J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 5(2):146-161, 2002
Role of integrative pharmacokinetic and pharmacodynamic optimization strategy in the management of Parkinson's disease patients experiencing motor fluctuations with levodopa.
Chukwuemeka S. Okereke1
Department of Clinical Pharmacology, Clinical Research and Development, Eisai Inc., Glenpointe Centre West, Teaneck, New Jersey, USAReceived 25 April 2002, Revised 14 June 2002, Accepted 24 June 2002
PDF version
Abstract
Parkinson's disease is a progressively debilitating motor neuron disease that affects the dopaminergic neurons within the nigral-striatal and surrounding pathways and which is characterized clinically by rigidity, resting tremor and bradykinesia with or without postural imbalance. Levodopa is the "gold standard" for the treatment and management of Parkinson's disease worldwide. However, following prolonged use of the drug, the "honey-moon" which was once enjoyed by patients on levodopa begins to wane. The clinical as well as the socio-economic costs associated with such failure in response to levodopa is enormous. Various approaches in the management of Parkinson's disease patients experiencing motor fluctuations with levodopa treatment have been suggested and include both pharmacologic and non-pharmacologic strategies involving invasive surgical intervention. Currently, the non-pharmacological approach, which is invasive, remains to be fully perfected and is associated with high morbidity and mortality. The use of the non-invasive, pharmacological approach is currently the most widely accepted approach but would require a review of all possible drug regimens used. This entails evaluating the pharmacokinetics and pharmacodynamic actions of the drug regimens used and possibly, dosage form and route of administration of the drugs. The use of levodopa formulated for transdermal or intranasal administration might help improve the ease of use and compliance. Controversy abounds as to the role of plasma pharmacokinetics of levodopa in the management of Parkinson's patients, vis a vis its dynamics at the central nerve terminal and its receptor site. However, it is worthy of mention that an integrated optimal pharmacological approach involving the peripheral, and central pharmacokinetics of levodopa as well as its central pharmacodynamics would ensure better treatment and management of this disease. In addition, the choice of alternate formulations and routes of administration will not only improve on the bioavailability and overall pharmacokinetics of levodopa, but also increase compliance. Furthermore, monitoring of both plasma and central concentrations of levodopa and its metabolites might play a major role in individualization of pharmacotherapy in special Parkinsonian patients experiencing motor fluctuations with levodopa..
Introduction
Parkinson's disease is a common, progressively debilitating neurodegenerative disease that affects the dopaminergic neurons within the substantia nigra (SN), non-dopaminergic neurons and extra-nigral projection bundles that control sensory, cognitive, premotor and motor pathways (1). It is characterized by neuronal cell loss in the SN resulting in eventual depletion of dopamine in the nigral-striatal pathways culminating in pathologies of some key body systems and functions especially, the motor function (2).
The initial loss of dopaminergic neurons and subsequent decreased production of dopamine in the substantia nigra in Parkinson's disease distorts the normal dopaminergic and cholinergic balance, thus leading to the over-activation, of the cholinergic pathway that manifests as the characteristic abnormal motor symptoms often seen in Parkinson's disease (3). Other neurotransmitters are also affected such as norepinephrine, gamma-aminobutyric acid (GABA) and serotonin.
The prevalence of Parkinson's disease varies widely across different countries but it is more prevalent in Westernized countries (4-6). For instance in the Unites States of America with about 50,000 new cases being diagnosed annually, it is estimated that there are about 1 million persons with Parkinson's disease in the US today (7). In fact, some have estimated the prevalence of Parkinson's Disease to be about 1% of the United States population of persons older than 50 years (8). Demographically, the highest prevalence of PD worldwide is observed in white Caucasian and lowest in black Africans (9, 10).
Parkinson's disease occurs in individuals in their middle age or older age (i.e. 30 years or above). The incidence of the disease increases with age with greater prevalence in individuals older than 65 years (11, 12). Hence, with the increasing longevity due to improvements in medical science, healthcare delivery, and standard of living, it is expected that the number of people living with Parkinson's disease in the world would rise. Parkinsonism in the elderly is associated with increased morbidity and mortality (12-18) with attendant personal and societal consequences (19). Late phase Parkinson's disease is associated with dementia, hallucinations, respiratory dysfunction, and delusions (20-23).
The cause of Parkinson's disease remains essentially unknown. Although an inverse relationship has been demonstrated in a number of studies with cigarette smoking, coffee drinking, and Parkinson's disease (23-26), others have insisted on the contrary or have maintained that the relationship between cigarette smoking and Parkinson's disease is equivocal (27, 28). However, a multi-factorial etiology involving interplay between environmental and genetic risk factors has been hypothesized and supported by some findings (29-33).
Other potential related causes include infection, nutritional and free radical mechanisms, inflammation, nitric oxide as well as apoptosis have been proposed (34-38). This is due in part to the observation that Parkinson's disease is characterized by the decrease in substantia nigra (SN) levels of the thiol antioxidant molecule, glutathione (GSH); a decrease that appears to parallel the disease severity and progression (36, 38, 39). Depletion of GSH, among other antioxidants, tends to precede decreases in mitochondrial complex 1 activity and dopamine levels (38, 39).
Following the initiation of levodopa therapy (LD) in levodopa-naïve patients, motor symptoms associated with the disease tend to be easily controlled. At this stage, no clear-cut relationship between drug concentration and drug effect can be easily observed or appreciated (40). However, with advancing disease and prolonged exposure to LD, problems arise due to L-dopa use and correlation between levodopa plasma concentration and clinical effect becomes vital (41). In advanced disease stages, the rapid rise (upswing) and fall in plasma concentration after each dose of levodopa tend to correlate more with effect (the end-of -dose wearing-off phenomenon). Therefore, this review will discuss the role of pharmacokinetics and pharmacodynamics strategies that may be vital in the management of Parkinson's disease patients experiencing fluctuations in motor function while taking levodopa.
Presentation and Clinical diagnosis of Parkinson's disease
Parkinson's disease is mainly due to lack of dopamine in the striatum. According to Gibb (42), and others (43), the pathological hallmark of the disease is a loss of the pigmented, dopaminergic neurons of the subtantia nigra pars compacta, and the subsequent appearance of intracellular inclusions referred to as Lewy bodies. In Parkinson's disease, this loss of dopamine producing neurons in the substantia nigra culminates in a decrease in the production of dopamine which can be partially reversed by the administration of levodopa, a precursor of the CNS neurotransmitter, dopamine.
Clinical diagnosis has been based on the presence of two of the four cardinal symptoms for Parkinson's disease (i.e. "cog-wheel" type rigidity, resting tremor, bradykinesia and postural instability). Secondary features of Parkinson's disease which may be attributed to degeneration of the nervous system include cardiovascular, gastrointestinal, and genitourinary systems dysfunction, orthostatic hypotension, arrhythmia, constipation, hyper-salivation, urinary frequency, impotence, hallucinations, depression and psychosis (44-48).
In addition, Parkinson's disease affects the quality of life of the patients tremendously (19, 48-51). Increase in daytime somnolence and decrease in ability to operate motorized vehicles in people with Parkinson's have been reported (49-51). Many studies have documented the psychological dimensions of Parkinson's disease with a view to ascertaining better ways of managing the psychological aspect of the disease (52, 53). Because Parkinson's disease is debilitating, it interferes with various physical, emotional and social aspects of the quality of life of the patients and imposes tremendous socio-economic stress on the patient in particular and the society in general (19, 52, 54). Without a cure for Parkinson's disease, the goal in its management is to improve the quality of life of the patients and enable them to carry on with basic daily function independent of caregivers. In deed, the loss of normal functions in the sensory organs, cognitive function, and most notably, motor functions have been described as the hallmarks of Parkinson's disease (55). Of the sensory impairments associated with Parkinson's disease, diminution in olfaction has been well studied (56, 57). Emotional problems with both the patients, caregivers and family members are commonplace. The quality of life as measured by the ability of an individual's sense of well-being, purpose in life, autonomy, and ability to assume worthwhile roles and participation in significant relationship (58), has been demonstrated to be adversely affected by Parkinson's disease (59).
Sexual function in Parkinson's disease patients has been reported to be highly impaired in both male and female patients compared to matching controls (60, 61) with the males being most affected.
Treatment and management of Parkinson's disease
Since Parkinson's disease is essentially a condition precipitated by decreased dopamine in the CNS, treatment is geared towards ensuring an adequate supply of dopamine to the striatum to rectify the imbalance. Various approaches in the treatment and management of patients with Parkinson's disease are currently being evaluated. These strategies may be sub-divided into two: (i) Non Pharmacological approaches and (ii) Pharmacological approaches. Most of the various non-pharmacological approaches adopted are aimed at either ameliorating the symptoms of the disease or correcting the pathological deficits associated with Parkinson's disease. Although some of the recent advances in the use of invasive surgical approaches are targeted at correcting the pathological changes, it is worthy of mention that none of these strategies has yielded convincing results (62-64) and may be associated with high morbidity and mortality (65). Hence, these non-pharmacologic approaches will not be discussed in this review.
Pharmacological approaches involve the use of exogenously administered xenobiotics in replacement therapy (dopamine agonists or its precursor such as levodopa) and anti-cholinergic agents, selegiline, amantidine and other symptomatic medications. The goal of pharmacotherapy in Parkinson's disease is to maintain the patient's functional ability by providing symptomatic relief and minimizing adverse events from concomitant medications and perhaps, slow the pace of disease progression. The choice of agents to use and when to initiate pharmacotherapy in Parkinson's disease (66) especially with levodopa regimens (67, 68) is challenging and controversial. Ferreira and Rascol (68) have suggested therapeutic strategies involving the use of combination agents for the management of Parkinson's disease patients experiencing levodopa-induced dyskinesias. Like most terminal diseases, patients tend to be knowledgeable about their condition and oftentimes are excellent assessors of the state of their health. The temptation to self-medicate, "dose-pinching" and misuse of antiparkinson medications have been reported with Parkinson's disease patients (69). However, recently, a publication in the official Journal of the American Academy of Neurology has suggested a treatment algorithm to be followed in the treatment and management of Parkinson's disease (7).
Table 1 lists some agents that are commonly used in the treatment of Parkinson's disease (70-82).
Table 1: List of some dopamine agonists and precursors that are commonly used in the treatment and Management of Parkinson's disease.
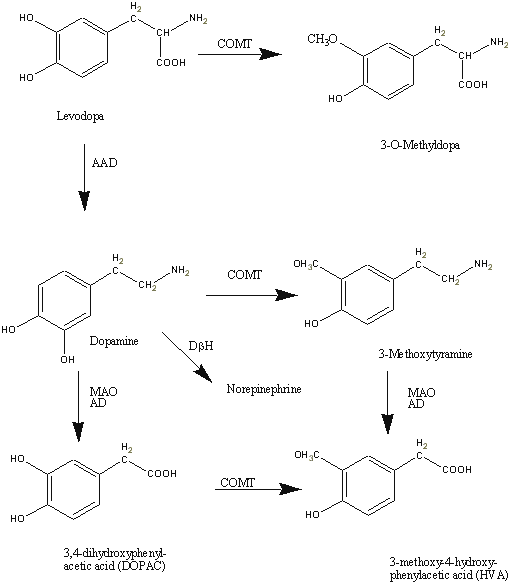
Of all the pharmacological agents available for use in the treatment and management of Parkinson's disease, levodopa (Figure 1) remains the "gold standard". The establishment of levodopa in late 1960s for the treatment of Parkinson's disease undoubtedly, has proved to be a monumental advance in the therapy and management of Parkinson's disease. The use of levodopa has been reported to improve the quality of life and extend the life span of Parkinson's disease patients. With the caveat that early use may lead to disabling dyskinesias and motor fluctuations, typically, use of a long acting dopamine agonist is recommended for initial use, followed later by combination therapy with levodopa plus dopamine agonist.
Figure 1: Metabolism of Levodopa
Bioanalytical Methods for the quantitation of levodopa in human plasma
The key to effective plasma sample monitoring and the development of reliable PK/PD relationship with leovdopa hinges in part on the availability of good, reliable, sensitive and rapid assay for the determination and quantitation of levodopa and other by-products in biological fluids. Being an endogenous compound, most assay methodologies available today would have to consider background levels vis a vis the total biogenic amine levels in order to obtain the amount of exogenously administered levodopa in any given biological sample. Various analytical methods for the analysis of levodopa in plasma samples exist. The separation and quantitation techniques involve ion-pair reversed phase high-performance liquid chromatography (HPLC) (83-85) with either electrochemical or colorimetroc detectors equipped with dual-electrode systems (83-86). Although sample preparations could be a little bit cumbersome and included difficult column-switching operations, nonetheless, these methods are fast, reliable and offer sensitivities in the nanogram range (86-88). The use of Gas chromatography coupled to mass spectrophotometer (GC/MS) offers another technique with better sensitivity but with higher expenses (89). With increasing automation in the pharmaceutical industry today, methologies involving the use of LC/MS/MS that offer easier, faster and more sensitivity are being explored.
Pharmacokinetics of Levodopa
Following oral administration, levodopa per se is rapidly absorbed from the GI tract by an active transport system, achieving peak plasma concentrations within 0.5 to 2 hours post -drug administration (79, 90). The rate and extent of absorption of standard levodopa tablets (immediate release formulation) is affected by food and other gastric factors that modulate gastric emptying (91) including gastric pH, intestinal transit time, and intestinal enzymes. Food modulates gastric motility and affects the delivery of Levodopa to the small intestines where drug absorption mainly occurs. In addition, diets high in protein content have been shown to reduce the absorption of levodopa from the intestines. In a study comparing the effect of a low-protein diet against a high-protein diet on Parkinson's disease patients with "on-off" syndrome, Eriksson et al (92) observed that the administration of low-protein diets significantly increased the total daily "on" state compared to high-protein diet.
The peak plasma concentrations and bioavailability of standard levodopa is highly variable with a half-life of about 1-hour when administered alone and 1.5-2 hrs upon concomitant administration with a dopa-decarboxylase inhibitor (90, 93-95). Following oral administration of 100 mg levodopa + 25 mg carbidopa tablet to young and elderly volunteers, levodopa was found to achieve 41 and 86 % bioavailability respectively. Both clearance and volume of distribution but not the half-life (t 1/2 ), peak plasma concentration (C max ) and time to peak plasma drug concentration (T max ) were affected by age (96).
Levodopa administered alone is widely distributed in body tissues with a volume of distribution about 65% the total body volume. However, distribution into the CNS is minimal, accounting for less than 1% of the dose. However, following co-administration with dopa-decarboxylase inhibitor, the amount of levodopa bioavailable is increased. The transport of levodopa across the blood brain barrier into the CNS is via a saturable sodium- independent facilitated transport mechanism that is shared with other amino acids (70, 97).
Also noteworthy is the bi-modal pharmacokinetic (multiple peak) profile of levodopa that has been observed by some authors (80, 98). The presence of multiple peaks in levodopa plasma concentration versus time profile has been attributed to changes emanating from gastric motility and gastric emptying. Levodopa has been reported to modulate the pattern of gastric emptying in man, thus affecting its own disposition (80). Pharmacokinetics of levodopa has been shown to be modulated by a host of factors chief of which is gastric emptying. Gastric emptying is delayed by fatty meals resulting in marked increase in the t- max of levodopa following oral administration. On the contrary, prokinetic drugs such as cisapride and domperidone, which increase gastric emptying, tend to improve levodopa absorption and its pharmacodynamic effect (99, 100). Effect on the absorptive factors poses the major obstacle in getting the drug into the systemic circulation (101-103). Modulation of gastric emptying affects levodopa absorption (104), its pharmacokinetic profile (101-103) and may cause random fluctuations of Parkinson's disease patient's mobility (104) and treatment failure (92, 105).
Levodopa is biotransformed to its active moiety, dopamine and to other by-products by aromatic amino acid decarboxylase (AAAD), an enzyme that utilizes pyridoxine as a co-factor. Copious amount of the administered dose (about 95%) is metabolized in the lumen of the stomach and intestines and on first pass through the liver (106, 107). Conversion of levodopa to dopamine in the periphery is disadvantageous as dopamine not only inhibits further absorption of levodopa in the GI tract but also cannot cross the blood brain barrier into the CNS site of action. Inhibition of the AAAD enzyme by co-administration of levodopa with a dopa-decarboxylase enzyme inhibitor (such as carbidopa or benserazide) increases the amount of intact drug in plasma that is available for distribution to the CNS (108, 109). Dopamine is, in turn, metabolized by monoamine oxidase (MAO) and Catechol-O-methyl-transferase (COMT) (figure 1).
Problems associated with prolonged Levodopa therapy in Parkinson's disease
Motor fluctuation associated with levodopa therapy is a major problem encountered in the treatment of Parkinson's disease. Following prolonged use of levodopa (usually > 5years), especially in advanced stages of the disease, the efficacy and benefits derived from levodopa begin to wane and motor complications occur regularly (110, 111).
Loss of therapeutic efficacy of Levodopa following long-term administration is a serious problem (112-114). After initial benefit from Levodopa, many Parkinson's disease patients develop (1) progressive, global loss of drug effectiveness; (2) the "wearing-off "phenomenon, in which daily periods of benefits after individual doses of levodopa become shorter; and (3) "on-off" phenomenon, which refers to abrupt and unpredictable fluctuations in responsiveness and which is thought to be unrelated to dosage schedule (115-117). Rajput et al (117) in a study to determine the continued benefit and pattern of motor complications sequel to long term levodopa reported dyskinesia as the most common occurring and earliest noticeable untoward effect of prolonged levodopa use, followed by wearing-off and on-off phenomenon.
Like most capacity limited or saturable systems, upon prolonged use of levodopa, the "law of diminishing returns" begins to set in. The usual "honey moon" once enjoyed upon initial introduction of therapy gradually disappears. Mechanisms of motor fluctuations associated with prolonged use of Levodopa are not fully understood. Various authors (111, 118) have suggested that it could be due to one or combination of the following factors: (a) modulation of central pharmacokinetics (delivery of L-dopa from pre-synaptic to post-synaptic receptors), (b) peripheral pharmacokinetics or delivery of levodopa from an exogenous source to the brain across the blood brain barrier (c) pharmacodynamics -alteration in the interaction between dopamine and striatal receptors.
The loss of benefit from levodopa therapy is usually associated with problems such as end-of-dose deterioration and dyskinesias. The mechanisms involving changes in levodopa pharmacokinetics and pharmacodynamics including changes in responsiveness of dopamine receptors in the CNS have been supported by others (98, 119-124). Hyperkinetic movement disorders, such as chorea, dystonia and myoclonus develop in most Parkinson's disease patients following prolonged use of levodopa (125). Most often, the dyskinesia may occur from an overshoot of the therapeutic concentration of L-dopa, thus calling for a careful monitoring of the therapeutic optimum of this drug in advanced Parkinson's disease patients.
A reduction in the capacity of dopaminergic cells to synthesize, take up, and store dopamine leads to a dependence on the bioavailability of exogenously administered levodopa. Thus, delivery of levodopa to the brain from the peripheral pool remains a major strategy to ensuring adequate availability of dopamine to the brain. Hence, it is expected that factors that modulate the peripheral concentrations as well as the central levels of dopamine will in turn affect the amount of drug available for delivery to the brain. Some studies aimed at ensuring adequate peripheral levels of Levodopa have demonstrated improvement in motor function in Parkinson's disease patients with increasing plasma concentration of levodopa (126, 127).
A good understanding of the reasons for the occurrence of motor dyskinesias and delineation of the pharmacokinetics and pharmacodynamics of levodopa in these subjects may provide clues to solving the problem. However, an ideal approach would be to determine central pharmacokinetics of levodopa. Currently, this is a tedious process with existing technology and is not plausible. However, a good monitoring of peripheral pharmacokinetics of levodopa would be much easier and may be helpful in the pharmacotherapy of patients experiencing motor fluctuations. The relationship between plasma concentrations of levodopa and its clinical response remains to be categorically defined.
Evidence for PK-PD interplay in the management of motor fluctuations in Parkinson's disease patients stabilized on levodopa regimen
Understanding the relationship between the PK and PD of levodopa has been at the center of most discussions of effective management of Parkinson's disease patients experiencing motor fluctuations. Some workers have reported lack of correlation between plasma levodopa concentrations and effect (128-130). Although some studies have failed to show a clear-cut correlation between plasma concentration of levodopa and effect in motor fluctuating patients (131, 132), some evidence as to the role of Pharmacokinetics and Pharmacodynamics in the "On-off" phenomenon in Parkinson's disease has been demonstrated by others (93, 133-138). In a study with Parkinson's disease patients dosed with levodopa/carbidopa (Sinemet®) tablets every 6 hours, Okereke et al (139) observed a time-dependent improvement in overall motor function (Figure 2). In addition, it has been reported that continuous intravenous infusion, constant-rate duodenal administration of liquid levodopa (140-145) and administration of controlled-release levodopa/carbidopa (Sinemet®) tablet (146) produced more stable plasma concentrations of levodopa. This resulted in better motor response and improved quality of life of patients (147). Thus, it can be inferred that increasing peripheral levels would increase the peripheral pool of drug for active transport across the blood brain barrier (136, 148-152).
Figure 2: Plasma concentration vs time curve of levodopa after administration of Sinemet® to Parkinson's disease patients (Top Panel). Change in Unified Parkinson's Disease Rating Scale (UPDRS) score from baseline (bottom panel). Adapted from Okereke et al 2000
Furthermore, various PK/PD studies have suggested the role of PK in explaining the observed effect occurring as a function of time and disease progression (91, 113, 136, 153). Also, the fact that inhibition of COMT activity with Entacapone has been documented to increase the plasma concentration of Levodopa, decrease the proportion of daily "off" time and improve patient's clinical condition, indicates the role of peripheral pharmacokinetics of levodopa and the net effect on Parkinson's disease treatment (127, 153, 155). Similarly, Baas et al (156) and others (157) have reported significant decrease in the "wearing off" phenomenon in Parkinson's disease patients following concomitant administration of Tolcapone with levodopa.
Although still controversial, the role of 3-O-methyldopa (figure 1) in the pharmacokinetics/pharmacodynamic relationship of levodopa has been investigated (138, 158) and may be helpful in elucidating the relationship between plasma pharmacokinetics of levodopa and its effects. Others have postulated that 3-O-Methyldopa, the inactive metabolite of levodopa can inhibit the uptake of levodopa across the blood-brain (159, 160) thus suggesting a relationship between the central and peripheral levels of levodopa. In fact, following a single dose administration of 100 mg levodopa and plus 25mg benserazide to Parkinson's disease patients at various stages of the disease, Harder and Baas (82) observed a positive correlation between pre-dose and post-dose increases in 3-O-methyldopa plasma concentration and plasma concentration of levodopa at steady-state that elicited half the maximum effect (EC50). In addition, others (161) have reported a competition between levodopa and its metabolite 3-O-Methyldopa for transport across the intestinal wall of experimental animals.
Although the phenomenon of motor fluctuation in Parkinson's disease patients is not fully understood, some authors have suggested some pharmacokinetics factors that may influence the delivery of levodopa to the brain and pharmacodynamic factors such as variations in dopamine receptor function as possible mechanisms (145, 162-164).
Pharmacokinetic/Pharmacodynamic considerations in the treatment of Parkinson's disease patients
Since the problem of levodopa-induced motor fluctuations rarely occurs at onset of therapy, the role of pharmacokinetics/pharmacodynamics may not be obvious. However, at advanced stages of the disease where small changes in plasma concentration and disposition of levodopa can greatly affect therapeutic response, pharmacokinetic properties of the drug and receptor dynamics for levodopa largely account for the motor dyskinesias observed. Thus, the need for pharmacokinetic optimization of dosage intake becomes essential to obtain plasma profiles of Levodopa and matched therapeutic responses (70, 165, 166). The role of PK/PD in the management of levodopa induced motor fluctuations cannot be overemphasized. In Parkinson's disease where the drug of choice remains levodopa, a proper understanding of the relationship between the pharmacokinetics of the drug and its effect would no doubt be beneficial in the management of this disease.
The pattern of the pharmacokinetic and pharmacodynamic relationship with levodopa during the course of Parkinson's disease is a changing one due largely to changes in the ability of dopamine nerve terminals to store and release dopamine, probable modulation of receptor sensitivity, and modulation of central and perhaps peripheral pharmacokinetics of levodopa (167, 168).
Various approaches for the management of motor fluctuations are under investigation. Based on a single dose study by Sweet et al (169) the use of drug holidays has been suggested as a means of solving the problem of motor fluctuations in Parkinson's disease. Others (170) have argued against this proposal, considering the discomfort, dangers of withdrawal and resultant cost of possible hospitalization. Suggested strategies to be adopted in the therapeutic management of Parkinson's disease involve modulating both the pharmacokinetics of levodopa and the receptor (site of action of the drug).
The idea that modified-release dosage forms of levodopa as a means of improving on the bioavalability of levodopa might play a significant role in the management of motor fluctuation in Parkinson's disease has also been suggested (165).
Currently, the most popular clinically available route for the administration of levodopa in Parkinson's disease patients is via the oral route. Development of newer formulations of levodopa for administration via alternate routes might be helpful to ensure adequate plasma levels of levodopa and thus help prevent excessive fluctuations in motor response in Parkinson's disease patients. The use of pharmaceutical delivery systems in combination with guidance for individualizing drug dosage has been adduced as a means of providing optimal and cost efficient pharmacotherapy (171, 172). Thus, the use of alternate delivery systems such as transdermal delivery systems are currently being investigated as a possible means of achieving constant flow of levodopa to the peripheral pool and eventual delivery across the blood brain barrier to the CNS (173-179). The use of transdermal preparations for drug delivery is gaining wide acceptance and has been reported to improve the quality of life of patients with chronic diseases (180-185). Today, different classes of drugs ranging from small molecules to large macromolecules such as used in hormonal replacement therapy, e.g. estrogen, can be administered through the skin and have achieved wide patient compliance (180, 184). The use of transdermal delivery systems such as patches, nasal sprays and buccal or subcutaneously administered devices that will offer rapid availability of levodopa in emergencies (such as cogwheel type dyskinesias as occurring during end-of-dose) may be of vital importance (186-188). In addition, these devices could ensure constant flow of levodopa to the peripheral pool once a patient is titrated and stabilized on a regimen.
The fact that some low molecular weight compounds as well as large molecular weight drugs such as insulin, can be administered subcutaneously, and others intranasally (189, 190), the need to develop an intranasal spray of levodopa that can be used in emergency situation where rapid delivery of drug to the peripheral pool would be necessary. In fact, some authors have reported increased neostriatal dopamine activity following intranasal, intraperitoneal or subcutaneous administration of L-dopa to experimental animals (186-188). The ease, safety and cost effectiveness demonstrated with intranasal administration of some drugs makes this route very attractive (191, 192).
Dosing regimens aimed at maximizing therapeutic concentrations of levodopa without overshooting the "therapeutic window" is the best approach in the management of Parkinson's disease patients experiencing motor complications with Levodopa. Minimal decrements in levodopa doses help in minimizing dyskinesias while "wearing off" effects are best managed by ensuring "constant" therapeutic level of Levodopa. The importance of Pharmacokinetic/pharmacodynamics in the management of Parkinson's disease patients experiencing motor fluctuations has been supported by data obtained from various studies (82, 150, 193) indicating that the equilibrium half-life of levodopa highly correlates with the duration of tapping response and this provides a reliable quantitative index of central mechanisms that affect the length of clinical effect. In a study involving both motor fluctuating and non-fluctuating Parkinson disease patients at different stages of the disease, Harder and Baas (82) observed no difference in the pharmacokinetics of levodopa between both groups of patients. However, the threshold level (EC50) required to obtain clinical response was larger in those patients experiencing motor fluctuations and they exhibited a steeper concentration-response curve. Maintenance of sustained therapeutic levels of levodopa in Parkinson's disease patients has been an issue that many believe could be achieved by changes in formulation of the drug.
Furthermore, individualization of therapy at reasonable intervals may be useful in optimizing bioavailability of levodopa during treatment (194). Some investigators have attempted to monitor blood levels of levodopa using microdialysis techniques as a means of optimizing levodopa therapy (195). Others have reported significant correlation between severity in motor symptoms and dopamine levels in the cerebrospinal fluid (196). Some have suggested approaches to facilitate transport of agents across the blood-brain barrier and improve bioavailability of levodopa via osmotic opening of the blood brain barrier (197). Currently, this approach has been successfully done in experimental animals. The search of such agents for clinical use as adjuncts to levodopa in this regard may be helpful.
Finally, considering the preponderance of information on the bioavailability of levodopa as a function of its activity, it can be deduced that optimization of the dosage and administration time of levodopa would be very helpful in controlling not only the untoward effect associated with levodopa use but its efficacy as well. To achieve this, an integrative PK and PD approach, which entails detailing or profiling of each patient's dosage regimen, would need to be worked out. Although it has been demonstrated that the plasma level of an amino acid alone is a poor predictor of its brain concentrations (97, 198, 199), but rather, dependent on the plasma concentration of other amino acids that share the same blood brain barrier transport system (97, 200-203); nonetheless, some researchers have advocated (204) the periodic monitoring of plasma concentrations of levodopa in patients experiencing motor fluctuation as a better way of managing the disease in such patients.
Generally, the flux of amino acids including levodopa across the blood brain barrier is bi-directional. The net flux of un-metabolized levodopa is from the brain to plasma pool when the plasma concentration of levodopa falls. Thus, it follows that factors that promote increase in plasma pool of un-metabolized levodopa may help increase the amount of levodopa available for transport into the brain compartment. Current techniques in modeling the Pharmacokinetics of levodopa and its effect is tedious as the process is often complicated by the presence of its metabolite, 3-O-methyldopa. Various studies have successfully used the E-max model to describe the possible relationships between the pharmacokinetics and dynamics of levodopa. Others (205) have attempted to use the Dixon equation with a characteristic 3-dimensional model to explain the relationship of levodopa pharmacokinetics and effect taking into cognizance the role of the 3-O-methyldopa metabolite. While much has been achieved in attempts to decipher the PK/PD relationships, the result from these studies still remain equivocal and a clear-cut delineation of the PK/PD relationship of levodopa continues to be a subject of further exploration.
Conclusion
Although the relationship between peripheral pharmacokinetics and motor response with levodopa has not been properly delineated, it does not preclude the existence of such an effect. Therefore, further exploration of PK-PD relationship of levodopa and its effect at all stages of the disease, especially at the advanced stage of Parkinson's disease, is important. No doubt, it would be very helpful in the pharmacotherapy and management of Parkinson's disease especially at advanced disease stage characterized by neuron loss, attendant loss of the storage and pulsatile release function, and where motoric control becomes dependent on exogenous delivery of dopamine from the plasma pool. Today, the challenge remains to develop a demonstrable correlation between effector site concentration of dopamine vs the plasma levels of levodopa and its effect.
Acknowledgements
The author acknowledges the contributions of Louis Kirby, M.D. in the preparation of this manuscript.
References
- Greenfield, J.G., Paralysis agitans (Parkinson's Disease), in Blackwood, McMenemy, Meyer et al. (Eds) Greenfield's Neuropathology. Williams and Wilkins, Baltimore, 2: pp582-585, 1963.
- Bernheimer, H, Birkmayer, W., Hornykiewicz, O., Jellinger, K., and Seitelberger, F., Brain dopamine and the syndrome of Parkinson and Huntington: Clinical morphological and neurochemical correlations. J. Neurological Sciences, 20: 415-455, 1973.
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci, 12:366-375, 1989.
- Schrag, A., Ben-Shlomo, Y., Quinn, NP. Cross sectional prevalence survey of idiopathic Parkinson's disease and Parkinsonism in London. BMJ, 321:21-22, 2000.
- Chan DK, Dunne M, Wong, A, Hu E, Hung WT, Beran RG. Pilot study of prevalence of Parkinson's disease in Australia. Neuroepidemiology, 20:112-127, 2001.
- De Rijk, MC, Launer LJ, Berger K, Breteler, MM, Dartrigues JF, Baldereschi, M, Fratiglioni L, Lobo, A., Martinez-Lage, J., Trenkwalder C., Hoffman, A. Prevalence of Parkinson's disease in Europe: A collaborative Study of population-based cohorts. Neurologic Diseases in elderly Research Group. Neurology; 54 (11 Suppl 5): S21-S23, 2000.
- Olanow CW, Watts RL, Koller WC. An algorithm (decision tree) for the management of Parkinson's Disease (2001). Treatment guidelines. Neurology; 56(Suppl5):S3-S29, 2001.
- Herndon, C.M., Young, K., Herndon, A.D., Dole, E.J. Parkinson's Disease revisited. J. Neurosci Nurs; 32: 216-221, 2000.
- Zhang Z, Roman G. Worldwide occurrence of Parkinson's disease: an overview. Neuroepidemiology, 12:195-208, 1993.
- Richards M, Chaudhuri KR. Parkinson's disease in populations of African origin: a review. Neuroepidemiology, 15:214-221, 1996.
- Waite LM, Broe GA, Creasy H. Grayson, D., Edelbrock D, O'Toole B. Neurological signs, aging, and the neurodegenerative syndromes. Archs Neurol, 53:498-502, 1996.
- Morens DA, Davis JW, Grandinelli A, Ross GW, Popper JS, White LR. Epidemiologic observations on Parkinson's disease: Incidence and mortality in a prospective study of middle-aged men. Neurology, 46:1044-1050, 1996.
- Bennett DA, Beckett LA, Murray AM, Shannon KM, Goetz CG, Pilgrim DM, Evans DA. Prevalence of Parkinsonian signs and associated mortality in a community population of older people. N Engl J Med., 334: 71-76, 1996.
- Diamond SG, Markham CH, Hoehn MM, Mcdowell FH, Muenter MD. Effect of age at onset on progression and mortality in Parkinson's disease. Neurology, 39:1187-1190, 1989.
- Imaizumi, Y, Kaneko R. Rising mortality from Parkinson's disease in Japan 1950-1952. Acta Neurol Scand, 91:169-176, 1995.
- Chio A, Magnani C, Tolardo G, Schiffer D. Parkinson's disease mortality in Italy, 1951 through 1987. Analysis of an increasing trend. Arch Neurol, 50: 149-153, 1993.
- Morgante L, Salemi G, Meneghini F, Di Rosa AE, Epifanio A, Grigoletto F, Ragonese P, Patti F, Reggio A, Di Perri R, Savettieri G. Parkinson disease survival: a population-based study. Arch Neurol, 57:507-512, 2000.
- Kurtzke JF, Murphy FM. The changing patterns of death rates in Parkinsonism. Neurology, 40: 42-49, 1990.
- Hewer R L. The economic impact of neurological illness on the health and wealth of the nation and of individuals. J Neurosurg Psychiatry, 63(Suppl 1):19-23, 1997.
- Zarkharov VV, Akhutina TV, Yakhno NN. Memory impairment in Parkinson's disease. Neurosci Behav Physiol, 31:157-163, 2001.
- Aarsland D, Anderson K, Larsen JP, Lolk A, Nielsen H, Kragh-Sorensen P. Risk of dementia in Parkinson's Disease: a community-based, prospective study. Neurology, 56:730-736, 2001.
- Holroyd S. Currie L, Wooten GF. Prospective study of hallucinations and delusions in Parkinson's disease. Neurol Neursurg Psychiatry, 70:743-748, 2001.
- Brown K. L. Respiratory dysfunction in Parkinson's disease. Clin Chest Med, 15:715-727, 1994.
- Behari M, Srivastava AK, Das RR, Pandey RM. Risk factors of Parkinson's disease in Indian patients. J Neurol Sci, 190: 49-55, 2001.
- Kelton MC, Kahn HJ, Conrath CL, Newhouse PA. The effects of nicotine on Parkinson's disease. Brain Cogn, 43: 274-282, 2000.
- Ross GW, Abbott RD, Petrovitch H, Morens DM, Grandinetti A, Tung KH, Tanner CM, Masaki KH, Blanchette PL, Curb JD, Popper JS, White LR. Association of coffee and caffeine intake with the risk of Parkinson's disease. JAMA, 283: 2674-2679, 2000.
- Nilsson A, Waller L, Rosengren A, Adlerberth A, Wilhelmsen L. Cigarette smoking is associated with abnormal involuntary movements in the general male population -a study of men born in 1933. Biol Psychiatry, 41: 717-723, 1997.
- Honig LS. Relationship between Caffeine intake and Parkinson's disease. JAMA, 284: 1378-1379, 2000.
- Elbaz A, Manubens-Bertran JM, Baldereschi M, Breteler MM, Grigoletto F, Lopez-Pousa S, Dartigues JF, Alperovitch A, Rocca WA, Tzourio C. Parkinson's disease, smoking, and family history. EUROPARKINSON Study Group. J Neurol, 247: 793-798, 2000.
- Liou, H. H., Tsai, M.C., Chen, C.J., Jeng, J.S., Chang, Y.C., Chen, S.Y. and Chen, R.C. Environmental risk factors and Parkinson's disease: A case-control study in Taiwan. Neurology, 48: 1583-1588, 1997.
- Smith CA, Gough AC, Leigh PN, Summers BA, Harding AE, Maraganore DM, Sturman SG, Schapira AH, Williams AC, et al. Debrisoquin hydroxylase gene polymorphism and susceptibility to Parkinson's disease. Lancet, 339:1375-1377, 1992.
- Checkoway H, Farin FM, Costa-Mallen P, Kirchner SC, Costa LG. Genetic polymorphisms in Parkinson's disease. Neurotoxicology, 19: 635-643, 1998.
- Seidler A, Hellenbrand W, Robra BP, Vieregge P, Nischan P, Joerg J, Oertel WH, Ulm G, Schneider E. Possible environmental, occupational, and other etiologic factors for Parkinson's disease: a case-control study in Germany. Neurology, 46:1275-1284, 1996.
- Mirsattari SM, Power C, Nath A. Parkinsonism with HIV infection. Mov. Disord., 13: 684-689, 1998.
- Kidd PM. Parkinson's disease as multifactorial oxidative neurodegeneration: implications for integrative management. Altern Med Rev., 5:502-529, 2000.
- Perry TL, Young VW. Idiopathic Parkinson's disease, progressive supranuclear palsy and glutathione metabolism in the substantia nigra of patients. Neurosci Lett 1986; 67:269-274.
- Jenner P. Oxidative mechanisms in nigral cell death in Parkinson's disease. Mov. Disord 1998; 13 (Suppl 1): 24-34.
- Reichmann H., Janetsky B. Mitochondrial dysfunction- a pathogenic factor in Parkinson's disease. J. Neurol, 247(suppl 2): 63-68, 2000.
- Harada S, Fujii C, Hayashi A, Ohkoshi N. An Association between Idiopathic Parkinson's Disease and Polymorphisms of Phase II Detoxification Enzymes: Glutathione S-Transferase M1 and Quinone Oxidoreductase 1 and 2. Biochem Biophys Res Commun, 288:887-892, 2001.
- Muenter MD, Tyce GM. L-Dopa therapy of Parkinson's disease: Plasma L-dopa concentration, therapeutic response, & side effects. Mayo Clin Proc, 46:231-239, 1971.
- Contin M, Riva R, Martinelli P, Cortelli P, Albani F, Baruzzi A. Longitudinal monitoring of the levodopa concentration -effect relationship in Parkinson's disease. Neurology, 44:1287-1292, 1994.
- Gibb WR. Neuropathology of Parkinson's disease and related syndromes. Neurol Clin., 10:361-376, 1992.
- Fearnley J, Lees A. Pathology of Parkison's disease. In Calne, D.B. eds.) Neurodegenerative Diseases. Saunders, Philadelphia, pp545-554, 1994.
- Tissingh G, Berendse HW, Bergmans P, DeWaard R, Drukarch B, Stoof JC, Wolters EC. Loss of olfaction in de novo and treated Parkinson's disease: possible implications for early diagnosis. Mov Disord, 16: 41-46, 2001.
- Soykan I, Lin Z, Bennett JP, McCallum RW. Gastric myoelectrical activity in patents with Parkinson's disease: evidence of a primary gastric abnormality. Dig Dis Sci, 44:927-931, 1999.
- Lamberg L. Psychiatric symptoms common in neurological disorders. JAMA, 286:154-156, 2001.
- Mesec A, Sega S, Trost M, Pogacnik T. The deterioration of cardiovascular reflexes in Parkinson's disease. Acta Neurol Scand, 100:296-299, 1999.
- Niimi Y, Ieda T, Hirayama M, Koike Y, Sohue G, Hasegawa Y, Takahashi A. Clinical and physiological characteristics of autonomic failure with Parkinson's disease. Clin Auton Res, 9:139-144, 1999.
- Martinez-Martin P. An introduction to the concept of "quality of life in Parkinson's disease". J Neurol, 245(Suppl 1): S2-S6, 1998.
- Ondo WG, Dat Vuong K, Khan H, Atassi F, Kwak C, Jankovic J. Daytime sleepiness and other sleep disorders in Parkinson's disease. Neurology, 57:1392-1396, 2001.
- Heikkla V-M, Turkka J, Korpelainen J, Kallaranta T, Summala H. Decreased driving ability in people with Parkinson's disease. J Neuro Neurosurg psychiatry, 64:325-330, 1998.
- Frazier LD. Coping with disease-related stressors in Parkinson's disease. Gerontologist, 40:53-63, 2000.
- Scheife RT, Schumock GT, Burstein A, Gottwald MD, Luer MS. Impact of Parkinson's disease and its pharmacologic treatment on quality of life and economic outcomes. Am J Health Syst Pharm, 57:953-962, 2000.
- Jost WH. Costs in the treatment of Parkinsonism. J Neurol, 247 (suppl 4):IV/31-IV33, 2000.
- Cubo E, Bernard B, Leurgans S, Raman R. Cognition and motor function in patients with Parkinson's disease with and without depression. Clin Neuropharmacol, 23:331-334, 2000.
- Sobel, N., Thomason, M.E., Stappen, I., Tanner, C.M et al. An impairment in sniffing contributes to the olfactory impairment in Parkinson's disease, Proc. Natl. Sci. USA., 98: 4154-4159, 2001.
- Lehrner J, Brucke T, Kryspin-Exner I, Asenbaum S, Podreka I. Impaired olfactory function in Parkinson's disease. Lancet, 345:1054-1055, 1995.
- Hobson P, Holden A, Meara J. Measuring the impact of Parkinson's disease with Parkinson's disease quality of life questionnaire. Age and Ageing, 28:341-346, 1999.
- Koplas PA, Gans HB, Wisely MP, Kuchibhatla M, Cutson TM, Gold DT, Taylor CT, Schenkman M. Quality of life and Parkinson's disease. J Gerentol, 54A:M197-M202, 1999.
- Chandler BJ, Brown S. Sex and relationship dysfunction in neurological disability. J Neurol Neurosurg Psychiatry, 65: 877-880, 1998.
- Brown, R. G., Jahanshahi, M., Quinn, N., Marsden CD. Sexual function in patients with Parkinson's disease and their partners. J. Neurosurg Psychiatry, 53:480-486, 1990.
- Chan DTM, Mok VCT, Hung KN, Zhu XL. Surgical management of Parkinson's disease: a critical review. HKMJ, 7:34-39, 2001.
- Fischbach, GD, McKham GM. Cell therapy for Parkinson's disease. N Engl J Med., 344: 763-765, 2001.
- Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, Eidelberg D, Fahn S. Transplantation of embryonic neurons for severe Parkinson's Disease. N Engl J Med , 344:710-719, 2001.
- Olanow CW, Koller W, Goetz CG, Stebbins GT, Cahill DW, Gauger LL, Morantz R, Penn RD, Tanner CM, Klawans HL, et al. Autologous transplantation of adrenal medulla in Parkinson's disease: 18 months results. Arch Neurol, 47: 1286-1289, 1990.
- Caraceni T, Scigliano G, Musicco M. The occurrence of motor fluctuations in Parkinson's patients treated long term with levodopa: role of early treatment and disease progression. Neurology, 41:380-384, 1991.
- Pfeiffer R. Optimization of levodopa therapy. Neurology, 42(suppl 1):39-43, 1992.
- Ferreira JJ, Rascol O. Prevention and therapeutic strategies for levodopa-induced dyskinesias in Parkinson's disease. Curr Opin Neurol, 13:431-436, 2000.
- Stein WM, Read S. Chronic pain in the setting of Parkinson's disease and depression. J Pain Symptom Manage, 14:255-258, 1997.
- Deleu D, Northway MG, Hanssens Y. Clinical pharmacokinetic and pharmacodynamic properties of drugs used in the treatment of Parkinson's disease Clin Pharmacokinet, 41:261-309, 2002.
- Friis ML, Gron U, Larsen NE, Pakkenberg H, Hvidberg EF. Pharmacokinetics of bromocriptine during continuous oral treatment of Parkinson's disease. Eur J Clin Pharmacol, 15:275-280, 1979.
- Fariello RG. Pharmacodynamic and pharmacokinetic features of cabergoline. Rationale for use in Parkinson's disease. Drugs, 55 Suppl 1:10-16, 1998.
- Wachtel H. Antiparkinsonian dopamine agonists: a review of the pharmacokinetics and neuropharmacology in animals and humans. J Neural Transm Park Dis Dement Sect, 3:151-201, 1991.
- Wright CE, Sisson TL, Ichhpurani AK, Peters GR. Steady-state pharmacokinetic properties of prampexole in healthy volunteers. J Clin Pharmacol, 37:520-525, 1997.
- Hubble J, Koller WC, Atchison P, Taylor AC, Citerone DR, Zussman BD, Friedman CJ, Hawker N. Linear pharmacokinetic behavior of ropinirole during multiple dosing in patients with Parkinson's disease. J Clin Pharmacol, 40:641-646, 2000.
- Ramji JV, Keogh JP, Blake TJ, Broom C, Chenery RJ, Citerone DR, Lewis VA, Taylor AC, Yeulet SE. Disposition of ropinirole in animals and man. Xenobiotica, 29:311-325, 1999.
- Kaye CM, Nicholls B. Clinical pharmacokinetics of ropinirole. Clin Pharmacokinet, 39:243-254, 2000.
- Miscellaneous. Antiparkinson Agents. In: Drug Facts and Comparison. Wolters Kluwer Publishers, St. Loius, MO, pp1083-1099, 2001.
- Standaert DG, Young AB. Treatment of central nervous system degenerative disorders. In: , Hardman JG, L.E. Limbird, AG Gilman, (eds), Goodman & Gilman's The pharmacological basis of therapeutics, 3rd edition, McGraw-Hill publishers, New York, New York, pp549-568, 2001.
- Robertson DRC, Renwick AG, Wood ND, Cross N, Macklin BS, Fleming JS, Waller DG, George CF. The influence of levodopa on gastric emptying in man. Br. J Clin Pharmacol, 29: 47-53, 1990.
- Gasser UE, Crevoisier C, Ouwerkerk M, Lankhaar G, Dingemanse J. Comparative single- and multiple-dose pharmacokinetics of levodopa and 3-O-methyldopa following a new dual-release and a conventional slow-release formulation of levodopa and benserazide in healthy subjects. Eur J Pharm Biopharm, 46:223-228, 1998.
- Harder S, Baas H. Concentration-response relationship of levodopa in patients at different stages of Parkinson's disease. Clin Pharmacol Ther, 64; 183-191, 1998.
- Deleu D, Sarre S, Herregodts P, Ebinger G, Michotte Y. Continuous intravenous monitoring of levodopa and 3-O-methyldopa by microdialysis and high-performance liquid chromatography with electrochemical detection. J. Pharm Biomed Anal, 9:159-165, 1991.
- Titus DC, August TF, Yeh KC, Eisenhandler R, Bayne WF, Musson DG. Simultaneous high-performance liquid chromatographic analysis of carbidopa, levodopa and 3-O-methyldopa in plasma and carbidopa, levodopa and dopamine in urine using electrochemical detection. J Chromatogr, 534:87-100, 1990.
- Blandini F, Martignoni E, Pacchetti C, Desideri S, Rivellini D, Nappi G. Simultaneous determination of L-dopa and 3-O-methyldopa in human platelets and plasma using high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Sci Appl, 700:278-282, 1997.
- Rondelli I, Acerbi D, Mariotti F, Ventura P. Simultaneous determination of levodopa methyl ester, levodopa, 3-O-methyldopa and dopamine in plasma by high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Appl, 653:17-23, 1994.
- Frayn KN, Maycock PF. Sample preparation with ion-exchange resin before liquid-chromatographic determination of plasma catecholamines. Clin Chem, 29:1426-1428, 1983.
- Zurcher G, Da Prada M. Simple automated high-performance liquid chromatographic column-switching technique for the measurement of dopa and 3-O-methyldopa in plasma. J Chromatogr, 530: 253-262, 1990.
- Michotte T, Deleu D, Ebinger G. The use of capillary gas chromatography/mass spectromertyfor the determination of acidic dopamine metabolites in human brain. Biomed Mass Spect, 12:704-706, 1985.
- American Society of Health-System Pharmacists Inc. Miscellaneous central nervous system agents. In: AHFS Drug information, American Society of Health System Pharmacists Publishers, Bethesda, MD, pp 2377-2412, 2001.
- Nutt JG, Fellman JH. Pharmacokinetics of Levodopa. Clin Neuropharmacol, 7:35-49, 1984.
- Eriksson T, Granerus A, Linde A, Carlsson A. "On-off' phenomenon in Parkinson's disease: relationship between dopa and other large neutral amino acids in plasma. Neurology, 38: 1245-1248, 1988.
- Tolosa ES, Martin WE, Cohen HP, Jacobson RL. Patterns of clinical response and plasma DOPA levels in Parkinson's disease. Neurology, 177-183, 1975.
- Abrams WB, Coutinho CB, Leon AS, Spiegel HE. Absorption and metabolism of levodopa. JAMA, 218: 1912-1914, 1971.
- Evans MA, Triggs, EJ Broe GA, Saines N. Systemic availability of orally administered L-Dopa in the elderly parkinsonian patient. Eur J. Clin Pharmacol, 17:215-221, 1980.
- Robertson DR, Wood ND, Everest H, Monk K, Waller DG, Renwick AG, George CF. The effect of age on the pharmacokinetics of levodopa administered alone and in the presence of carbidopa. Br. J. Clin Pharmacol, 28: 61-69, 1989.
- Pardridge WM. Kinetics of competitive inhibition of neutral amino acid transport across the blood brain barrier. J Neurochem, 28:103-108, 1977.
- Djaldetti R, Rosmarin V, Ziv I, Melamed E. The pharmacokinetic profile of the: first ever" oral dose of levodopa in de novo patients with Parkinson's disease. Clin Neuropharmacol, 24:95-98, 2001.
- Djaldetti R, Koren M, Ziv I, Chiron A, Melamed E. Effect of cisapride on response fluctuations in Parkinson's disease. Mov Disord, 10:81-84, 1995.
- Schindler JS, Finnerty GT, Towlson K, Dolan AL, Davies CL, Parkes JD. Domperidone and levodopa in Parkinson's disease. Br J Pharmac, 19:959-962, 1984.
- Andersson I, Granerus AK, Jagenburg R, Svanborg A. Intestinal decarboxylation of orally administered L-DOPA. Acta Med Scand, 198:415-420, 1975.
- Bianchine JR, Calimlim LR, Morgan JP, Dujovne CA, Lasagna L. Metabolism and absorption of L-3,4 dihydrophenylalanine in patients with Parkinson's disease. Ann NY Acad Sci, 179:126-140, 1971.
- Mearrick PT, Wade DN, Birkett DJ, Morris J. Metoclopramide, gastric emptying and the L-DOPA absorption. Aust NZ J Med, 4:144-148, 1974.
- Kurlan R, Nutt JG, Woodward WR, Rothfield K, Lichter D, Miller C, Carter JH, Shoulson I. Duodenal and gastric delivery of levodopa in Parkinsonism. Ann Neurol, 23:589-595, 1988.
- Rivera-Calimlim L, Dujovne CA, Morgan JP, Lasagna L, Bianchine JR. L-DOPA treatment failure: explanation and correction. Br Med J., 4: 93-94, 1970.
- Landsberg L, Berardino MB, Silva P. Metabolism of 3H-L-DOPA by the rat gut in vivo-evidence for glucuronide conjugation. Biochem Pharmacol, 1167-1174, 1975.
- Tyce GM, Owen CA. Administration of L-3,4-dihydroxyphenylalanine to rats after complete hepatectomy I: metabolites in tissues. Biochem Pharmacol 1979; 28:3271-3278.
- BonifatiV, Meco G. New, selective catechol-O-methyltransferase inhibitors as therapeutic agents in Parkinson's disease. Pharmacol Ther, 81: 1-36, 1999.
- Roberts JW, Cora-Locatelli G, Bravi D Amantea MA, Mouradian MM, Chase TN. Catechol-O-methyltransferase inhibitor tolcapone prolongs levodopa/carbidopa action in Parkinson's patients. Neurology, 43: 2685-2688, 1993.
- Poewe HW, Wenning GK. The natural history of Parkinson's disease. Neurology, 47(suppl 3):S146-S52, 1996.
- Sage JI, Mark MH. Basic mechanisms of motor fluctuations. Neurology, 44(suppl 6):S10-S14, 1994.
- Marooned, C. D. and Parkas, J. D. Success and problems of long -term levodopa therapy in Parkinson's disease. Lancet, 1: 345-349, 1977.
- Fahn, S., and Calne, D. B. Considerations in the management of Parkinsonism. Neurology (NY), 28: 5-7, 1978.
- Murata M, Kanazawa I. Effects of chronic levodopa therapy on dopa pharmacokinetics. Eur Neurol, 38(suppl 2):50-55, 1997.
- McDowell, F. H. and Sweet, R. D. The on-off phenomenon, in Birkmayer, W., Hornykiewics, O., (eds.), Advances in Parkinsonism. Editiones Roche., Basel, pp 603-612, 1976.
- Ludin, H. P. Bass-Varrey, F. Study of deterioration in long term treatment of parkinsonism with L-dopa plus decarboxylase inhibitor. J. Neural. Transm. , 38:249-258, 1976.
- Rajput AH, Fenton ME, Birdi S, Macaulay R, George D, Rozdilsky B, Ang LC, Senthilselvan A, Hornykiewicz O. Clinical-pathological study of levodopa complications. Mov. Disord, 17: 289-296, 2002.
- Stocchi F, Bonamartini A, Vacca L, Ruggieri S. Motor fluctuations in levodopa treatment: clinical pharmacology. Eur Neurol, 36(suppl 1):38-42, 1996.
- De La Fuente-Fernandez R, Pal PK, Vingerhoets FJ, Kishore A, Schulzer M, Mak EK, Ruth TJ, Snow BJ, Calne DB, Stoessl AJ. Evidence for impaired presynaptic dopamine function in parkinsonian patients with motor fluctuation. J Neural Transm., 107:49-57, 2000.
- Obeso JA, Granadas F, Vaemorde J. Motor complications associated with chronic levodopa therapy in Parkinson's disease. Neurology, 11 (suppl1):11-18, 1989.
- Rinne UK, Lonnberg P, Koskinen V. Dopamine receptor changes in Parkinson's disease. J. Neural Transm., 51:97-106, 1981.
- Marsden CD, Parkes JD, Quinn N. Fluctuations of disability in Parkinson's disease: clinical aspects. In Marsden CD, S. Fahn eds. Movement disorders., Butterworth, Cornwall, UK: pp 96-122, 1981.
- Hardie RJ, Lees AJ, Stern GM. On-off fluctuations in Parkinson's disease: a clinical & neuropharmacological study. Brain, 107:487-506, 1984.
- Fabbrini G, Mouradian MM, Juncos JL, Schlegel J, Mohr E, Chase TN. Motor fluctuations in Parkinson's disease: central pathophysiologic mechanisms. Part I. Ann Neurol, 24:366-371, 1988.
- Nutt JG. Levodopa-induced dyskinesia: review, observations, and speculations. Neurology, 40:340-345, 1989.
- Lyytinen J, Kaakkola S, Ahtila S, Tuomainen P and Teravainen Heikki. Simultaneous MAO-B and COMT inhibition in L-Dopa-treated patients with Parkinson's disease. Movement Disorders, 12:497-505, 1997.
- Heikkinen H, Nutt JG, LeWitt PA, Koller WC, Gordin A. The effects of different repeated doses of entacapone on the pharmacokinetics of L-dopa and on the clinical response to L-dopa in Parkinson's disease. Clin Neuropharmacol, 24:150-157, 2001.
- Rinne UK, Sonninen V, Siirtola T. The plasma concentration of levodopa in patients with Parkinson's disease. Eur Neurol, 10:301-310, 1973.
- Pilling JG, Baker J, Iverson LL, Iverson SD, Robbins T. Plasma concentration of L-DOPA and 3-methoxydopa and improvement in clinical ratings and motor performance in patients with parkinsonism treated with L-DOPA alone or in combination with amantidine. J Neurol Neurosurg Psychiatry, 38:129-135, 1975.
- Hare TA, Beasely BL, Chambers RA, Boehme DH, Vogel WH. DOPA and amino acid levels in plasma and cerebrospinal fluid of patients with Parkinson's disease before and during treatment with L-DOPA. Clin Chim Acta, 45: 273-280, 1973.
- Gancher ST, Nutt JG, Woodward WR. Peripheral pharmacokinetics of levodopa in untreated, stable, and fluctuating Parkinsonian patients. Neurology, 37:940-944, 1987.
- Rainero I, Gilli M, DeGennaro T, Chiado I, Delsedime M, Riccio A, Bergamasco B. Peripheral pharmacokinetic parameters of levodopa/carbidopa and the on-off phenomenon in parkinsonian patients. Ital J Neurol Sci., 9: 225-259, 1988.
- Kurth MC, Tetrud JW, Irwin I, Lyness WH, Langston JW. Oral levodopa/carbidopa solution versus tablets in Parkinson's patients with severe fluctuations: a pilot study. Neurology, 43:1036-1039, 1993.
- Shoulson I, Glaubiger GA, Chase TN. On-off response: clinical and biochemical correlations during oral and intravenous levodopa administration in Parkinsonian patients. Neurology, 25:1144-1148, 1975.
- Rossor MN, Watkins J, Brown MJ, Reid JL, Dollery CT. Plasma levodopa, dopamine and therapeutic response following levodopa therapy of parkinsonian patients. J Neurol Sci, 46:385-392, 1980.
- Nutt JG, Woodward WR, Hammerstad JP. The "On-off" phenomenon in Parkinson's disease: Relation to levodopa absorption and transport. N Engl J Med., 310: 4830488, 1984.
- Sage JI, Mark MH, McHale DM, Sonsalla PK, Vitagliano D. Benefits of monitoring plasma levodopa in Parkinson's disease patients with drug-induced chorea. Ann Neurol, 29:623-628, 1991.
- Troconiz IF, Naukkarinen TH, Ruottinene HM, Rinne UK, Gordin A, Karlsson MO. Population pharmacodynamic modeling of levodopa in patients with Parkinson's disease receiving entacapone. Clin Pharmacol Ther, 64: 106-116, 1998.
- Okereke CS, Pratt R, Cullen E, Kirby L, Kumar D, Hane W. A safety and pharmacokinetic study of Aricept ® and Sinemet in Parkinson's disease patients. Unpublished data, 2000.
- Kurth MC, Tetrud JW, Tanner CM, Irwin I, Stebbins GT, Goetz CG, Langston JW. Double-blind, placebo-controlled, crossover study of duodenal infusion of levodopa/carbidopa in Parkinson's disease patients with 'on-off' fluctuations. Neurology, 43:1698-1703, 1993.
- Sage JI, Shuh L, Heikkila RE, Duovisin RC. Continuous duodenal infusions of levodopa: plasma concentrations and motor fluctuations in Parkinson's disease. Clin Neuropharmacol, 11:36-44, 1988.
- Quinn N, Parkes JD, Marsden CD. Control of on/off phenomenon by continuous intravenous infusion of levodopa. Neurology, 34: 1131-1136, 1984.
- Kurlan R, Rubin AJ, Miller C, Rivera-Calimlim L, Clarke A, Shoulson I. Duodenal delivery of levodopa for on-off fluctuations in Parkinsonism. Ann Neurol, 20:262-265, 1986.
- Kurlan R, Rothfield KP, Woodward WR, Nutt JG, Miller C, Lichter D, Shoulson I. Erratic gastric emptying of levodopa may cause "random" fluctuations of parkinsonian mobility. Neurology, 38: 419-421, 1988.
- Deleu D, Ebinger G, Michotte Y. Clinical and Pharmacokinetic comparison of oral duodenal delivery of levodopa /carbidopa in patients with Parkinson's disease with a fluctuating response to levodopa. Eur J Clin Pharmacol, 41: 453-458, 1991.
- McHale DM, Sage JI, Sonsalla PK, Heikkila RE, Duvoisin RC. Steady plasma levodopa concentrations required for good clinical response to CR_4 in patients with "on-off". Eur Neurol, 30: 90-92, 1990.
- Pahwa R, Lyons K, Mcguire D, Silverstein P, Zwiebel F, Robischon M, Koller WC. Comparison of standard carbidopa-levodopa and sustained-release carbidopa-levodopa in Parkinson's disease: pharmacokinetic and quality-of-life measures. Mov. Disord, 12:677-681, 1997.
- Fabrini G, Juncos J, Moradian MM: Levodopa pharmacokinetic mechanisms and motor fluctuations in Parkinson's disease. Neurology, 21: 370-376, 1987.
- Cedarbaum JM, Silvestri M, Clark M, Harts A, Kutt H. L-Deprenyl, levodopa Pharmacokinetics, and response fluctuations in Parkinson's disease. Clin Neuropharmacol, 13:29-35, 1990.
- Contin M, Riva R, Martinelli P, Cortelli P, Albani F, Baruzzi A. Pharmacodynamic modeling of oral levodopa: clinical application in Parkinson's disease. Neurology, 43:367-371, 1993.
- Dietz M, Harder S, Graff J, Kung G, Vontobel P, Leenders KL, Baas H. Levodopa pharmacokinetics-pharmacodynamic modeling and 6-[18F]levodopa positron emission tomography in patients with Parkinson's disease. Clin Pharmacol Ther, 70:33-41, 2001.
- Rinne UK, Molsa P. Levodopa with benserazide or carbidopa in Parkinson's disease. Neurology, 29:1584-1589, 1979.
- Djaldetti R, Ziv I, Melamed E. Impaired absorption of oral levodopa: a major cause for response fluctuation in Parkinson's disease. Isr J Med Sci, 32: 1224-1227, 1996.
- Merelo M, Lees AJ, Webster R, Bovington M, Gordin A. Effect of entacapone, a peripherally acting catechol-O-methyl transferase inhibitor, on the motor response to acute treatment with levodopa in patients with Parkinson's disease. J Neurol Neurosurg, 57: 186-189, 1994.
- Ruottinen H, Rinne UK. Effect of one month's treatment with a peripherally-acting catechol-O-methyltransferase inhibitor, entacapone on pharmacokinetics and motor response to levodopa in advanced parkinsonian patients. Clin Neuropharmacol, 19: 222-233, 1996.
- Baas H, Beiske AG, Ghika J, Jackson M, Oertel WH, Poewe W, Ransmayr G. Catechol-O-methyltransferase inhibition with tolcapone reduces the "wearing off" phenomenon and levodopa requirements in fluctuating parkinsonian patients. Neurol Neurosurg Psychiatry, 63: 421-428, 1997.
- Jorga KM, Sedek G, Fotteler B, Zurcher G, Nielsen T, Aitken JW. Optimizing levodopa pharmacokinetics with multiple tolcapone doses in the elderly. Clin Pharmacol Ther, 62: 300-310, 1997.
- Olanow CW, Gauger LL, Cederbaum JM. Temporal relationships between plasma and cerebrospinal fluid pharmacokinetics of levodopa and clinical effects in Parkinson's disease. Ann Neurol, 29:556-559, 1991.
- Mouradian MM, Juncos JL, Fabrini G, Schegel J, Bartko JJ, Chase TN. Motor fluctuations in Parkinson's disease: central pathophysiological mechanisms: II. Ann Neurol, 24:372-378, 1988.
- Wade LN, Katzman R. 3-O-Methyldopa inhibition of L-dopa at the blood-brain barrier. Life Sci, 17:131-136, 1975.
- Reches A, Mielke LR, Fahn S. 3-O-Methyldopa inhibits rotations induced by levodopa in rats after unilateral destruction of the nigrostriatal pathway. Neurology, 32:887-888, 1982.
- Kempster PA, Frankel JP, Bovingdon M, Webster R, Lees AJ, Stern GM. Levodopa peripheral pharmacokinetics and duration of motor response in Parkinson's disease. J Neurol Neurosurg Psychiatry, 52:718-723, 1989.
- Mouradian M, Chase TN. Central mechanisms and levodopa response fluctuations in Parkinson's disease. Clin. Neuropharmacol, 11:378-385, 1988.
- Nutt JG. On-off phenomenon: relation to levodopa pharmacokinetics and pharmacodynamics. Ann Neurol, 22: 535-540, 1987.
- Contin M, Riva R, Albani F, Baruzzi A. Pharmacokinetic optimization in the treatment of Parkinson's disease. Clin Pharmacokinet; 30:463-481, 1996.
- Montgomery EB. Pharmacokinetics and Pharmacodynamics of levodopa. Neurology, 42(1 Suppl1): 17-22, 1992.
- Baas H, Harder S, Demisch L, Burklin F, Stecher K, Fischer PA. Fluctuations in Parkinson's disease. Pathogenic significance of levodopa's cerebral pharmacokinetics and pharmacodynamics. J Neural Transm Suppl, 46: 367-379, 1995.
- Piccini P, Del Dotto P, Pardini C, D'Antonio P, Rossi G, Bonuccelli U. Diurnal worsening in Parkinson patients treated with levodopa. Riv Neurol, 61: 219-224, 1991.
- Sweet RD, Lee JD, Spiegel HE, McDowell F. Enhanced response to low doses of levodopa after withdrawal from treatment. Neurology, 22: 520-525, 1972.
- Mayeux R, Stern Y, Mulvey K, Cote L. Re-appraisal of temporary levodopa withdrawal ("drug holiday") in Parkinson's disease. N Engl J Med, 313: 724-728, 1985.
- Levy G. Predicting effective drug concentrations for individual patients. Determinants of pharmacodynamic variability. Clin Pharmacokinet, 34: 323-333, 1998.
- Torres-Lugo M, Peppas NA. Transdermal delivery systems for calcitonin: A review. Biomaterials, 21:1191-1196, 2000.
- Hutton JT, Metman LV, Chase TN, Juncos JL, Koller WC, Pahwa R, LeWitt PA, Samii A, Tsui JK, Calne DB, Waters CH, Calabrese VP, Bennett JP, Barrett R, Morris JL. Transdermal dopaminergic D (2) receptor agonist therapy in Parkinson's disease with N-0923 TDS: A double -blind, placebo -controlled study. Mov Disord , 16:459-463, 2001
- LeWitt PA, Albanese A. New delivery systems for antiparkinsonian drugs. Adv Neurol, 80:549-554, 1999.
- Kao HD, Traboulsi A, Itoh S, Ditter L, Hussain A. Enhancement of the systemic and CNS specific delivery of L-dopa by the nasal administration of its water soluble prodrugs. Pharm Res, 17: 978-984, 2000.
- Cooper SD, Ismail HA, Frank C. Case report: Successful use of rectally administered levodpa-carbidopa. Can Fam Physician, 47: 112-113, 2001.
- Brime B, Ballesteros MP, Frutos P. Preparation and in vitro characterization of gelatin microspheres containing levodopa for nasal administration. J Microencapsul, 17: 777-784, 2000.
- Stahl SM. Applications o f new drug delivery technologies to Parkinson's disease and dopaminergic agents. J Neural Transm Suppl, 27: 123-132, 1988.
- Metman LV, Hoff J, Mouradian MM, Chase TN. Fluctuations in plasma levodopa and motor responses with liquid and tablet levodopa/carbidopa. Mov Disord, 9: 463-465, 1994.
- Allan L, Hays H, Jensen N, et al. Randomised crossover trial of transdermal fentanyl and sustained release oral morphine for treating chronic non-cancer pain. BMJ, 322: 1-7, 2001.
- Cacciatore B, Paakkaari I, Hasselblatt R, Nieminen MS, Toivonen J, Tikkanen MI, Ylikorkala O. Randomized comparison between orally and transdermally administered hormone replacement therapy regimens of long-term effects on 24-hour ambulatory blood pressure in postmenopausal women. Am J Obstet Gynecol, 184: 904-909, 2001.
- Ramachandran C, Fleisher D. Transdermal delivery of drugs for the treatment of bone diseases. Adv Drug Deliv Rev, 31: 197-223, 2000.
- Wang C, Swedloff RS, Iranmanesh A, Dobs A, Snyder PJ, Cunningham G, Matsumoto AM, Weber T, Berman N. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. Testosterone gel Study Group. J Clin Endocrinol Metab, 85: 2839-2853, 2000.
- Chen FP, Lee N, Soong YK, Huang KE. Comparison of transdermal and oral estrogen-progestin replacement therapy: effects on cardiovascular risk factors. Menopause, 8: 347-352, 2001.
- Sudo J, Iwase H, Terui J, Kakuno K, Soyama M, Takayama K, Nagai T.
Transdermal absorption of L-dopa from hydrogel in rats. Eur J Pharm Sci, 7: 67-71, 1998.- Djaldetti R, Atlas D, Melamed E. Effect of subcutaneuos administration of levodopa ethyl ester, a soluble prodrug of levodopa, on dopamine metabolism in rodent striatum: implication for treatment of Parkinson's disease. Clin Neuropharmacol, 19: 65-71, 1996.
- Silva MA, Mattern C, Hacker R, Tomaz C, Houston JP, Schwarting RK. Increased neostriatal dopamine activity after intraperitoneal or intranasal administration of L-dopa: on the role benserazide pretreatment. Synapse, 27: 294-302, 1997.
- Illum, L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci, 11: 1-18, 2000.
- Kern W, Born J, Schreiber H, Fehm HL. Central nervous effects of intranasally administered insulin during euglycemia in men. Diabetes, 48: 557-563, 1999.
- Pontiroli AE, Calderara A, Perfetti MG, Bareggi SR. Pharmacokinetics of intranasal, intramuscular and intravenous glucagon in healthy subjects and diabetic patients. Eur J Clin Pharmacol, 45: 555-558, 1993.
- Luce BR, Zangwill KM, Palmer CS, Mendelman PM, Yan L, Wolff MC, Cho I, Marcy SM, Iacuzio D, Belshe RB. Cost effectiveness of analysis of an intranasal influenza vaccine for the prevention of influenza in healthy children. Pediatrics, 108: E24, 2001.
- Lalej-Bennis D, Boillot J, Bardin C, Zirinis P, Coste A, Escudier E, Chast F, Peynegre R, Slama G, Selama JL. Efficacy and tolerance of intranasal insulin administered during 4 months in severely hyperglycemic Type 2 diabetic patients with oral drug failure: a crossover study. Diabet Med, 18: 614-618, 2001.
- Montgomery EB. Pharmacokinetics and Pharmacodynamics of levodopa. Neurology, 42(1 Suppl1): 17-22, 1992.
- Gottwald MD. Maximizing the benefit: risk ratio of levodopa therapy in Parkinson's disease. Pharmacotherapy, 19(11 Pt 2): 162S-168S, 1999.
- O'Connell MT, Tison F, Quinn NP, Patsalos PN. Clinical drug monitoring by microdialysis: applications to levodopa therapy in Parkinson's disease. Br. J. Clin Pharmacol, 42: 765-769, 1996.
- Tohgi H, Abe T, Saheki M, Yamazaki K, Murata T. Concentration of catecholamines and indoleamines in the cerebrospinal fluid of patients with vascular Parkinsonism compared to Parkinson's disease patients. J Neural Transm, 104: 441-449, 1997.
- Kroll RA, Neuwalt EA. Outwitting the blood-brain barrier for therapeutic purposes: osmotic opening and other means. Neurosurg, 42: 1083-1099, 1998.
- Daniel PM, Moorhouse Sr, Pratt OE. Do changes in blood levels of other aromatic amino acids influence levodopa therapy? Lancet, 1: 95, 1976.
- Markovitz DC, Fernstrom JD. Diet and uptake of aldomet by the brain: competition with natural large neutral amino acids. Science, 197: 1014-1015, 1977.
- Fernstrom JD. Diet-induced changes in plasma amino acid pattern: effects on the brain uptake of large neutral amino acids, and on brain serotonin synthesis. J Neural Transm Suppl, 15: 55-67, 1979.
- Fernstrom JD, Faller DV. Neutral amino acids in the brain: changes in response to food ingestion. J Neurochem, 30: 1531-1538, 1978.
- Pincus JH, Bary K. Control of the "On-off" Phenomenon with dietary manipulation. Ann Neurol, 20: 149-150, 1986.
- Murata M, Mitzusawa H, Yamanouchi H, Kanazawa I. Chronic levodopa therapy enhances dopa absorption: contribution to wearing-off. J Neural Transm, 103: 1177-1185, 1996.
- Furlanut M, Furlanut Jr. M, Bentello P. Monitoring of L-dopa concentrations in Parkinson's disease. Pharmacol Res, 43: 423-427, 2001.
- Wu G, Furlanut M. Pharmacodynamic modeling of levodopa, 3-O-methyldopa and their effects: An application of the Dixon equation. Pharmacol Res, 33: 203-210, 1999.
Corresponding Author: Chukwuemeka S. Okereke, Department of Clinical Pharmacology, Clinical Research and Development, Eisai Inc., Glenpointe Centre West, Teaneck, New Jersey, USA. Chuk_Okereke@eisai.com
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps