J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 5(3):226-230, 2002
Inhibitory activity of Cinnamomum cassia bark-derived component against rat lens aldose reductase.
Hoi-Seon Lee1
Research Center for Industrial Development of Biofood Materials and Institute of Agricultural Science and Technology, College of Agriculture, Chonbuk National University, Chonju, South KoreaReceived 18 June 2002, Revised 7 July 2002, Accepted 29 August 2002
PDF version
Abstract
PURPOSE. To evaluate the inhibitory activity of active compounds isolated from Cinnamomum cassia bark against lens aldose reductase and compare to that of three commercially available compounds (cinnamyl alcohol, trans -cinnamic acid, and eugenol) and quercitrin as aldose reductase inhibitors. The IC 50 value of cinnamaldehyde was determined. METHODS. Active compound was purified on repeated silica gel column and HPLC (Waters Delta Prep 4000). Aldose reductase was prepared from lenses of Sprague-Dawley male rat eyes. The incubation mixture contained 135 mM Na, K-phosphate buffer (pH 7.0), 100 mM lithium sulfate, 0.03 mM NADPH, 0.04 mM DL-glyceraldehyde and 50mL of an enzyme preparation, with or without a plant extract. The reaction was initiated by adding NADPH at 37°C and stopped by adding 0.5 N hydrochloric acid. Subsequently, 6 N NaOH containing 10 mM imidazole was added, and the mixture was incubated at 60°C for 10 min to convert NADP into a fluorescent product. The fluorescence was measured with a spectrofluorophotometer. RESULTS. The biologically active constituents of C. cassia extract against lens aldose reductase were characterized as trans -cinnamaldehyde by spectral analysis. The IC 50 value of cinnamaldehyde is 0.003 mg/mL. However, cinnamyl alcohol, trans -cinnamic acid and eugenol exhibited only weak inhibition against aldose reductase. In comparison, quercitrin was 6 times more potent than cinnamaldehyde. CONCLUSIONS. These results suggest that cinnamaldehyde isolated from C. cassia barks may be useful as a lead compound and a medicinal foodstuff for aldose reductase inhibition.
Introduction
Aldose reductase, as a key enzyme in the polyol pathway, is reported to catalyze the reduction of glucose to sorbitol. In normal tissue, aldose reductase has low substrate affinity for glucose, so that the conversion of glucose to sorbitol is little catalyzed. However, in diabetes mellitus, the increased availability of glucose in insulin-insensitive tissues such as lens, nerve, and retina leads to the increased formation of sorbitol through the polyol pathway. Sorbitol does not readily diffuse across cell membranes and the intracellular accumulation of sorbitol has been implicated in the chronic complications of diabetes such as cataract, neuropathy, and retinopathy. These findings suggest that aldose reductase inhibitors prevent the conversion of glucose to sorbitol and may have the capacity of preventing and/or treating several diabetic complications (1).
Plants constitute a rich source of bioactive chemicals against aldose reductase (2-7). Since many of them are largely free from adverse effects and have excellent pharmacological actions, they could lead to the development of new classes of possibly safer antidiabetic agents. Additionally, some flavonoids and polyphenols as well as sugar derivatives are found to be effective inhibitors of aldose reductase (2-7). Therefore, much effort has been focused on the plants for potentially useful products as commercial aldose reductase inhibitors or as lead compounds. In the course of our characterization studies on antidiabetic principles of natural medicines, we have found that the extracts of several natural medicines and medicinal foodstuffs, such as Canavalia lineata, Cinnamomum cassia, Coptis japonica, Curcuma longa, Fagopyrum esculentum, Sorghum bicolor , and Vicia tetrasperma exhibited potent inhibitory activity against rat lens aldose reductase (2). In this study, active components from the extract of Cinnamomum cassia were isolated by bioassay-guided separation. This plant species, the commercial source of cinnamon, is not only important as a spice, but in East Asia is considered to have some medicinal properties, e.g. as a stomachic agent, an antimicrobial agent, an astringent, and a carminative agent (8-10). It is rich in essential oils and tannins (11). However, relatively little work has been done on aldose reductase inhibitory activity of C. cassia despite its excellent pharmacological action in East Asia (9). Aldose reductase inhibitor isolated from C. cassia bark may be a good source for medicinal foodstuffs and lead compounds as alternatives for aldose reductase inhibitors currently used. The importance of finding effective aldose reductase inhibitors led us to further investigate these natural compounds.
MATERIALS AND METHODS
Chemicals and Reagents
Bovine serum albumin, trans -cinnamic acid, eugenol, DL-glyceraldehyde, imidazole, lithium sulfate, NADPH, phenylmethylsolfonyl fluoride, and quercitrin were purchased from Sigma Chemical (St. Louis, MO, USA). Cinnamyl alcohol was supplied by Tokyo Kasei (Tokyo, Japan). Coomassie Blue reagent was purchased from Bio-Rad (Hercules, CA, USA). Sprague Dawley male rats were purchased from Dong Nam Laboratory Animal Research Center Co. (Chonju, Chonbuk, South Korea), and all other chemicals were of reagent grade.
Isolation and Identification
The bark from Cinnamomum cassia (3.6 kg) purchased as a commercially available product was dried in an oven at 60°C for 2 d, finely powdered, extracted twice with methanol (10 L) at room temperature and filtered (Toyo filter paper No. 2, Japan). The combined filtrate was concentrated in vacuo at 35oC to yield about 10.4% (based on the weight of the bark: gum). The extract (20 g) was sequentially partitioned into hexane (3.9 g), chloroform (4.5 g), ethyl acetate (1.9 g), and water-soluble (9.7 g) portions for subsequent bioassay against rat lens aldose reductase. The organic solvent portions were concentrated to dryness by rotary evaporation at 35°C, and the water portion was freeze-dried.
The hexane portion (10 g) was chromatographed on a silica gel column (Merck 70-230 mesh, 500 g, 5.5 i.d. x 70 cm), and successively eluted with a stepwise gradient of hexane-ethyl acetate (0, 10, 30, 50, 80, and 100%). The active 50% fraction (4.1 g) was chromatographed on a silica gel column and eluted with hexane-ethyl acetate (2:1). Twenty-five column fractions were collected and analyzed by TLC (hexane-ethyl acetate, 3:1). Fractions with similar TLC patterns were combined. The active fraction (2.4 g) was chromatographed on a silica gel column and eluted with hexane-ethyl acetate (8:2). For further separation of the bioactive substance(s), prep HPLC (Waters Delta Prep 4000) was used. The column was 29 i.d. x 300 mm Bondapak C 18 (Waters) using methanol-water (3:7) at a flow rate of 10 ml/min and detection at 260 nm. Finally, a potent active principle (0.1 g) was isolated.
Structural determination of the active isolate was based on spectral analysis. 1 H- and 13 C-NMR spectra were recorded with a Bruker AM-500 spectrometer and chemical shifts were given in ppm. UV spectra were obtained on a Waters 490 spectrometer, IR spectra on a Biorad FT-80 spectrophotometer, and mass spectra on a JEOL JMS-DX 30 spectrometer.
Isolation of Aldose Reductase from Sprague Dawley Rats.
Crude aldose reductase was prepared from rat lenses. Lens were removed from the eyes of 8-week-old Sprague-Dawley male rats each weighing 100-150 g and were homogenized in 12 volumes of 135 mM Na, K-phosphate buffer (pH 7.0) containing 0.5 mM phenylmethylsulfonyl fluoride and 10 mM 2-mercaptoethanol. The homogenate was centrifuged at 100,000 x g for 30 min, and the resulting supernatant was retained as an enzyme preparation. All procedures were carried out at 4°C. The activity of this preparation was determined by measuring the amount of NADP converted from NADPH per unit time at 37 °C and pH 7.0. One unit (U) of activity is defined as the amount of the enzyme catalyzing the oxidation of 1mmol of NADPH per minute under our experimental conditions.
Enzyme Inhibitory Assay
Aldose reductase activity was assayed according to the method described by Lee and Kim (3). The incubation mixture contained 135 mM Na, K-phosphate buffer (pH 7.0), 100 mM lithium sulfate, 0.03 mM NADPH, 0.04 mM DL-glyceraldehyde and 50mL of an enzyme preparation, with or without a plant extract, at a total volume of 1.0 mL. Each sample was dissolved in dimethyl sulfoxide, which was found to have no effect on the enzyme activity at less than 1%. Appropriate blanks contained all of the above-mentioned compounds, except DL-glyceraldehyde. The reaction was initiated by adding NADPH at 37°C and stopped by adding 0.5 N hydrochloric acid (0.3 mL). Subsequently, 6 N NaOH (1 mL) containing 10 mM imidazole was added, and the mixture was incubated at 60°C for 10 min to convert NADP into a fluorescent product. The fluorescence was measured at room temperature with a spectrofluorophotometer (Aminco Bowman series 2, Spectronic Instruments, Rochester, NY, USA) with an excitation wavelength of 360 nm and an emission wavelength of 460 nm. Standards of NADP (0.1-5mM) were treated in the same manner. All determinations were performed in triplicate. The concentration of each test sample giving 50% inhibition of the enzyme activity (IC 50 ) was estimated from the least-squares regression line of the logarithmic concentration plotted against the remaining activity. The protein content of the enzyme preparation was 0.021 g/L, and aldose reductase activity in the preparation was 7.38 U/L or 370.1 U/g of protein at 37°C. The protein content of the enzyme preparation was determined using Coomassie Blue reagent (Bio-Rad) according to the manufacturer's instructions, with bovine serum albumin as a standard.
RESULTS AND DISCUSSION
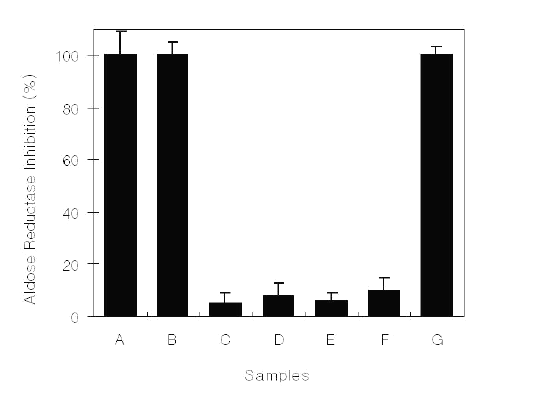
Fractions obtained from methanol extracts of C. cassia bark were assessed for inhibitory activity against lens aldose reductase isolated from Sprague Dawley male rats (Figure 1).
Figure 1: Aldose reductase inhibitory activity of C. cassia bark-derived materials at a concentration of 0.1 mg/mL. A, Methanol extract; B, Hexane fraction; C, Chloroform fraction; D, Ethyl acetate fraction; E, Butanol fraction; F, Water fraction; G, Quercitrin.
At 0.10 mg/mL, the hexane fraction showed 100% inhibition against aldose reductase, whereas other fractions exhibited weak or no inhibition. Purification of the biologically active compound(s) from the fraction was done by silica gel column chromatography and HPLC, and the isolates were bioassayed. One active isolate showed inhibitory activity. Structural determination of the isolate was made by spectral techniques, and it was characterized as trans -cinnamaldehyde. The compound was identified based on the following evidence: C9H8O (MW, 132); EI-MS (70 eV) m/z (% rel. int.): M+ 132 (3), 103 (2), 74 (83), 59 (100), 58 (75); IR (neat) max cm-1: 2920, 1680, 1630, 1130; 1H-NMR (CD3OD, 400 MHz): 6.60 (dd, J = 8 and 18 Hz), 7.35 (d, J = 18 Hz), 7.1-7.7 (m), 9.52 (d, J = 8 Hz); 13C-NMR (CD3OD, 100 MHz): 195.6, 154.4, 135.0, 132.1, 129.9 129.7, 129.5, 129.0, 128.9. This spectral data is identical with some references cited (9, 12).
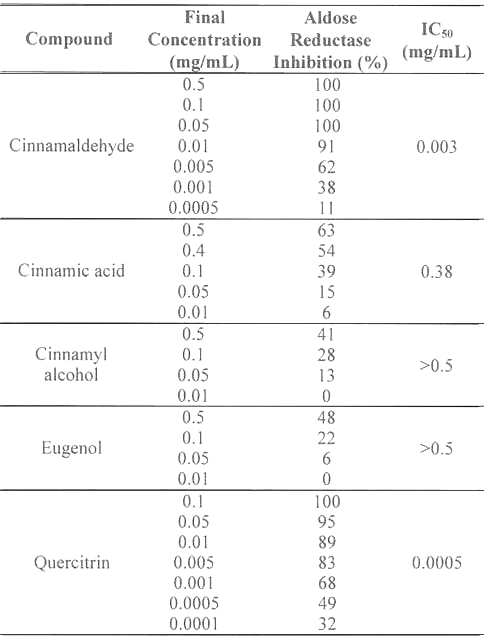
The inhibitory activities of cinnamaldehyde, cinnamic acid, cinnamyl alcohol, and eugenol against aldose reductase were compared to quercitrin, a naturally occurring flavonoid which has been demonstrated to be a potent aldose reductase inhibitor in vitro (Table 1).
Table 1: Aldose reductase inhibitory activities of C.cassia bark-derived compounds.
Inhibitory responses varied with the chemicals tested. At 0.5 mg/mL, cinnamaldehyde completely inhibited (100%) aldose reductase, whereas the inhibitory activities of cinnamic acid, cinnamyl alcohol, and eugenol were 63, 41, and 48%, respectively. These results indicate that the inhibitory activity of C. cassia extract against aldose reductase is primarily caused by cinnamaldehyde. The IC 50 values of cinnamaldehyde, cinnamic acid, cinnamyl alcohol, and eugenol were determined to be 0.003, 0.38, >0.5, and >0.5 mg/mL, respectively. However, quercitrin (IC50, 0.0005 mg/mL) was 6 times more potent an inhibitor than cinnamaldehyde.
It has been well acknowledged that plant-derived extracts and phytochemicals are potential alternatives to synthetic inhibitors against aldose reductase (2-7). Currently, the compounds isolated from plants known to inhibit aldose reductase are flavonoids and flavonoid-related compounds. These include 5,7,4'-trihydroxy-3,6-dimethoxyflavone from Acanthospermum australe (13), myricetin 3-O-(4"-acetyl)-fucoside from Anthocepharus chinensis (14), and dihydroflavonol rhamnosides and quercitrin 3-rhamnoside from Engelhardtia chrysolepis (15). In this study, the active component isolated from C. cassia bark against aldose reductase was identified as trans -cinnamaldehyde, a low molecular weight cinnamic acid analogue. It has been reported that the C. cassia bark-derived materials including cinnamaldehyde, cinnamic acid, cinnamyl alcohol, and eugenol have antibacterial, astringent, carminative, and stomachic effects (8-10). It might be expected that the active components isolated from of C. cassia bark would have some pharmacological actions against diabetes.
Aldose reductase inhibitors including quercitrin are currently the most commonly used oral agents for their good penetrations through cellular membranes and fast metabolism of sorbitol by sorbitol dehydrogenase. They are considered more importantly as therapeutic prospects for treatment of diabetic complications such as retinopathy, cataracts, neuropathy and nephropathy (1). In this study, cinnamaldehyde has been identified as a lead compound for treatment of diabetic complications, although the inhibitory activity of cinnamaldehyde was lower than that of quercitrin. Cinnamaldehyde was approved with a conditional acceptable daily intake for man (ADI) of 1.25 mg/kg by FDA/WHO (16). Furthermore, The oral LD50 of cinnamaldehyde for rat and mice varies from 3.4 g/kg to greater than 5.0 g/kg (17). Many investigations have shown that cinnamaldehyde has antibacterial (18), antibotulinal (19), and intestinal modulating effects (9).
In conclusion, these results indicate that C. cassia bark-derived materials have an inhibitory effect in vitro against rat lens aldose reductase. Based upon our limited data and some earlier findings, cinnamaldehyde may be useful as a lead compound for an antidiabetic agent and a medicinal foodstuff, although in vivo efficacy and clinical utility remain to be evaluated.
Acknowledgments
This research was supported by Research Center for Industrial Development of Biofood Materials in Chonbuk National University, Chonju, Korea. Research Center for Industrial Development of Biofood Materials is designated as a Regional Research Center appointed by the Korea Science and Engineering Foundation (KOSEF), Chollabukdo Provincial Government and Chonbuk National University.
References
Williamson, J., Kilo, C. and Tilton, R.G., Mechanism of glucose- and diabetes-induced vascular dysfunction, in: Ruderman, N., Williamson, J. and Brownlee, M. (eds.), Hyperglycemia, Diabetes, and Vascular Disease. American Physiological Society, New York, pp 107-132, 1992.
Kim, M.K., Kim, S.Y. and Lee, H.S., Rat lens aldose reductase inhibitory activities of oriental medicinal plants. Agric Chem Biotechnol, 45:84-88, 2001.
Lee, H.S. and Kim, M.K., Rat intestinal a-glucosidase and lens aldose reductase inhibitory activities of grain extracts. Food Sci Biotechnol, 10:172-177, 2001.
Haraguchi, H., Ohmi, I., Sakai, S. and Fukuda, A., Effect of Polygonum hydropiper sulfated flavonoids on lens aldose reductase and related enzymes. J Nat Prod, 59:443-445, 1996.
Yoshikawa, M., Shimada, H., Norihisa, N., Li, Y., Toguchida, I., Yamahara, J. and Matsuda, H., Antidiabetic principles of natural medicines. II. Aldose reductase and a-glucosidase inhibitors from Brazilian natural medicine, the leaves of Myrcia multiflora DC. (Myrtaceae): Structures of Myrciacitrins I and II and Myrciaphenones A and B. Chem Pharm Bull, 46:113-119, 1998.
Matsuda, H., Murakami, T., Yashiro, K., Yamahara, J. and Yoshikawa, M., Antidiabetic principles of natural medicines. . Aldose reductase and -glucosidase inhibitors from the roots of Salacia oblonga Wall. (Celastraceae): Structure of a new friedelane-type triterpene, kotalagenin 16-acetate. Chem Pharm Bull, 47:1725-1729, 1999.
Kador, P.F., Robison, W.G. and Kinoshita, J.H., The pharmacology of aldose reductase inhibitors. Ann Rev Pharmacol Toxicol, 25:691-714, 1985.
Namba, T., Colored Illustrations of Wakan-Yaku (The Crude Drugs in Japan, China and the Neighbouring Countries). Hoikusha Publishing, Osaka, 1986.
Lee, H.S. and Ahn, Y.J., Growth-inhibiting effects of Cinnamomum cassia bark-derived materials on human intestinal bacteria. J Agric Food Chem, 46:8-12, 1998.
Kim, M.K., Lee, S.E. and Lee, H.S., Growth-inhibiting effects of Brazilian and oriental medicinal plants on human intestinal bacteria. Agric Chem Biotechnol, 43:54-58, 2000.
Morimoto, S., Nonaka, G. and Nishioka, I., Tannins and related compounds XXXVIII. Isolation and characterization of flavan-3-ol glucosides and procyanidin oligomers from Cassia bark (Cinnamomum cassia Blume). Chem Pharm Bull, 34:633-642, 1986.
Park, I. K., Lee, H. S., Lee, S. G., Park, J. D. and Ahn, Y. J., Insecticidal and fumigant activities of Cinnamomum cassia bark-derived materials against Mechoris ursulus. J Agric Food Chem, 48:2528-2531, 2000.
Shimizu, M., Horie, S., Arisawa, M., Hayashi, T., Suzuki, S., Yoshizaki, M., Kawasaki, M., Terashima, S., Tsuji, H., Wada, S., Ueno, H., Morita, N., Berganza, L.H., Ferro, E. and Basualdo, I., Chemical and pharmaceutical studies on medicinal plants in Paraguay. I. Isolation and identification of lens aldose reductase inhibitor from "Tapecue" Acanthospermum australe O. K. Chem Pham Bull, 35:1234-1237, 1987.
Haraguchi, H., Kaanada, M. and Fukuda, A., An inhibitor of aldose reductase and sorbitol accumulation from Anthocepharus chinensis. Planta Medica, 64:68-69, 1998.
Haraguchi, H., Ohmi, I., Masuda, H., Tamura, Y., Mizutani, K., Tanka, O. and Chou, W.H., Inhibition of aldose reductase by dihydroflavonols in Engelhardtia chrysolepis and effects on other enzymes. Experientia, 52:564-567, 1996.
FAO/WHO, Specifications for the identity and purity of food additives and their toxicological evaluation: some flavoring substances and non-nutritive sweetening agents. Eleventh Report of the Joint FAO/WHO Expert Committee on Food Additives, FAO Nutrition Meetings Report Series No. 44, Wld Hlth Org. techn. Rep. Ser. No. 383, 1968.
Opdyke, D.L.J., Fragrance raw materials monographs. Food Cosmet. Toxicol., 16:687-689, 1978.
Moleyar, V. and Narasimham, P., Antibacterial activity of essential oil components. Int J Food Microbiol, 16:337-342, 1992.
Bowles, B. L. and Miller, A. J., Antibotulinal properties of selected aromatic and aliphatic aldehydes. J Food Prot, 56:788-794, 1993.
Corresponding Author: Hoi-Seon Lee, Professor, Research Center for Industrial Development of Biofood Materials, College of Agriculture, Chonbuk National University, Chonju 561-756, South Korea. hoiseon@moak.chonbuk.ac.kr
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps