J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 5(2):135-145, 2002
A novel hplc assay for pentamidine: comparative effects of creatinine and inulin on gfr estimation and pentamidine renal excretion in the isolated perfused rat kidney
Nagaraju R. Poola, Dider Bhuiyan, Stephan Ortiz, Ishani A. Savant, Madiha Sidhom and David R. Taft1
Division of Pharmaceutics and Industrial Pharmacy, Long Island University, Brooklyn, New York, USAHarold Kirschenbaum
Division of Pharmacy Practice, Long Island University, Brooklyn, New York, USAMichelle Kalis
Massachusetts College of Pharmacy and Health Sciences, Boston, Massachusetts, USAReceived March 14, 2002, Revised May 28, 2002, Accepted June 5, 2002
PDF version
Abstract
PURPOSE . 1. To develop and validate an analytical method for pentamidine (PTM) by reversed-phase HPLC. 2. To compare the effects of creatinine and inulin on PTM excretion in the isolated perfused rat kidney.
METHODS . The HPLC method utilized a base deactivated, 5 μ , C18 column and a mobile phase containing acetonitrile (24%) and 0.025M monobasic phosphate buffer, pH 3.2 (76%). Mobile phase flow rate and UV detection wavelength were 1mL/min and 270 nm, respectively. Sulfadiazine (SDZ) was used as the internal standard. The method was used to measure pentamidine in perfusate and urine samples generated from studies with the isolated perfused rat kidney (IPK) model. Perfusion experiments were conducted in the presence of two different GFR markers: creatinine and inulin (PTM dose 800 μ g). Both creatinine and inulin were assayed using colorimetric methods.
RESULTS . The HPLC assay is rapid, sensitive and reproducible. The method was validated over two standard concentration ranges: 0.1 to 1 μ g/mL, and 1 to 10 μ g/mL. In control (drug-naïve) IPK perfusions, creatinine clearance was approximately 15% greater than inulin clearance (0.80 ± 0.21 mL/min vs. 0.69 ± 0.17 mL/min, p > 0.05). In the presence of PTM, however, creatinine clearance was reduced to 0.56 ± 0.27 (p < 0.05 compared to control). Inulin clearance was not altered by PTM administration (0.76 ± 0.26 mL/min). Cumulative urinary excretion of PTM (% dose) was 3.0 ± 0.47% and 9.6 ± 4.2% in the presence of creatinine and inulin, respectively. PTM clearance was significantly reduced (0.06 ± 0.01 mL/min vs. 0.13 ± 0.01 mL/min, p < 0.05) and % kidney accumulation significantly enhanced (66 ± 4.7% vs. 37 ± 9.7%, p < 0.05) by creatinine.
CONCLUSIONS . Creatinine overestimated GFR in the IPK. The altered renal excretion of PTM by creatinine is consistent with inhibition of PTM tubular secretion. Because of increased kidney accumulation, detrimental effects of PTM on renal function were observed. Based on these findings, creatinine should be used cautiously as an indicator of GFR in IPK experimentation.
Introduction
Pentamidine (PTM), an aromatic diamidine, is an antiprotozoal agent active against a number of organisms including Cryptosporidium (1), Giardia lamblia (2), and Toxoplasma gondii (3). Clinically, PTM is used in the treatment of African trypanosomiasis (4), antimony resistant leishmaniais (5), and Pneumocystis carinii pneumonia (PCP) (6), an opportunistic infection in patients with Acquired Immune Deficiency Syndrome (AIDS). However, the use of this medication is limited by side effects, the most severe being nephrotoxicity (7). In a 1991 retrospective study of 37 patients receiving PTM, two patients developed nephrotoxicity that was directly attributed to the drug (8). Presently, the mechanism of PTM-nephrotoxicity is unknown. Although renal excretion is a minor route of PTM elimination, accumulation of the drug within the kidney has been suggested, analogous to aminoglycoside-induced renal toxicity (9).
A number of different PTM assays have been reported including several high-performance liquid chromatography (HPLC) assays for the detection of PTM in plasma, serum, and urine (10-17). Berger et al. summarized the problems and pitfalls with these reported methods (18). Among the drawbacks of the methods is the need for a large sample volume that is not obtainable in smaller species such as rodents. Moreover, the mobile phase of many of the reported methods contained one or more ion-pairing agents. Several assays involve fluorescent detection that is normally not recommended for routine clinical analysis. In addition, most reported assays utilized hexamidine as an internal standard. Hexamidine is not available commercially.
In this report, an alternative HLPC assay for PTM is presented. The method is rapid, sensitive, and reproducible. The assay was used to support ongoing drug excretion studies in the isolated perfused rat kidney (IPK). The IPK has been utilized in a number of drug disposition studies over the past two decades and investigators have demonstrated a correlation between IPK results and human data (19-22). Thus, the IPK represents a useful model to study the renal excretion of pentamidine. In a series of experiments, the reliability of creatinine and inulin as GFR markers was determined. Creatinine is the most commonly used indicator of GFR in the clinical setting, primarily because it is a more convenient and does not require invasive tactics as does inulin. Likewise, creatinine has been widely used in IPK investigations. Nevertheless, the accuracy of creatinine clearance as an estimation of GFR is compromised by a reported secretory component to creatinine excretion (23). Furthermore, assuming that the creatinine is indeed secreted, an additional aim of the current study was to assess a possible interaction between creatinine, a cation, and PTM in the kidney.
Experimental
Chemicals
Bovine serum albumin (Fraction V), dextran (clinical grade, molecular weight range 60,000-90,000 Da), inulin (from Chicory root), amino acids, potassium chloride, sodium chloride, sodium bicarbonate, magnesium sulfate, calcium chloride, glucose, pentamidine isethionate, sulfadiazine, and diagnostic test kits for glucose (Kit #315) and creatinine (Kit #555-A) were purchased from Sigma Chemical Company (St. Louis, MO, USA). Acetonitrile (HPLC Grade), methylene chloride, and anthrone were purchased from EM Science (Gibbstown, NJ, USA). Potassium phosphate (monobasic) and phosphoric acid were purchased from J. T. Baker (Phillipsburg, NJ, USA). HPLC grade water was available in the laboratory.
Chromatography
Instrumentation
The HPLC system consisted of a system controller (SCL-6B, Shimadzu Scientific Instruments, Columbia, MD), an auto injector (SIL-6B, Shimadzu Scientific Instruments), and a scanning ultraviolet detector (SPD-10AV UV-Vis, Shimadzu Scientific Instruments). The output was processed using a Hewlett- Packard personal computer with Turbochrome integration software (version 4.0, Perkin Elmer Instruments, Norwalk, CT, USA) and with a PE Nelson 900 series interface.
Chromatographic Conditions
The chromatographic separation of PTM was performed isocratically using a base deactivated reversed-phase C18 column (250 x 2.1 mm, Alltima, 5m, Alltech Associates, Deerfield, IL, USA). The mobile phase consisted of 76% 0.025M monobasic potassium phosphate solution (pH adjusted to 3.2 with dilute phosphoric acid) and 24% acetonitrile. The flow-rate was maintained at 1 mL/min with an operating pressure of 150 kgf/cm 2 . The UV spectra of the eluting peaks were determined at a wavelength of 270 nm ( l max for PTM). The injection volume was 100mL. The assay was conducted at ambient temperature (25°C).
Aliquots of an aqueous stock solution of PTM were added to matrix (IPK perfusate or Krebs Henseleit (KHS) buffer) to generate standard samples. KHS buffer was used for analysis of urine samples generated from perfusion studies. The composition of each matrix is provided below (see IPK experiments). Two ranges of standard concentrations were utilized: 0.1-1mg/mL (0.1, 0.25, 0.5, 0.75, and 1mg/mL) and 1-10mg/mL (1, 2.5, 5, 7.5, and 10mg/mL). Check samples representing two concentrations within each standard range were prepared prior to assay validation. For the lower concentration range, check samples of 0.2mg/mL and 0.8 μ g/mL were utilized, and concentrations of 2mg/mL and 8mg/mL were used for the upper standard range.
Sample extraction procedure
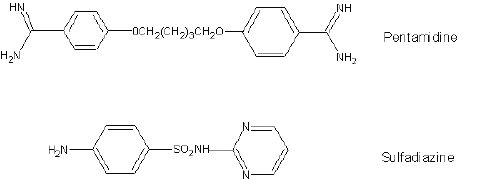
Fresh perfusate and KHS buffer was made before each experiment. The required amount of PTM stock solution was pipetted into a 1.5 mL microcentrifuge tube and the calculated amount of matrix (perfusate or KHS buffer) was added to produce a sample volume of 250mL. A 25 mL internal standard solution [25mg/mL of sulfadiazine (SDZ) stock solution] was added and the sample was vortex-mixed for 1 minute. A 250mL solution of 1% (w/v) of zinc sulfate was added to the microcentrifuge tube, vortex-mixed for 1 minute, and centrifuged for 10 minutes at 8000 x g. The layer of protein was easily separated at the bottom and 100mL of the clear supernatant was used for analysis. Chemical structures of PTM and SDZ are provided in Figure 1.
Figure 1: Chemical structures of pentamidine (analyte) and sulfadiazine (internal standard).
Assay Validation
The method was validated in accordance with United States Pharmacopoeia (USP 23) <1225> guidelines. The parameters essential to ensure the acceptability of the performance of the analytical method included linearity and range, precision (method precision and system precision), accuracy or recovery, and specificity. Limits of detection and quantitation were determined. Stability studies were also conducted. Each of these tests is described below.
Standard curve and linearity
Two PTM concentration ranges were studied in both perfusate and KHS buffer: 0.1-1 μ g/mL and 1-10mg/mL. Each sample was analyzed in triplicate and the mean relative standard deviation (RSD) of the peak area ratio (PTM: SDZ) was determined. Standard curves were constructed for each concentration range by fitting a regression line to the test results (peak area ratio vs. analyte concentration) using the method of least squares. Blank (drug-naïve) samples were analyzed to demonstrate lack of interfering peaks in the matrix.
Precision and accuracy
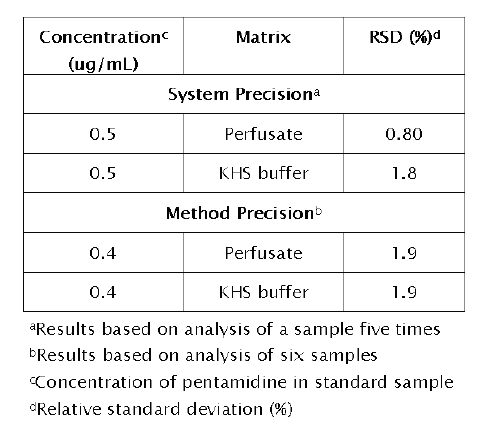
System precision was assessed by analyzing five injections from the same sample (concentration 0.5mg/mL). The RSD for the peak area ratio was calculated. Method precision was determined by analyzing five identical samples (prepared through identical sample treatment) of PTM (concentration 0.4mg/mL) and calculating the percent RSD for the peak area ratio. Inter-day precision of the method in perfusate and KHS buffer was evaluated by assaying calibration standards at five consecutive days.
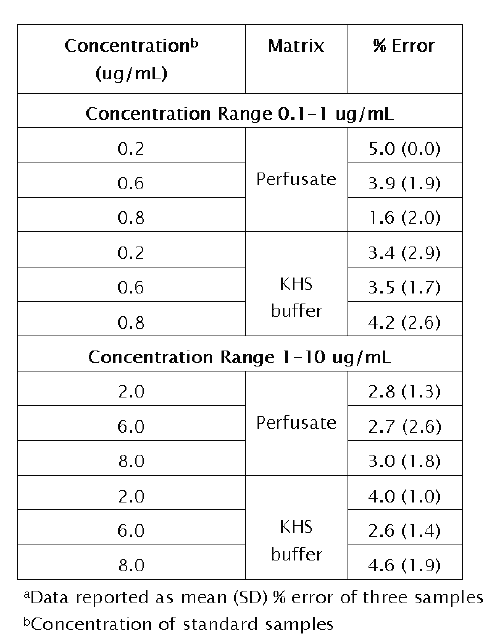
Accuracy of the method was determined two ways. First, the efficiency of the extraction procedure (recovery) was assessed. Concentrations representing the middle of the standard ranges (0.5mg/mL and 5mg/mL) were selected. The absolute extraction ratio was measured by comparing the peak area ratio of PTM (with internal standard) in the matrix with the peak area ratio of an aqueous solution of PTM (with internal standard). Second, the accuracy of the method was studied over the two standard concentration ranges. Concentrations representing the lower (0.2mg/mL and 2mg/mL) middle (0.4mg/mL and 4mg/mL) and upper (0.8mg/mL and 8mg/mL) end of each concentration range were selected. The percent error of the method was calculated based upon differences between actual and predicted concentrations.
Specificity
The specificity of the method was determined by comparing the chromatograms obtained from the samples containing PTM and SDZ with those obtained from blank samples.
Limit of detection (LOD) and limit of quantitation (LOQ)
LOD is a parameter that provides the lowest concentration of analyte in a sample that can be detected, but not quantitated, under the stated experimental conditions. LOD was determined using the signal-to-noise ratio by comparing test results from samples with known concentrations of analyte to blank samples. The analyte concentration that produced a signal-to-noise ratio of 3:1 was accepted as the LOD. LOQ is defined as the lowest concentration of analyte that can be determined with acceptable precision and accuracy under the stated experimental conditions. LOQ was identified as the concentration that produced a signal-to-noise ratio of 10:1.
Stability
Samples from three concentrations within both the low (0.2, 0.6 and 0.8mg/mL) and high (2, 6 and 8mg/mL) standard ranges were used to investigate the stability of PTM over a period of 7 days. Two sets of samples were prepared at each concentration. One set of samples was stored under ambient conditions in the absence of 1 % (w/v) zinc sulfate (protein precipitating agent). The second set of samples was stored at -2°C (also in the absence of zinc sulfate). The samples in the low concentration range were analyzed for PTM concentration on Days 1, 2, 3, 4 and 7. Samples in the high concentration range were analyzed on Days 1, 2, 5 and 7. No internal standard was added prior to the analysis.
IPK Studies
Isolated Perfused Rat Kidney Preparation
The Institutional Animal Care and Usage Committee of Long Island University approved the experimental protocol for this investigation. Male Sprague-Dawley rats were used for perfusion experiments. Animals were housed in stainless steel cages and fed a standard diet including water ad libitum . The surgical technique employed was the Bowman modification (24) of the Nishiitsutsuji-Uwo procedure (25). Anesthesia was induced with an intraperitoneal injection of sodium pentobarbital (40 mg/kg). A midline incision was made and the renal segment of the aorta exposed. A ligature was passed under the right renal artery close to the aorta, and distal and proximal ligatures placed around the superior mesenteric artery. The right ureter was catheterized with polyethylene-10 (PE-10) tubing, in order to facilitate urine collection. A cannula was then threaded through the mesenteric artery, across the aorta, and into the right renal artery in situ . The ligatures were tied, securing the cannula in place. The right kidney was then excised from the animal, trimmed of adhering tissue, and transferred to the in vitro recirculating perfusion apparatus.
Perfusion of the kidney proceeded according to a method described previously (26). Perfusate consisted of the following: KHS buffer (pH 7.4), 4.00% Fraction V bovine serum albumin, 1.69 g/dL clinical grade dextran, 110 mg/dL glucose, and a 13 mM amino acid solution. Inclusion of amino acids improves viability of the preparation (27). The volume of recirculating perfusate was 80 mL. The composition of KHS buffer was as follows: NaCl (0.117 M), NaHCO3 (0.025 M), KCl (0.0047 M), CaCl2 (0.0025 M), MgSO4 (0.0021 M) and KH2PO4 (0.0012 M).
Study Groups
PTM excretion was studied in the presence of creatinine or inulin. The dose of PTM utilized in these experiments was 800 mg, yielding an initial perfusate concentration of 10 mg/mL. Additionally, three sets of control (drug-naïve) perfusions were carried out using one or both GFR markers: inulin alone (60 mg/dL), creatinine alone (60 mg/dL), and both inulin (60 mg/dL) and creatinine (60 mg/dL). These control studies were used to elucidate the effects of PTM on renal function, and allowed for comparison of GFR estimation between inulin and creatinine. Three perfusion experiments were conducted for each study group (n = 3).
Study Design
Following kidney excision and transfer to the recirculating perfusion system, a stabilization period (usually 10 minutes) preceded any pharmacokinetic experimentation. A bolus dose of PTM was added to the perfusate, and a perfusate sample (0.8 mL) was then collected 15 minutes post-dose and every 10 minutes thereafter. Urine was collected in 10-minute intervals over the entire experiment (90 minutes) Volume lost due to sampling or urine excretion was replaced as needed with a 50:50 mixture of perfusate and deionized water. All samples were stored at -20°C until analyzed.
Aliquots of both perfusate and urine were analyzed for sodium (ion specific electrode), glucose (glucose oxidase reaction, Sigma Chemical diagnostic test kit #315), creatinine (picric acid reaction, Sigma Chemical diagnostic test kit# 555-A), and/or inulin (colorimetric assay described below). Preliminary studies found no interaction of analyte (PTM) with the colorimetric assays for creatinine and inulin. Parameters monitored to assess kidney function throughout an IPK perfusion included glomerular filtration rate (GFR, estimated as inulin/creatinine clearance), fractional reabsorption of glucose (FR glucose ) and sodium (FR sodium ), urine flow rate, and urine pH. Perfusion pressure was maintained at 100 ± 10 mm Hg by adjusting the perfusate flow rate as necessary.
Colorimetric Assay for Inulin
Inulin was measured in perfusate and urine samples by colorimetric analysis using anthrone complexation. This assay was modified from published methods (28-30). To prepare the anthrone reagent, 70 mL of concentrated sulfuric acid (95%-98%) was slowly added to 30 mL of distilled water (placed in an ice-bath), and 200 mg of anthrone was added with continuous stirring. Anthrone reagent was prepared fresh prior to analysis.
The assay methodology is as follows: 30mL of the perfusate samples was added to 30mL of glucose oxidase (2U/mL) in 1.5 mL disposable micro-centrifuge tubes (for urine analysis, 10 mL of sample was added to 10mL of glucose oxidase). The tubes were vortexed for 5 seconds and placed in a shaker water bath at 37°C for 15 minutes. The tubes were then cooled to ambient temperature (25°C) and the sample volume adjusted to 250mL with deionized water. A total of 250mL of 1% (w/v) aqueous zinc sulfate solution was then added and the mixture vortex-mixed for 15 seconds. The tubes were then centrifuged for 10 min at 9000 x g. Aliquots of 350mL were then transferred to 15-mL test tubes in an ice bath. Four mL of ice-cold anthrone reagent was added and the tubes were sealed with parafilm and vortex-mixed for 30 seconds. The tubes were then incubated in a shaker water bath at 57°C for 10 minutes. During this period, samples exhibited differences in color (green) as a function of inulin concentration. The test tubes were then placed in ice-cold water for 5 minutes to stop the reaction. Within 40 minutes of stopping the reaction, samples were analyzed by a UV/Vis spectrophotometer at a wavelength of 620 nm.
Perfusate Binding Determination
The perfusate binding of PTM was determined by ultrafiltration. Aliquots (1 mL) of perfusate were sampled during each perfusion at 45 and 75 minutes post-dose and were added to Amicon Centrifree Micropartition Systems (Millipore Corporation, Bedford, MA, USA) and centrifuged at 1500 x g for 15 minutes. The resultant ultrafiltrate contained free PTM and was stored as described previously. Preliminary experiments determined that binding of the drug to the membrane was negligible.
Kidney Accumulation of PTM
Upon completion of a perfusion experiment, the kidney was weighed and homogenized in 2 mL of 0.3M sucrose solution using a DUALL tissue grinder (Kontes, Vineland, NJ). The homogenized kidney tissue was stored at -20°C until analyzed. Prior to analysis of PTM in the kidney homogenate, samples were brought to ambient temperature. The homogenate sample was vortex-mixed and a 250mL sample of the homogenate was added to a 1.5 mL microcentrifuge tube. The pH was adjusted to approximately 10 with 20mL of 1N sodium hydroxide and the sample was vortex-mixed for 30 seconds. To the sample, 500mL of methylene chloride was added and vortex-mixed for 30 seconds. The sample was centrifuged for 15 minutes at 15,000 x g, after which the aqueous supernatant was removed by means of suction. The remaining water was removed by freezing and the organic phase (methylene chloride) was transferred to a clean 1.5 mL microcentrifuge and evaporated under reduced pressure at -108°C using a vacuum evaporator (Speed Vacplus® SC 210, Savant Instruments, Holbrook, NY, USA) attached to a refrigerated vapor trap (Model RVT 4104, Savant Instruments). The residue was dissolved in 250mL of the mobile phase. SDZ (25mL stock solution of 12.5mg/mL) was added and the tube was vortex-mixed for 15 seconds. A volume of 100mL was injected into the HPLC system. Samples were analyzed by HPLC using a standard curve from samples prepared using drug-naïve kidney homogenate (PTM concentration range of 10 to 100mg/mL).
Data Analysis
PTM renal clearance (Cl total ) during each perfusion was calculated as the ratio of dose and AUC (0-90 minutes). AUC (0-90 minutes), representing AUC over the period of sample collection post-dose, was calculated using trapezoidal rule. Filtration clearance (Cl filtration ) was defined as the product of glomerular filtration rate (GFR) and unbound fraction of PTM in perfusate (fu). Excretion ratio (XR), an indication of net mechanisms of elimination, was calculated as the ratio of Cl total and Cl filtration (31).
Statistical Analysis
Mean parameter estimates of IPK viability criteria (e.g., GFR, urine flow rate) for control and drug treatment groups were compared using analysis of variance (ANOVA). Pairwise comparison tests were performed to identify those study groups that differed in terms of viability criteria. Consequently, alterations in kidney function as a function of drug administration were determined. Results were considered statistically significant if a p-value less than 0.05 was obtained.
Mean values for PTM excretion parameters were analyzed by Student's t-test. Significant differences between treatment groups were identified in an effort to elucidate the differential effects of creatinine and inulin on PTM disposition.
Results and Discussion
HPLC Method Validation
A reversed-phase column was chosen for analysis of PTM because it is a polar compound and exhibits low solubility in organic media. A microbore column (2.1 mm I.D.) was chosen to provide increased sensitivity when analyzing samples with drug concentrations less than 500 ng/mL. The pH of mobile phase was made acidic (pH = 3.2) to ensure that PTM was completely protonated. In the development of this method, there was a need to have an internal standard to correct for the variable recovery of analyte following sample preparation or expected differences in the volume of sample injected. Published methods have used hexamidine as internal standard. However, hexamidine is not commercially available. In this study, SDZ was found to be a useful internal standard. SDZ eluted shortly after PTM and its peak was completely resolved from other peaks in the chromatogram. The composition and pH of the mobile phase was optimized to ensure peak resolution among analyte, internal standard, and other materials present in the matrix.
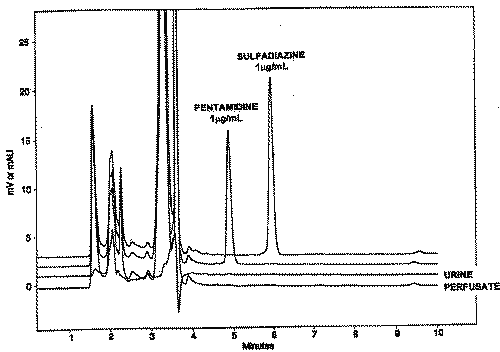
A sample chromatogram is presented in Figure 2.
Figure 2: Sample chromatograms of perfusate containing analyte (PTM) and internal standard (SDZ). Also presented in the figure are chromatograms for blank (drug-naïve) perfusate and urine samples.
The retention times of PTM and SDZ were 4.8 minutes and 5.8 minutes, respectively. As seen in the figure, the method completely separated PTM and SDZ. Furthermore, no interfering peaks were identified in drug-naïve samples.
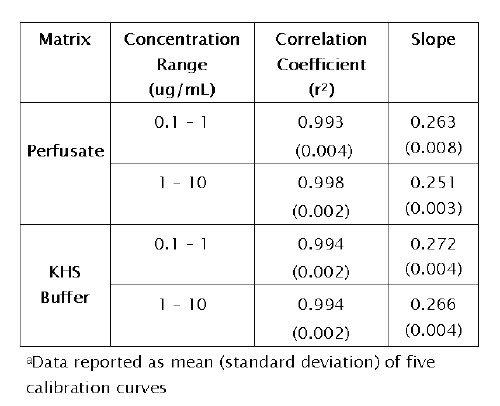
Calibration data are presented in Table 1.
Table 1: Calibration data for pentamidine in IPK perfusate and Krebs Henseleit (KHS) buffer a
The correlation coefficients of the regression lines generated over 5 consecutive days were greater than 0.993 and the slopes were relatively constant among all study groups. The linearity of the method was also verified by constructing response factor plots. The plots followed horizontal behavior in each matrix (perfusate and KHS buffer) at both concentration ranges tested, indicating that the assay was linear.
Representative response factor plots are provided in Figure 3.
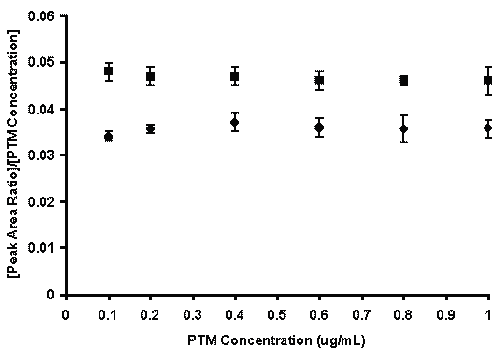
Figure 3: Response factor plots (peak area ratio/[PTM concentration] vs. PTM concentration) for HPLC assay of PTM in perfusate (●) and KHS buffer (■). Data is presented as mean ± SD of five calibration curves (analyte concentration range 0.1 - 1 mg/mL).
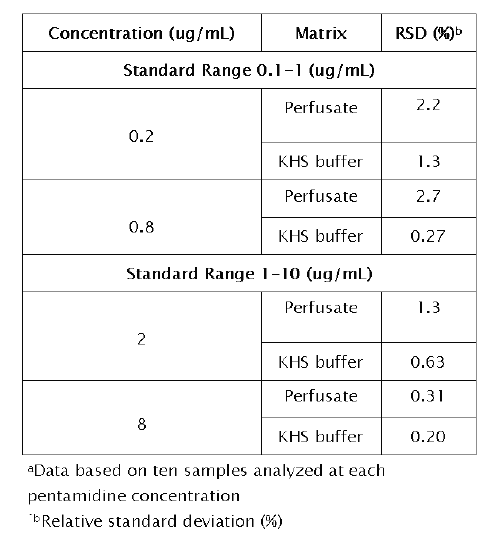
Intra-day reproducibility of the method was determined upon replicate analysis of 10 samples representing two concentrations within each standard range.The results are provided in Table 2. Intra-day variability was less than 2.2%.
Table 2: Intra-day reproducibility of the method for IPK perfusate and Krebs Henseleit (KHS) buffer a
Demonstration of system precision and method precision is provided in Table 3.
Table 3: Precision of the method in IPK perfusate and Krebs Henseleit (KHS) buffer
The accuracy of the method is illustrated in Table 4.
Table 4: Accuracy of the method in IPK perfusate and Krebs Henseleit (KHS) buffer a
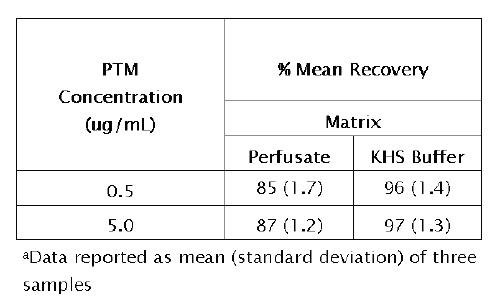
The error obtained was less than 5% in perfusate and KHS buffer over all concentrations tested. These results indicate that the method developed for the determination of PTM is accurate within the concentration range studied. The recovery of PTM from each matrix was greater than 85% (range 85-98%).
These data are presented in Table 5.
Table 5: Recovery of pentamidine (PTM) from IPK perfusate and KHS buffer a
The absolute extraction ratio of PTM was better from KHS buffer, presumably the result of protein binding in perfusate. The limits of quantitation and detection of the method were 50 ng/mL and 10 ng/mL, respectively, for the two sample matrices. Because of the need to store samples before analysis, the stability of PTM was assessed during development and validation of the method.
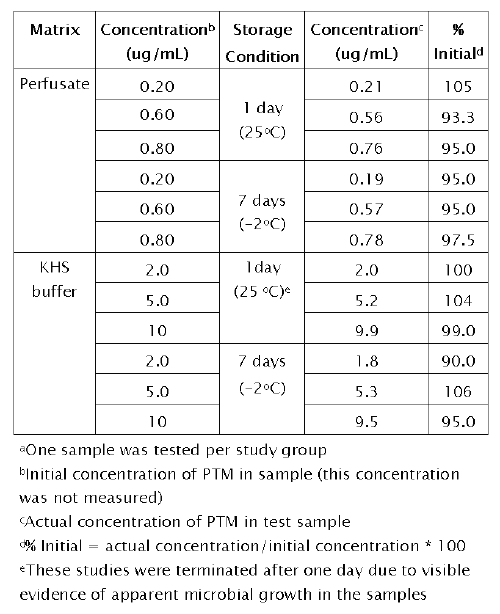
Table 6 indicates the stability data for PTM when stored under ambient and frozen conditions (-2°C) for seven days.
Table 6: Stability of pentamidine (PTM) in IPK perfusate and Krebs Henseleit (KHS) buffer a
No significant degradation of PTM in perfusate was observed during this period under the storage conditions. There was also no significant degradation of PTM in KHS buffer stored at -2oC. However, stability studies in KHS buffer were conducted for only one day at room temperature. Beyond this storage period, there was visible evidence of apparent microbial growth in the KHS buffer samples.
Comparative Effects of Creatinine and Inulin on GFR Estimation and PTM Excretion in the IPK
A specific aim of this research was to study the excretion of PTM in the presence of different GFR markers, creatinine and inulin. Creatinine is the most commonly used indicator of GFR in the clinical setting, primarily because it is a more convenient and less invasive method than inulin. Nevertheless, the accuracy of creatinine clearance as an estimation of GFR is compromised by a reported secretory component to creatinine excretion (23). Furthermore, assuming that the kidney secretes creatinine, the possibility of an interaction between creatinine, a cation, and PTM existed in this investigation. Given that the perfusate concentration of creatinine in IPK studies (60 mg/dL) is approximately 50-fold greater than in vivo levels (1.4 mg/dL, reference 32), it was important to establish whether PTM excretion was influenced by creatinine in the IPK.
A nonradioactive analytical method for inulin (described above) was used for analysis of perfusate and urine samples generated from IPK studies. With this methodology established in our laboratory, an investigation was conducted to compare the effects of creatinine and inulin on PTM excretion.
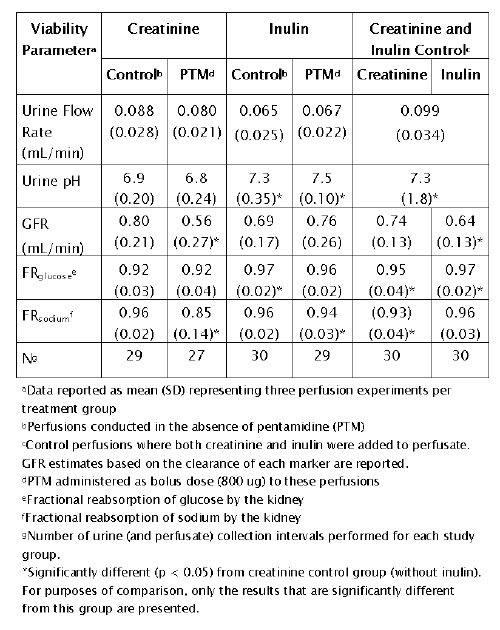
A summary of renal function parameters for the IPK experiments is provided in Table 7.
Table 7: IPK viability parameters (comparison of creatinine and inulin as GFR markers)
Collectively, these parameters provide an assessment of kidney viability. Overall, the values reported (for the control group) in Table 7 are consistent with those reported by other investigators (33-35).
Two important inferences can be made from the data in Table 7. First, creatinine clearance appears to over estimate GFR in comparison to inulin. The GFR estimate from the creatinine control group (drug naïve perfusion) is approximately 15% greater than the inulin control group (0.80 ± 0.21 vs. 0.69 ± 0.17 mL/min, p > 0.05). To eliminate the possible influence of inter-kidney variability on these findings, IPK perfusions containing both creatinine and inulin (drug naïve perfusion) were conducted. Again, creatinine-based GFR estimations were roughly 15% greater than inulin. Although these differences were not statistically significant within the study group (0.74 ± 0.13 vs. 0.64 ± 0.13, p > 0.05), they suggest that creatinine overestimates GFR. Toto (23) reported that 10 to 20% of urinary excretion of creatinine is derived by secretion, consistent with these observations. Therefore, it appears that inulin is the preferred marker for GFR in IPK experimentation.
A second important observation from Table 7 is the impaired kidney viability in the PTM-creatinine treatment group. Significant (p < 0.05) reductions in GFR and sodium reabsorption were noted in kidneys perfused with PTM and creatinine. Since there were no observed drug effects in the inulin treatment group, it appeared that creatinine was an important determinant of PTM-induced toxicity. Previous studies in rodents support these physiologic findings. Intravenous administration of PTM (10 mg/kg) produced a 43.5% reduction in GFR in anesthetized rats (36). Kleyman et al. (37) demonstrated that luminal PTM inhibits sodium reabsorption in the distal tubule in a manner similar to potassium sparing diuretics.
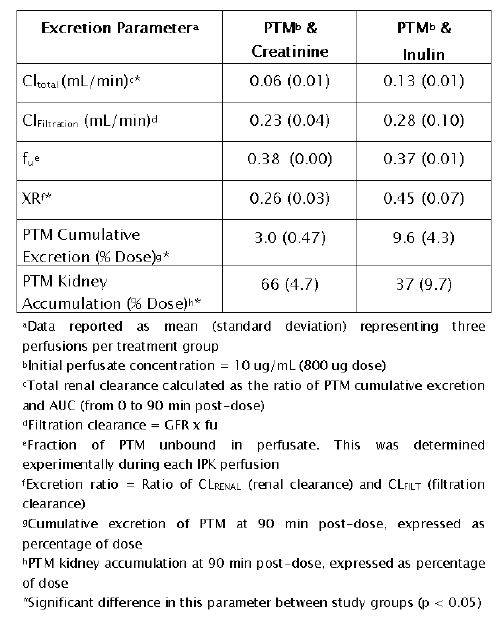
Evidence of an apparent interaction between creatinine and PTM is provided in Table 8.
Table 8: Pentamidine (PTM) renal excretion parameters in the IPK: Comparison of creatinine and inulin
With creatinine as a GFR marker, a significant reduction (p < 0.05) in PTM Cl total was noted. Although XR values for both study groups were indicative of net PTM reabsorption in the IPK, the decrease in XR for creatinine suggests an inhibition of tubular transport of PTM. Furthermore, cumulative PTM excretion was significantly reduced (p < 0.05) and PTM kidney accumulation was significantly increased (p < 0.05) by creatinine. Collectively, these findings are consistent with a reduction in tubular secretion of PTM in the presence of creatinine, resulting in increased kidney accumulation of drug and, consequently, detrimental effects on kidney function (GFR and sodium reabsorption, Table 7).
Conclusions
This report describes a sensitive and reliable HPLC method for analysis of PTM. The method was validated in accordance with USP guidelines. This method offers several advantages compared with previously published HPLC assays including a mobile phase devoid of an ion-pairing agent, a short run time, and use of a commercially available internal standard.
The method was applied to a preliminary study of PTM disposition in the IPK and several important results were obtained. First, compared with creatinine, inulin is a superior marker for GFR in IPK experimentation. Not only was creatinine found to overestimate GFR by roughly 15%, but creatinine also influenced the renal disposition of PTM. Whereas creatinine significantly altered the renal disposition of PTM in this study, this finding is not likely to be clinically significant as much higher creatinine levels were used in IPK compared to in vivo . Nevertheless, creatinine should be used cautiously in IPK methodology because of the potential for interaction with the compound(s) being studied. Studies are ongoing to elucidate the mechanisms of renal excretion and kidney accumulation of PTM further.
ACKNOWLEDGEMENTS
The authors thank The Burroughs Wellcome Fund and Berlex Laboratories Inc., Montville, NJ for the support of this project.
References
Blagburn, B.J., Reddy, V.V., Le, S.T., Lombardy, R.J., Hall, J.E, and Tidwell, R.R., Inhibition of Cryptosporidium parvum in neonatal Hsd (ICR) BR Swiss mice by polyether ionophores and aromatic amidines. Antimicrob Agents Chemother, 35:1520-1523, 1991.
Bell, C.A., Cory, M., Fairley, T.A., Hall, J.E., and Tidwell, R.R., Structure-activity relationships of pentamidine analogs against Giardia lamblia and correlation of antigiardial activity and DNA binding affinity. Antimicrob Agents Chemother, 35:1099-1107, 1991.
Lindsay, D., Blagburn, B., Hall, J.E., and Tidwell, R.R., Activity of pentamidine and pentamidine analogs against Toxoplasma gondii in cell cultures. Antimicrob Agents Chemother, 35:1914-1916, 1991.
Apted, F., Present status of chemotherapy and chemophylaxis of human trypanosomiasis in the Eastern Hemisphere. Pharmaco. Ther, 11:391-413, 1980.
Bryceson, A.D.M., Chulay, J.D., Mugambi, M., Were, J.B., Gachihi, G., Chunge, C.N., Muigai, R., Bhatt, S.M., Ho, M., Spencer, H.C., Meme, J., and Anabwani, G., Visceral Eishmaniasis unresponsive to antimonial drugs: II. Response to high dosage sodium stibogluconate or prolonged treatment with pentamidine. Tran R. Soc Trop Med Hyg, 79:705-714, 1985.
Ivady, V., and Paldy, L., Ein neues behandlungsverfahren der interstitiellen plasmazelligan pneumonia fruhgeborener mit funfwertigem stibium und aromatischen diamidinen. Monotsschr Kinderheilkd, 106:10-14, 1958.
Wong, R.J., and Hardy, W.D., Focus on aerosolized pentamidine: An agent for prophylaxis of pneumocystis carinii pneumonia. Hosp Formul, 25:1061-1075, 1990.
Briceland, L.L., and Bailie, G.R., Pentamidine-associated nephrotoxicity and hyperkalemia in patients with AIDS. DICP Ann Pharmacother, 25:1171-1174, 1991.
Sack, K., Schultz, E., and Marre, R., Nierenverträglichkeit von Antibiotika. Fortschritte der Antimikrobiellen und Antineoplastischen Chemotherapie, 67:1085-1093, 1987.
Dickinson, C.M., Navin, T.R., and Churchill, F.C., High performance liquid chromatographic method for quantitation of pentamidine in blood serum. J Chromatogr, 345:91-97, 1985.
Vinet, B., Comtois, R., Gervais, A., and Lemieux, C., Clinical usefulness of HPLC determination of serum pentamidine in AIDS patients. Clin Biochem, 25:93-97, 1992.
Ericsson, O., and Rais, M., Determination of pentamidine in whole blood, plasma, and urine by high-performance liquid chromatography. Ther Drug Monit, 12:362-365, 1990.
Conte, J.E., Upton, R.A., Phelps, R.T., Wofsky, C.B., Zurlinden, E., and Lin, E.T., Use of a specific and sensitive assay to determine pentamidine pharmacokinetics in patients with AIDS. J Infect Dis, 154:923-929, 1986.
Lin, J.M., Shi, R.J., and Lin, E.T., High performance liquid chromatographic determination of pentamidine in plasma. J Liq Chromatogr, 9:2035-2046, 1986.
Dusci, L.J., Hackett, L.P., and Forbes, A.M., High performance liquid chromatographic method for measurement of pentamidine in plasma and its applications in an immunosuppressed patient with renal dysfunction. Ther Drug Monit, 9:422-425, 1987.
Rabanal, B., De Arriba, R.G., Garzon, M.J., Reguera, R.M., Balana-Fouce, R., and Negro, A., Determination of pentamidine in leishmania infantum promastigotes by ion-paired liquid chromatography. J. Liq Chromatogr, 17:2017-2029, 1994.
Yeh, T-K., Dalton, J.T., and Au, J.L.S., High-performance liquid chromatographic determination of pentamidine in plasma. J Chromatogr, 662:255-261, 1993.
Berger, B.J., Henry, L., Hall, J.E., and Tidwell, R.R., Problems and pitfalls in the assay of pentamidine. Clin Pharmacokinet, 22:163-168, 1992.
Bekersky, I., Poynor, W.J., and Colburn, W.A., Pharmacokinetics of saccharin in the rat: Renal clearance in vivo and in the isolated perfused kidney. Drug Metab. Dispos. 8:64-67, 1980.
Bekersky, I. and Colburn, W.A., Renal clearance of carprofen in the isolated perfused rat kidney. Drug Metab Dispos, 9:25-29, 1981.
Taft, D.R., Fournier, D.J., Chapron, D.J., and Sweeney, K.R., Concentration dependent tubular secretion of acetazolamide and its inhibition by salicylic acid in the isolated perfused rat kidney. Drug Metab Dispos, 24:456-451, 1996.
Statkevich, P., Fournier, D.J., and Sweeney, K.R., Characterization of methotrexate elimination and interaction with indomethacin and flurbiprofen in the isolated perfused rat kidney. J Pharmacol Exp Ther, 265:1118-1124, 1993.
Toto, R.D., Conventional measurement of renal function utilizing serum creatinine, creatinine clearance, inulin and para-aminohipppuric acid clearance. Curr Opin Nephrol and Hypertens, 4:505-509, 1995.
Bowman, R.H., The perfused rat kidney. Methods Enzymol, 39:3-11, 1975.
Nishiitsutsuji-Uwo, J.M., Ross, B.D., and Krebs, H.A., Metabolic activities of the isolated perfused rat kidney. Biochem J, 103:852-862, 1967.
Bowman, R.H., Methodology for study of isolated perfused rat kidney in vitro. In: Methods in Pharmacology, Vol. 4B, Renal Pharmacology, Plenum, New York, 1978 pp. 50-57.
Epstein, F.H., Brosnan, J.T., Tange, J.D., and Ross, B.D., Amino acids improve viability of the perfused rat kidney. Am J Physiol, 243:F384-F292, 1982.
Symes, A.L., and Gault, M.H., Assay of inulin in tissues using anthrone. Clin Biochem, 8:67-70, 1975.
Young, M.K., and Prudden, J.F., Simultaneous determination of sucrose and inulin in biological fluids. J Lab Clin Med, 44:160-165, 1954.
Handelsmann, M.B., and Sass, M., The use of capillary blood for estimating renal clearance of inulin and glucose excretion by means of an anthrone procedure. J Lab Clin Med, 48:759-768, 1956.
Tucker, G.T., Measurement of the renal clearance of drugs. Br J Clin Pharmacol, 12:761-770, 1981.
Waynforth, H.B.; Flecknell, P.A., Experimental and Surgical Techniques in the Rat. Academic Press, London, UK, 1992.
Bekersky, I., Popick, A.C., and Colburn, W.A., Influence of protein binding and metabolic interconversion on the disposition of sulfisoxazole and its N14-acetyl metabolite by the isolated perfused rat kidney. Drug Metab Dispos, 12:607-613, 1984.
Taft, D.R., and Sweeney, K.R., The influence of protein binding on the elimination of acetazolamide by the isolated perfused rat kidney: Evidence of albumin-mediated tubular secretion. J Pharm Exp Ther, 274:752-760, 1995.
Maack, T., Renal clearance and isolated kidney perfusion techniques. Kidney Int, 30:142-151, 1986.
Gabriels, G., Stockem, E., and Greven, J., Potassium-sparing renal effects of trimethoprim and structural analogues. Nephron, 86:70-78, 2000.
Kleyman, T.R., Roberts, C., and Ling, B.N., A mechanism for pentamidine-induced hyperkalemia: inhibition of distal nephron sodium transport. Ann Intern Med, 122:103-106, 1995.
Corresponding Author: David R. Taft, Division of Pharmaceutics, Long Island University, 75 KeKalb Avenue, Brooklyn, New York, USA. dtaft@liu.edu
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps