B. Molinoff, R.W. Ruddon, (ed.) The Goodman & Gilman's pharmacological basis of therapeutics. 9th. edition, Mc Graw-Hill, pp 659-682(1996)
- Pokrajac M., Varagic V.M., Spectrodensitometric determination of theophylline in plasma. Acta. Pharm. Jugosl. 33, 23-27(1983)
- Davis J.D., Aarons L., Houston J.B. (1993) Simultaneous assay of fluoroquinolones and theophylline in plasma by high-performance liquid chromatography. J. Chromatogr. 621, 105-9
- Iwase H., Gondo K., Koike T., Ono I. (1994) Novel precolumn deproteinization method using a hydroxyapatite cartridge for the determination of theophylline and diazepam in human plasma by high-performance liquid chromatography with ultraviolet detection. J. Chromatogr. B. Biomed. Appl. 655, 73-81
- Moncrieff J. (1991) Determination of theophylline in serum and saliva in the presence of caffeine and its metabolites. J. Chromatogr. 568, 177-85
- Devarajan P.V., Sule P.N., Parmar D.V. (1999) High-performance thin-layer chromatographic determination of theophylline in plasma. J. Chromatogr. B. Biomed. Sci. Appl. 736, 289-93
- Lee B.L., Jacob P., Benowitz N.L. (1989) Sulfonium salts as derivatizing agents. Part 2. Determination of theophylline in plasma by automated gas chromatography. J. Chromatogr. 494, 109-117
- Desage M., Soubeyrand J., Soun A., Brazier J.L., Georges Y. (1984) Automated theophylline assay using gas chromatography and a mass selective detector. J. Chromatogr. 336, 285-291
- Torrecilla-Junyent M.T., Escobar-Cava P., Navarro-Ruiz A., Izquierdo-Maria R., Navarro-Polo J.N. (1992) Determination of theophylline plasma levels: comparison of two analytical techniques. Farm. Clin. Spain 9: 168-170, 172-173
- Climent E., Valiente F., Selva J., Alamo J.M. (1994) Comparative study of the analytical methods TDx/TDxFLx and EMIT/COBAS-MIRA in the determination of theophylline concentration and establishment of the model of analytical error. Farm. Clin. Spain 11: 428-430, 432-433
- Canto-Pascual M.M., Alos-Alminana M., Magraner-Gil J., Ezquer-Borras J., Torrecilla-Junyent M.T. (1987) Influence of most common theophylline metabolites in their determination through immunochemical methods. Farm. Clin. Spain; 4: 40-42, 44-45, 47
- R. Roscoe and T. Vitti, Determination of theophylline serum levels by radioimmunoassay. Can. J. Pharm. Sci. 15: 69-70(1980)
- Zhang Z.Y., Fasco M.J., Kaminsky L.S. (1995) Determination of theophylline and its metabolites in rat liver microsomes and human urine by capillary electrophoresis. J. Chromatogr. B. Biomed. Appl. 665: 201-8
- Johansson I.M., Gron-Rydberg M.B., Schmekel B. (1993) Determination of theophylline in plasma using different capillary electrophoretic systems. J. Chromatogr. A. 652: 487-93
- Kuzuya T., Ogura Y., Hasegawa M., Nakamura T.A., Kitazawa S. (1986) Clinical evaluation of a newly developed Seralyzer reagent strip system for theophylline determination in body fluids. J. Nippon. Hosp. Pharm. Assoc. Sci. Ed. 12: 362-368
- Mirfazaelian A., Mahmoudian M. (1995) A comprehensive computer programme for evaluation and teaching drug pharmacokinetics, Abst. 12th Iranian Cong. Physiol. Pharmacol. Paper P3-18
- Massoud N., Munzenberger P., Sarniak A. (1986) Comparison of the Seralyzer and EMIT systems for determination of theophylline concentrations. Am. J. Hosp. Pharm. 43: 1722-1726
- Tsunekawa Y., Hasegawa T., Nadai M., Yamaki K., Takagi K., Nabeshima T. (1996) Clinical application of the Biotrack 516 system for determination of theophylline by a turbidimetric latex agglutination inhibition reaction. Ther. Drug Monit. 18: 86-91
- Malliaros D.P., Wong S.S., Wu A.H., Campbell J., Leonard H., Houser S., Berg M., Gornet T., Brown C., Feng Y.J. (1997) Quantitative determination of theophylline by an automated chemiluminescent immunoassay in serum and plasma: comparison to other methods of analysis. Ther. Drug Monit. 19: 224-9
Corresponding Author: M. Mahmoudian, Department of Pharmacology, School of Medicine, Iran University of Medical Sciences, P.O. Box 14155-6183, Tehran, Iran. masmah99@iums.ac.ir
JPPS Contents
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps