J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 5(2):185-189, 2002
Effect of several Chinese natural health products of human cytochrome P450 metabolism.
Brian C. Foster1
Office of Science, Therapeutic Products Directorate, Health Canada, Ottawa, Ontario, Canada; Centre for Research in Biopharmaceuticals, University of Ottawa, Ottawa, Ontario, CanadaSusan Vandenhoek, Rainy Tang, Jason W. Budzinski, Anthony Krantis
Centre for Research in Biopharmaceuticals, University of Ottawa, Ottawa, Ontario, CanadaK.Y. Li
Office of Science, Therapeutic Products Directorate, Health Canada, Ottawa, Ontario, CanadaReceived 31 January 2002, Revised 24 June 2002, Accepted 24 July 2002
PDF version
Abstract
Purpose: Traditional Chinese medicines (TCM) are believed by many to be safe and used for self-medication without supervision. Although the risk appears to be low, certain TCM have been associated with a number of serious adverse reactions. A preliminary study was undertaken with 12 products using a human cytochrome P450 (CYP450) isozyme assay to determine if these products could affect human drug metabolism.
Methods. Aliquots of samples were analyzed directly or as extracts for their potential to affect CYP450 2C9, 2C19, 2D6, and 3A4 mediated-metabolism of marker substrates using an in vitro fluorometric microtiter plate assay.
Results. One product was found to be a Chinese Proprietary Medicine (CPM). Most aqueous extracts inhibited CYP450 mediated-metabolism of at least 3 isozymes (ranging from 25 - 100%). All liquid samples markedly inhibited the metabolism of all 4 isozymes. De le ke chuan kang and Rensheng dao were the strongest CYP450 inhibitors.
Conclusions. Our in vitro findings demonstrate that TCM can inhibit CYP450 2C9, 2C19, 2D6 and 3A4 mediated-metabolism. TCM need to be examined further under clinical settings to determine if potential interactions will occur that affect the safety and efficacy of conventional therapeutic products.
Introduction
Traditional Chinese medicines (TCM) include both crude medicinal materials from plants, animal parts and minerals, as well as Chinese proprietary medicine (CPM). Herbal products cause few adverse effects, and may have beneficial pharmacological and therapeutic uses in a number of illnesses, including HIV where they have been examined for their capacity to reduce symptoms and improve quality of life (1). Although there is widespread belief that all TCM are of natural origin and hence are safe without adverse effects, Weber et al. (1) found that patients taking Chinese herbs reported significant gastrointestinal disturbances. Of 35 patients admitted to a medical unit for drug interactions, 19 received drugs or folk medicines which could interact with warfarin (2). Infants and children may be more susceptible than adults to some adverse effects (3). In another case study, a patient developed invasive transitional cell carcinoma of the urinary tract associated with the presence of aristolochic acid-DNA adducts after previously developing nephropathy due to ingestion of Chinese herbs (4).
Factors affecting the safety of CPM include intrinsic toxicity, adulteration, substitution, contamination, misidentification, lack of standardization, incorrect preparation, dosage, inappropriate labelling and advertising (5). Some TCM can also contain undeclared drugs and heavy metals (5). Case reports have been identified which substantiate previous reports, including liver problems following the use of Chinese herbal medicine for skin disorders, allergic reactions to royal jelly and propolis, and heavy metal poisoning caused by remedies from the Indian subcontinent (6). Lau et al. (7) reported a case of severe poisoning where the patient was admitted comatose resulting from the use of CPMs found to contain the anticonvulsants phenytoin, carbamazepine and valproate.
Liu (8) reported that many Chinese medicinal herbs were inhibitors and/or inducers of hepatic CYP450. Scutellariae Radix (Huangqin) increased benzo(a)pyrene hydrolase activity whereas Gentianae scabrae Radix (Longdan) effected a similar decrease in pentoxyresororufin O-dealkylase activity in rat liver microsomes (9). Neither had an effect on the CYP450 2E1 protein. Ge-gen ( Radix puerariae; Pueraria lobata (Willd. Ohwi) crude extracts and its main isoflavin puerarin significantly altered rat hepatic CYP-linked monooxygenases (10) with both induction and inhibition observed. Bufalin, a constituent of Chan Su and Lu-Shen-Wan was noted to displace digitoxin from the protein binding site (11). Ishihara et al. (12) reported the inhibitory effects of Angelica dahurica root extract on rat liver microsomal cytochrome CYP450 2C, 2D1 and 3A activity. Initially the extract altered the intrinsic clearance of tolbutamide but not diazepam administered intravenously. However the diazepam Cmax after oral dosing increased 4 fold in the presence of the A. dahurica extract. Tin Chuan Tang and Hsiao Ching Long Tang pretreatment did not affect the pharmacokinetic parameters of theophylline in 3 different age groups of Sprague Dawley rats (13). Paeoniae radix , a TCM used for the treatment of epilepsy, did not affect the pharmacokinetics in a small clinical trial with 6 healthy subjects for 7 days with valproic acid being given on the last day (14). This finding is similar to that of short term studies with St. John's wort in limited numbers of subjects (15) where the clinically significant effect was only noted after extended use (16,17).
Chen et al (18) reported that 16% of the epileptic patients under treatment were also using TCM concomitantly. Phenytoin pharmacokinetics in rat after a single dose with Paeoniae Radix were not affected except for significant differences in Tmax and Vd/F (19). A similar delay in drug absorption was also reported between Sho-seiryu-to and carbamazepine (20). The main focus connected with these health risks lies primarily with the possibility of unknown natural product-drug interactions (21) and product variation (22) which can confound direct comparative analysis.
There is less awareness of the potential interactions of Chinese herbal preparations on human cytochrome P450 isozymes associated with drug metabolism. The objective of this study was to establish the potential for a variety of Chinese natural products (Table 1) to affect the in vitro metabolism of a marker substrate by 4 selected cDNA-expressed human cytochrome P450 isozymes.
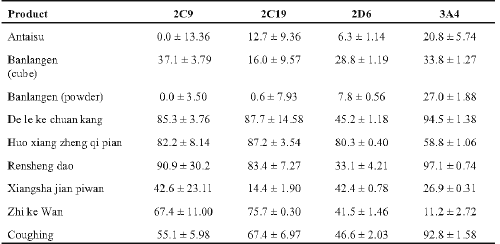
Table 1: The commercially available Chinese herbal products examined and their label information.
MATERIALS AND METHODS
Substrates and Reference Compounds
Three products (De le ke chuan kang, Rensheng dao and "coughing") had been imported into Canada for personal use. They were used concomitantly by an elderly Chinese woman for a 2 year period with conventional therapeutic agents including ciprofloxacin, colchicine, furosemide, prednisone, clavulin, salbutamol, quinine, and ranitidine who subsequently developed acute kidney failure. Nine additional products were obtained from local commercial outlets for personal use by laboratory members or their families. 7-Benzyloxyresorufin (7BR) and 7-ethoxy-3-cyanocoumarin (7EC) were obtained from Molecular Probes, Eugene, OR. 7-Methoxy-4-trifluoromethylcoumarin (7-MFC) was obtained from Fluka Chemicals (Sigma) and 3-[2-(N,N-diethyl-N-methylamino)ethyl]-7-methoxy-4-methylcoumarin (AMMC) was obtained from GENTEST Corp. All other chemicals and solvents were of analytical grade.
Assay Procedures
Tablets and cubes were finely ground to a powder using a mortar and pestle. The resulting powders (25 mg/ml) were suspended in distilled water and vortexed on high for 1 min, and then centrifuged for 18 min at 13,000 rpm. The supernatant was carefully removed for testing. Aliquots (5 μ L) of these stock solutions and other test solutions were screened for their ability to inhibit 2C9, 2C19, 2D6 and 3A4 marker substrates using an in vitro fluorometric microtiter plate assay (23, 24). Briefly, assays were performed in clear-bottomed, opaque-welled microtiter plates (96 well, Corning Costar, model # CSOO-3632, Corning, NY) using GENTEST supersomes according to the manufacturers directions (24). Enzyme solution was added to all wells. Inactivated enzyme was added to the blank wells. All supersomes were stored at - 80°C until used and were not subjected to more than 2 freeze-thaw cycles. Only data sets yielding the highest readings without saturation were used to calculate percent inhibition.
All samples were prepared in triplicate with the resultant percent inhibition calculations based on the mathematical combinations for the differences in fluorescence between the test/test-blank wells and the mean difference between each control and blank well. Thus, nine experimental values were achieved for each sample. Controls were run with every assay. Each assay was repeated at least once. All assays were performed under gold fluorescent lighting (Industrial Lighting, Ottawa, ON).
RESULTS
Aliquots from 3 of the stock solutions (25 mg/ml) of the powdered products tested had strong activity greater than 80% inhibitory activity against at least 3 of the isozymes tested (Table 2). Five solutions had marked activity of between 40 - 80% inhibition against at least 2 isozymes. Only Antaisu and Banlangen (compressed cube and powder) had weak activity of less than 35% inhibition against these isozymes. Banlangen powder was considerably less inhibitory than the compressed product.
Table 2: Percent inhibition of human cytochrome CYP450 isozymes by aqueous extracts of powdered Chinese herbal products (n > 6 ± SD).
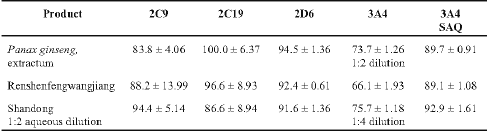
Aliquots of the 3 liquid formulations (Table 3) were strongly inhibitory against the CYP450 isozymes examined.
Table 3: Percent inhibition of human cytochrome CYP450 isozymes by liquid Chinese herbal products (n > 6 ± SD) alone or in combination with saquinavir (SAQ).
To examine the effects of these products with a protease inhibitor on 3A4-mediated metabolism, saquinavir (SAQ; 1.275 mg/ml) was then examined alone and in combination with these products (Table 3). SAQ alone had an inhibitory effect of 68.9 ± 2.0% (mean ± SD) but when added together with each product separately, the inhibitory effect was greater than each product alone.
De le ke chuan kang, Rensheng dao and "coughing" were analyzed chemically by standardized methods using atomic adsorption for heavy metals and by HPLC for scheduled drugs. Analysis found arsenic (1 ppm), cadmium (< 1 ppm), mercury (<1 ppm) but no scheduled drugs in De le ke chuan kang or Rensheng dao. In addition, both contained lead (De le ke chuan kang, 1 ppm; and Rensheng dao 2 ppm). Pharmaceutical analysis revealed that "coughing" was a CPM containing 38.2 mg/capsule theophylline, 1.16 mg/capsule chlorpheniramine, and 3.18 mg/capsule bromhexine.
DISCUSSION
The aim of this study was to assess whether TCM would affect CYP450-mediated metabolism of selected reference substrates. Of the 11 TCM products and single CPM examined in this preliminary screen, nine markedly or strongly inhibited the metabolism of at least 2 major CYP450 isozymes associated with human Phase I drug metabolism. Most of these had an effect on at least 3 isozymes The results from this study may suggest that a single exposure of TCM with conventional therapeutic products may increase the plasma levels of some therapeutic agents which would increase the risk for serious drug adverse events in patients (26). Conversely, this interaction between TCM and conventional therapies may also effect the safety profile of the TCM.
Of the 3 products submitted for examination, 2 were TCM with the third being a CPM, all would be contraindicated on the basis of this study with the conventional therapies taken by this elderly individual. As an increasing number of individuals, particularly older persons (25), use natural products including TCMs, there is a need for health care professionals to recognize and understand the potential risk of drug -TCM interactions.
The effect of repeated use of TCMs on induction of drug metabolism is not well understood, but some inhibitory compounds may become inducers after repeated administration (8). When the suggested daily ingestion of these products (about 1 - 75 g/day) is taken into consideration with the findings of this study, all products with the possible exclusion of the Banlangen powdered tea, may result in clinically significant drug interactions affecting safety and efficacy if taken concomitantly with therapeutic products. The potential for these products to affect drug disposition may increase if used in combination with one or more conventional therapeutic products or other TCM.
Many of plants used as natural products contain numerous classes of chemical constituents, including pyrrolizidine alkaloids (PAs) which are hepatotoxic, pneumotoxic, genotoxic, neurotoxic, and cytotoxic (27). The observed inhibitory effect on drug metabolism can be highly variable as the constituent content can differ with plant species, source, environment, and processing and storage conditions. A major difficulty in correlating the specific components in these products is distinguishing between the biomarker components associated with the pharmacological activity and affect CYP450-mediated metabolism, and those components that just affect CYP450-mediated metabolism. In studies with other natural products, we found that the concentration of known biomarkers does not correlate with the determined inhibitory effect (28). This suggests that the inhibition is a combined effect of several chemical classes of phytochemicals.
Individuals taking a wide number of conventional and TCM products would be expected to be at a higher risk of experiencing a clinically significant event arising from an interaction, particularly as duration of use is increased. The moderate to high inhibition observed with CYP2C9, CYP2C19 and CYP2D6 may also suggest that individuals and populations with a pharmacogenetic predisposition towards poor or slower drug metabolism may be at a higher risk of developing serious adverse effects. The results reported are specific to these single lot products and cannot be extrapolated to other lots or similar products from other manufacturers. Additional studies are required to examine lot-to-lot variability and consistency between manufacturers to determine the effect of these products on drug disposition and clinical outcome.
Acknowledgements
This research was supported in part by the Positive Action Program, AIDS Bureau of Ontario and the National Science and Engineering Research Council of Canada (Strategic Program).
Weber, R., Christen, L., Loy, M., Schaller, S., Chisten, S., Joyce, C.R., Ledermann, U., Ledergerber, B., Cone, R., Luthy, R. and Cohen, M.R., Randomized, placebo-controlled trail of Chinese herb therapy for HIV-1-infected individuals. J Acquir Immune Defic Syndr, 22(1):56-64, 1999.
Chan, T.Y., Drug interactions as a cause of overanticoagulation and bleedings in Chinese patients receiving warfarin. Int J Pharmacol Ther, 36(7): 403-5, 1998.
Tomassoni, A.J. and Simone, K., Herbal medicines for children: an illusion of safety? Curr Opin Pediatr, 13(2):162-9, 2001.
Lord, G.M., Cook, T., Arlt, V.M., Schmeiser, H.H., Williams, G., Pusey, C.D., Urothelial malignant disease and Chinese herbal nephropathy. Lancet Nov 3;358(9292):1515-6, 2001.
Koh, H.L. and Woo, S.O., Chinese proprietary medicine in Singapore: regulatory control of toxic heavy metals and undeclared drugs. Drug Saf, 23(5):351-62, 2000.
Shaw, D., Leon, C., Kolev, S. and Murray, V., Traditional remedies and food supplements. A 5-year toxicological study (1991-1995). Drug Saf, 17(5):342-56, 1997.
Lau, K.K., Lai, C.K. and Chan, A.W., Phenytoin poisoning after using Chinese proprietary medicines. Human & Experimental Toxicology, 19: 385-386, 2000.
Liu, G-T., Effects of some compounds isolated from Chinese medicinal herbs on hepatic microsomal cytochrome P-450 and their potential biological consequences. Drug Metab Rev, 23(3&4): 439-465, 1991.
Kang, J.J., Chen, Y.C., Kuo, W.C., Chen, T., Cheng, Y.W., Kuo, M.L. and Ueng, T.H., Modulation of microsomal cytochrome P450 by Scutellariae Radix and Gentianae scabrae Radix in rat liver. Am J Chin Med, 24(1): 19-29, 1996.
Guerra, M.C., Speroni, E., Broccoli, M., Cangini, M., Pasini, P., Minghett, A., Crespi-Perellino, N., Mirasoli, M., Cantelli-Forti, G. and Paolini, M., Comparison between Chinese medical Pueraria lobata crude extract and its main isoflavine puerarin antioxidant properties and effects on rat liver CYP-catalysed drug metabolism. Life Sci, 67(24): 2997-3006, 2000.
Datta, P. and Dasgupta, A., Interactions between drugs and Asian medicine: displacement of digitoxin from protein binding site by bufalin, the constituent of Chinese medicines Chan Su and Lu-Shen-Wan. Ther Drug Monit, 22(2):155-9, 2000.
Ishihara, K., Kushida, H., Yuzurihara, M., Wakui, Y., Yanagisawa, T., Kamei, H., Ohmori, S. and Kitada, M., Interaction of drugs and Chinese herbs: pharmacokinetic changes of tolbutamide and diazepam caused by extract of Angelica dahurica. J Pharmacol, 52(8): 1023-9, 2000.
Lin, S.Y., Hou, S.J., Wu, W.H., Chen, S.M. and Young, T.K., Effect of traditional Chinese herbal medicines on the pharmacokinetics of western drugs in SD rats of different ages. I. Aminophylline-Tin chuan Tang and aminophylline-Hsiao Ching Long Tang. J Pharmacobiodyn, 14(4):201-6, 1991.
Chen, L.C., Chou, M.H., Lin, M.F. and Yang, L.L., Lack of pharmacokinetic interaction between valproic acid and a traditional Chinese medicine, Paeoniae Radix, in healthy volunteers. J Clin Pharm Ther, 25(6):453-9, 2000.
Markowitz, J.S., DeVane, C.L., Boulton, D.W., Carson, S.W., Nahas, Z. and Risch, S.C., Effect of St. John’s wort (Hypericum perforatum) on cytochrome P-450 2D6 and 3A4 activity in healthy volunteers. Life Sci, 66: 133-139, 2000.
Piscitelli, S.C., Burstein, A.H., Chaitt, D., Alfaro, R.M. and Falloon, J., Indinavir concentrations and St. John’s Wort. The Lancet, 355: 547-548, 2000.
Ruschitzka, F., Meier, P.J., Turina, M., Luscher, T.F. and Noll, G., Acute heart transplant rejection due to St. John’s Wort. The Lancet, 355:548-549, 2000.
Chen, L.C., Chen, Y.F., Yang, L.L., Chou, M.H. and Lin, M.F., Drug utilization pattern of antiepileptic drugs and traditional Chinese medicines in a general hospital in Taiwan- a pharmaco-epidemiologic study. J Clin Pharm Ther, 25(2):125-9, 2000.
Chen, L.C., Chou, M.H., Lin, M.F. and Yang, L.L., Effects of Paeoniae Radix, a traditional Chinese medicine, on the pharmacokinetics of phenytoin. J Clin Pharm Ther, 26 (4): 271-278, 2001.
Ohnishi, N., Yonekawa, Y., Nakasako, S., Nagasawa, K., Yokoyama, T., Yoshioka, M. and Kuroda, K., Studies on interactions between traditional herbal and Western medicines. I. Effects of Sho-seiryu-to on the pharmacokinetics of carbamazepine in rats. Biol Pharm Bull, 22(5): 527-31, 1999.
Miller, L. G., Herbal medicinals: selected clinical considerations focussing on known or potential drug-herb interactions. Arch Intern Med, 158(20): 2200-11, 1998.
Fukuda, K., Guo, L.,Ohashi, N., Yoshikawa, M. and Yamazoe, Y., Amounts and variation in grapefruit juice of the main components causing grape-fruit-drug interactions. J Chromatogr, 741(b):195-203, 2000.
Foster, B.C., Foster, M.S., Vandenhoek, S., Budzinski, J.W., Gallicano, K.D., Choudri, S., Krantis, A. and Arnason, J.T., An in vitro evaluation of human cytochrome P450 3A4 and P-glycoprotein inhibition by garlic. J Pharm. Sci, 4(2):159-167, 2001
Methodology of a high throughput method for measuring cytochrome P450 inhibition (version 2). Woburn: GENTEST Technical Bulletin , GENTEST Corporation, 1998.
Ness, J., Sherman, F. T. and Pan, C. X., Alternative medicine: what the data say about common herbal therapies. Geriatrics, 54(10): 33-8, 40, 43, 1999.
Foster, B. C., Vandenhoek, S., Hanna, J., Akhtar, M. H. and Krantis, A., Effect of natural health products on cytochrome P-450 drug metabolism. Can J Infectious Dis Supp, 10(B): 18, 1999.
Roeder E., Medicinal plants in China containing pyrrolizidine alkaloids. Pharmazie 55(10):711-26, 2000.
Foster, B. C., Vandenhoek, S., Hanna, J., Budzinski, J.W., Akhtar, M. H., Bryan, M., Krantis, A. and Arnason, J.T. Effects of natural health products on cytochrome P-450 drug metabolism. Accepted Phytomedicine, 2001.
Corresponding Author: Brian C. Foster, Health Canada, Therapeutic Products Directorate, Holland Cross 3102C3, 1600 Scott Street, Ottawa, Ontario, Canada, K1A 1B6. brian_foster@hc-sc.gc.ca
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps