J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 6(2):215-222, 2003
Attenuation of benzodiazepine dependence in mice by a tri-substituted benzoflavone moiety of Passiflora incarnata Linneaus: A non-habit forming anxiolytic.
Kamaldeep Dhawan1
Department of Drugs Control, Government of Haryana, Panchkula, IndiaSanju Dhawan
University Institute of Pharmaceutical Sciences, Panjab University, Chandigarh, IndiaSumit Chhabra
Department of Pharmaceutical Sciences, Maharishi Dayanand University, Rohtak, Haryana, IndiaReceived 9 December 2001, Revised 11 April 2003, Accepted 13 June 2003
PDF version
Abstract
PURPOSE: A tri-substituted benzoflavone moiety (BZF) recently isolated from the methanol extract of aerial parts of the plant Passiflora incarnata Linneaus had exhibited encouraging results in countering the dependence produced by addiction-prone substances like morphine, nicotine, cannabinoids and ethyl alcohol, during the studies performed by the authors. Since the BZF moiety had exhibited significant anxiolytic properties at 10 mg/kg p.o. dose in mice, therefore, it was desirable to evaluate this potential phyto-moiety (BZF) for its own dependence-liabilities It was also deemed viable to evaluate BZF moiety for its possible usefulness in countering the dependence-liabilities associated with the chronic use of benzodiazepines keeping in light their tremendous clinical use in the management of anxiety and insomnia. METHODS. Different groups of mice were administered BZF alone (10, 50 or 100 mg/kg, p.o.), and concomitantly with diazepam (20 mg/kg, p.o.) in a 21-days treatment regimen, followed by no treatments for the next 72-hours. The withdrawal effects in the form of ambulatory behavior of the treated animals were recorded on the 25th day using an Actophotometer. RESULTS. The BZF-alone (three doses) treated mice exhibited a normal ambulatory behavior on 25th day. Mice groups receiving co-treatments, i.e., BZF-diazepam concomitantly, also exhibited a normal ambulatory behavior in a dose-dependant manner, i.e., the higher dose of BZF (100 mg/kg) being more effective in countering the withdrawal effects of chronically administered diazepam than the lower doses (10 or 50 mg/kg). CONCLUSIONS. The studies revealed that the chronic administration of the BZF moiety (three doses), did not exhibit any dependence-liability of its own, even upon an abrupt cessation. Additionally, the BZF co-treatments with diazepam also prevented the incurrence of diazepam-dependence, which might be because of the aromatase enzyme inhibiting properties associated with the BZF moiety..
Introduction
Benzodiazepines (e.g., diazepam, nitrazepam, lorazepam, alprazolam) are the most frequently prescribed synthetic chemical drugs for a variety of conditions, particularly anxiety, depression, epilepsy and insomnia (1, 2). The prevailant prescription pattern of benzodiazepines is symptomatic which results in their potential overuse (3). Chronic use of benzodiazepines causes deterioration of cognitive functioning (4), physical dependence (5) and tolerance (6, 7). Besides addiction-liabilities, benzodiazepines adversely affect the respiratory, digestive and immune systems of the body (8). Since, the chronic treatment with benzodiazepines often proves more harmful in the longer run, there has been an increasing thrust worldwide to opt for safer and effective plant-derived anxiolytics and hypno-sedatives mentioned in the traditional medical systems (9, 10). The plant Passiflora incarnata Linneaus (family Passifloraceae, synonyms: passionflower, maracuja, maypops) has been put to use as an anxiolytic, sedative, anti-convulsant, nervine and anti-spasmodic in the oriental system of healing in several countries since time immemorial (11, 12). The plant extract is popular homoeopathic medicine for anxiety and insomnia (13). Monographs of P. incarnata in the herbal pharmacopoeias of several countries mention the plant to be a safe and non-habit forming medicine for long term management of insomnia, anxiety, depression, epilepsy and morphine de-addiction (14-17).
In recent studies by the authors, a methanol extract of the leaves of P. incarnata was confirmed to possess anxiolytic (18, 19), anti-tussive (20), anti-asthmatic (21) and aphrodisiac properties (22) in experimental animals at a dose of 100 mg/kg per oral. The bioactive methanol extract was further subjected to bioactivity-directed-fractionation and standard chromatographic procedures to isolate a pure bioactive fraction from which a tri-substituted benzoflavone moiety (BZF) was isolated and its chemical identity was established using the standard spectroscopic techniques (23-25). Our subsequent pharmacological studies on the BZF moiety confirmed the significant usefulness of P. incarnata in countering the dependence and development of tolerance upon administration with substances like morphine (26), cannabinoids (27), nicotine (28) and ethanol (29) in mice at a dose-range of 10-50 mg/kg. A 30-days treatment with 10 mg/kg of the BZF moiety exhibited significant increase in the libido, sperm count, fertilization potential and the sexual virility in 2-years old male rats (30). The co-administration of 10 mg/kg dose of BZF along-with chronically administered (30-days treatment regimen) ethanol, nicotine, ethanol-nicotine combinations (31), as well as Δ9-tetrahydrocannabinol (32) prevented substance induced azospermia, mating efficiency and virility in male rats.
In the present study, the preventive effects of the BZF moiety of P. incarnata upon the diazepam dependence have been evaluated in mice. In addition to this, the BZF moiety has also been evaluated for its own dependence-liability in mice upon a chronic treatment-regimen (21-days) at three different doses (10, 50 and 100 mg/kg, po). One group of mice was given the 20 mg/kg dose of diazepam (i.e., ten times of the anxiolytic dose of 2 mg/kg of diazepam in mice) for 21 days. The other groups were given BZF (10, 50 and 100 mg/kg) concurrently with the 20 mg/kg dose of diazepam for 21 days. No drug was administered for the next 72 hours. The ambulatory activity exhibited by each of the treated group was recorded 72 h after the administration of the last treatment on the 21st day (i.e., on the 25th day). The ambulatory activity exhibited by different groups of mice reflects the physiological and physical manifestation of diazepam-dependence, abrupt-diazepam-deprival oriented withdrawal effects, and the preventive effects of various doses of BZF upon development of diazepam-dependence and expression of diazepam-cessation withdrawal effects in mice (33, 34).
Materials and methods
Plant material
Aerial parts of P. incarnata were picked up in January 1999 from cultivated source Rati Ram Nursery at village Khurammpur via Kalsia in district Saharanpur (Uttar Pradesh, India). The identity of the procured plant material was confirmed from the Department of Systematic Botany, Forest Research Institute, Dehradun, India (FRI). A voucher specimen (code no. 1325/2000) was deposited in the Herbarium-cum-Museum of the FRI.
Extraction, fractionation and isolation of the bioactive BZF
The aerial parts were dried in the shade and powdered ( # 60 mesh size) and 100 g of the dried powder was Soxhlet extracted successively with petroleum ether (60-80OC), chloroform (Ranbaxy Laboratory Chemicals), methanol (sd Fine-Chem Limited) and distilled water. All these extracts were dried using a Buchi RE-121 Rotavapor (Buchi Laboratoriums-Technik AG, CH-9230 Flawyl/Schweiz, Switzerland) and were preserved in a vacuum desiccator containing anhydrous silica blue. The weight of various extracts after drying was calculated as: petroleum ether extract ( 6.8875 g), chloroform extract ( 8.2314 g), methanol extract ( 11.8787 g) and water extract ( 4.8876 g). The four different extracts of P. incarnata were suspended in a vehicle comprising simple syrup I.P. (Indian Pharmacopoeia 1996) and 1% w/w carboxymethylcellulose (CMC) as suspending agent. Five sets of doses viz., 300, 200, 125, 100 and 75 mg/kg of each extract of P. incarnata were prepared by suspending the dried extracts in the vehicle under vigorous stirring to form a uniform suspension. The weight of the dried extracts was so adjusted as to administer 0.25 ml of the suspension of the extracts. Simple syrup containing CMC was used as control. Amongst the four extracts, only the methanol extract of P. incarnata showed a significant anxiolytic activity at a dose of 125 mg/kg. The remaining three extracts did not exhibit anxiolytic activity statistically comparable to that of the standard anxiolytic (diazepam, 2 mg/kg in vehicle, p.o.). The bioactive methanol extract was processed and purified further by resorting to bioactivity directed fractionation using column chromatographic procedures until a fraction, which exhibited significant anxiolytic activity at a dose of 10 mg/kg in mice, was obtained (23). This fraction (yield =332 mg, 0.33%) gave positive tests for the presence of flavones (35). UV, LC-MS, GC-MS, IR, 1 H-NMR, 13C-NMR characterization studies have confirmed the presence of a benzoflavone moiety (BZF), never reported from P. incarnata earlier, that has been accounted for the CNS effects of P. incarnata (23-25) by the authors. The exact structure and complete chemical identity of BZF is not being presented here due to patent considerations.
Animals
Swiss albino mice (either sex) procured from the Disease-Free Small Animals House, College of Veterinary Sciences, Haryana Agriculture University, Hisar, India, were bred at the Central Animal House of the Panjab University, Chandigarh. The mice were allowed standard laboratory feed and water ad libitum. Groups of ten mice (20-24 g) were used in all sets of experiments.
Treatment schedule and measurement of ambulatory activity
The eight groups of mice were subjected to the following treatment for 21 days as follows:
Diazepam treated group (20 mg/kg) D
BZF treated group (10 mg/kg) P-10
BZF treated group (50 mg/kg) P-50
BZF treated group (100 mg/kg) P-100
Diazepam + BZF co-treatment group (20 mg/kg and 10 mg/kg respectively) DP-10
Diazepam + BZF co-treatment group (20 mg/kg and 50 mg/kg respectively) DP-50
Diazepam + BZF co-treatment group (20 mg/kg and 100 mg/kg respectively) DP-100
Control group that received vehicle only C
All treatments were administered orally for 21 days at 09.00 hour daily. No treatment was given during the next 3 days. The locomotor (ambulatory) activity of all the treated groups was determined on 25th day using an Animal Activity Meter (Opto Varimex Mini, Columbus Instruments, Ohio, USA). An array of 15 infrared emitter/detector pairs, spaced at 2.5 cm intervals, measured the animal activity along a single axis of motion, the digital data being displayed on the front panel meters as ambulatory activity. The animals were placed individually in a transparent plastic cage (29 cm x 22 cm x 22 cm) for 5 min and the ambulatory activity was recorded 72 hours after the administration of the last dose on the 21day, i.e., on the 25th day (36).
Statistical analysis
The data expressed as mean ± S.D. were analyzed by analysis of variance (ANOVA) followed by a Fischer LSD test, and P values < 0.05 were considered statistically significant (37).
Results
Effect of chronic treatment with diazepam
Chronic administration produced dependence and withdrawal effects appeared when the drug was discontinued. On abrupt termination of diazepam after 21-days treatment, the animals show relapse of severe anxiety, evident from the significant increase in ambulatory activity. The maximum withdrawal effects (manifested by increase in ambulatory activity) were apparent 72 hours after the cessation of 21-days treatment with diazepam.
Effect of chronic treatments with BZF
Chronic administration of BZF (10, 50 and 100 mg/kg, po) for 21 days did not produce any dependence. After the discontinuation of 21-days treatment with the three doses of BZF, the pattern of ambulatory activity reflected a normal physiological behavior on the 25th day.
Effect of BZF on the expression of diazepam-dependence oriented withdrawal effects
The three doses of BZF (10, 50, 100 mg/kg) upon concurrent administration with 20 mg/kg diazepam, significantly blocked the expression of withdrawal effects of diazepam-dependence, in a dose dependent manner. The group DP-100 showed less ambulatory activity relative to the group DP-50 and DP- 10 respectively on the 25th day.
Discussion
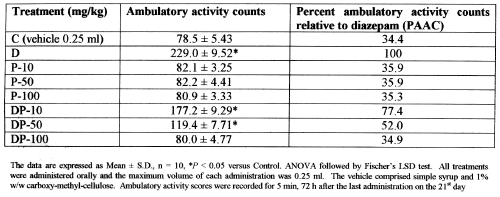
In rodents, an abrupt cessation of a chronic treatment with a proven anxiolytic agent (e.g., ethanol or diazepam) is manifested in the form of increased anxiety (4, 38) that has a direct bearing on the locomotor behavior of the animals (34, 39, 40). In several studies on behavioral manifestation of ethanol-dependence in mice, it has been hypothesized that an abrupt cessation of chronic treatment with ethanol leads to a relapse of anxiety, which is manifested in the form of increased ambulatory behavior in mice (29, 41, 42). The results of the present study with diazepam and BZF dependence also favor the same hypothesis. It is evident from Table 1 that administration of 10, 50 or 100 mg/kg doses of BZF for 21 days did not induce any dependence which is apparent from the normal ambulatory behavior of the BZF-treated mice, even after the cessation of BZF treatments for the next 3 days.
Table 1: Ambulatory activity scores recorded 3 days after administration of vehicle, diazepam, BZF, and diazepam-BZF combinations for 21 days.
The percent ambulatory activity counts (PAAC) after cessation of BZF-treatments, being statistically similar to the PAAC of the control group mice, further confirm that there are no physical symptoms of aggression or relapse of anxiety on 25th day, after the treatment with BZF for 21 days. Co-treatments of diazepam (20 mg/kg) concomitantly with the three doses of BZF for 21 days, also attenuate the development of diazepam dependence, in a dose-dependent-manner. The co-treatment of diazepam (20 mg/kg) with 100 mg/kg of BZF (DP-100) affords the complete suppression of the withdrawal-induced-anxiety, as the PAAC after this co-treatment (34.9) is statistically equivalent to that of the PAAC exhibited by the control group (34.4) of animals on the 25th day. The PAAC of the other co-treatments, i.e., DP-50 (20 mg/kg diazepam + 50 mg/kg BZF) and DP-10 (20 mg/kg diazepam + 10 mg/kg BZF) being 52.0 and 77.4 respectively.
Scientific studies have proved that chronic use of psychotropic substances like morphine (43), nicotine (44), ethanol (45), cannabinoids (46) and benzodiazepines (47-50) causes decline in blood-androgen levels depending upon the duration and quantity of the substance consumed. Benzodiazepines have also been reported to be implicated in male fertility and levels of testosterone upon chronic treatments (51-56). Treatment with 20 mg/kg dose of diazepam for 21 days is postulated to induce a significant decrease in testosterone levels that is manifested in the form of the anxiogenic behavior of the diazepam-treated-mice (57). However, co-treatments with BZF and diazepam are hypothesized to prevent decline in blood-testosterone levels, especially in light of the similar studies performed by the authors where the BZF moiety has been reported to prevent chronic ethanol and nicotine-induced azospermia, sterility and decreased libido, in healthy male rats (30, 31).
Benzodiazepines and barbiturates have long been hypothesized to modulate receptors in the gama amino-butyric acid (GABA) system (58). Over the past more than a decade, neuro-steroids (testosterone, progesterone, pregnanolone, dehydro-epi-androsterone and tetra-hydro-corticosterone, etc.) have been acknowledged as the key brain chemicals which regulate the neuro-biology of various psychiatric disorders, including drug-dependence and tolerance (59, 60). The identification of a steroid binding site on the GABA receptors and its implication in the sedative actions of neuroactive steroids has been a landmark discovery which facilitated the better understanding of various behavioral changes during drug addiction and other mental disorders (61-63). The neuro-steroidal implications of the BZF moiety isolated from the plant P. incarnata have provided an interesting hypothesis in understanding the multifarious biological effects of this potent plant-derived phyto-moiety. The benzoflavone compounds are the strongest (64, 65) inhibitors of the liver microsomal enzyme aromatase - a member of cytochrome P-450 family (66). Aromatase (P-450 3A4 enzyme) metabolizes testosterone to estradiol (67, 68). A specific functional aromatase gene `CYP 19' is necessary for the expression of aromatase enzyme in mice and human beings (69). Increased estrogen due to chronic benzodiazepine-administration increases the production of sex-hormone binding globulin (SHBG) (70). The SHBG binds testosterone and reduces the free testosterone in the blood. The increased estrogen levels cause the disruption of normal testicular production of testosterone and saturation of testosterone receptors in the hypothalamus in brain, thereby, reducing the signal sent to the pituitary gland which in turn reduces the secretion of LH. This ultimately reduces testosterone production in gonads (71). The testosterone levels in the plasma have an effect upon the gonadotropins [luteinizing (LH) and follicle-stimulating-hormone (FSH)], which regulate spermatogenesis and maturation of sperms (72). The BZF moiety isolated from aerial parts of P. incarnata is hypothesized to prevent the decline in androgens (a) through its anti-aromatase function, and (b) by eliminating estrogen's negative feedback loop, in light of the various reports (72-74). Further, the BZF moiety can be speculated to inhibit the metabolic conversion of androgens (testosterone) to estrogens (estradiol), thus, increasing free testosterone and decreasing free estrogen (75). The role of free testosterone in regulating behavioral anxiety (57) and stress in human beings and animals has been well established and well documented during the last 10 years (76, 77). High testosterone accounts for normalization of behavioral changes, that otherwise appear, in most of the mental disorders. BZF moiety influences the testosterone synthesis and maintains the optimum levels of free testosterone. Normalization of testosterone levels means health, joviality, and a normal behavior.
The beneficial effect of the co-treatment of diazepam with BZF is therefore apparent, as there are practically very little withdrawal effects even upon chronic intake of diazepam. The BZF moiety of P. incarnata affords a useful anxiolytic agent being free from dependence-liabilities even on a long-term chronic use. Secondly, the concurrent administration of BZF and diazepam affords a useful combination therapy, as the BZF moiety also prevents the development of diazepam-dependence and the subsequent appearance of withdrawal effects. The BZF moiety from passion flower offers a very useful phyto-moiety in countering the menace of substance dependence at-least in preliminary animal studies.
Acknowledgements
The authors are grateful to the Council of Scientific and Industrial Research, New Delhi, India for the award of Senior Research Fellowship to Kamaldeep Dhawan.
References
Baldessarini, R.J., Drugs and the treatment of psychiatric disorders. In, Hardman, J.G. and Limbird, L.E. (editors) Goodman & Gilman's The Pharmacological Basis of Therapeutics. The McGraw-Hill Companies, New York, the USA: 399-427, 1996.
Longo, L.P. and Johnson, B., Addiction: Part I. Benzodiazepines: Side effects, abuse risk and alternatives. American Family Physician, 61: 2121-2128, 2000.
Lader, M.H., Limitations on the use of benzodiazepines in anxiety and insomnia: are they justified? European Neuropsychopharmacology, 9 (Suppl 6): S399-S404, 1999.
Rickels, K. , Case, W. G. , Downing, R. W. and Winokur, A., Long-term diazepam therapy and clinical outcome. Journal of American Medical Association, 250: 767-771, 1983.
Woods, J.H., Katz, J.L. and Winger, G., Abuse liabilities of benzodiazepines. Pharmacology Reviews, 39: 251-413, 1987.
Ashton, H., Benzodiazepine withdrawal: an unfinished story. British Medical Journal, 288: 1135-40, 1984.
Busto, U., Sellers, E.M., Naranjo, C. A., Cappell, H. P., Sanchez, C. M. and Sykora, K., Withdrawal reaction after long-term therapeutic use of benzodiazepines. New England Journal of Medicine, 315: 654-59, 1986.
O'Brien, C. P., Drug Addiction and Drug Abuse. Wonsiewicz, M.J. and McCurdy, P. (eds). In:. Goodman & Gilman's The Pharmacological Basis of Therapeutics. The McGraw-Hill Companies, Inc., Nashville, Tennessee: 572-573, 1996.
Ernst, E., The risk-benefit profile of commonly used herbal therapies: Ginkgo, St. John's Wort, Ginseng, Echinacea, Saw Palmetto, and Kava. Annals of Internal Medicine, 136: 42-53, 2002.
Dechamp, J. F., Herbal Medicinal Products and Patient's Needs in Europe. Drug Information Journal, 33: 309-313, 1999.
Bartram, T., Encyclopedia of Herbal Medicine. Ed Grace Publishers, Dorset, England: Through http://www.raintree. com/maracuja.htm, 1995.
Blumenthal, M., Busse, W.R., Goldberg, A., Gruenwald, J., Hall, T., Riggins, C.W. and Rister, R. S. (eds.), Therapeutic Guide to Herbal Medicines. The American Botanical Council, Austin; copyright of Integrative Medicine Communications, Boston: 1998.
Reynolds, J.E.F. (ed), Martindale, The Extra Pharmacopoeia. Royal Pharmaceutical Society, London: 1738, 1996.
Passionflower, British Herbal Pharmacopoeia. British Herbal Medicine Society, Exeter, England: 154, 1983.
Passionbluenkraut, Deutsches Arzneibuch. Deutscher Apotheker Verlag., Stuttgart: 1997.
Passionflower, The Homoeopathic Pharmacopoeia of United States. American Institute of Homoeopathy, Arlington, VA: 440, 1981.
Passiflora incarnata, Homoeopathic Pharmacopoeia of India. Ministry of Health, Government of India, New Delhi: 116-117, 1974.
Dhawan, K., Kumar, S. and Sharma, A., Comparative biological activity study on Passiflora incarnata and P. edulis. Fitoterapia, 72: 698-702, 2001.
Dhawan, K., Kumar, S. and Sharma, A., Anxiolytic activity of aerial and underground parts of Passiflora incarnata. Fitoterapia, 72: 922-926, 2001.
Dhawan, K. and Sharma, A., Antitussive activity of the methanol extract of leaves of Passiflora incarnata. Fitoterapia, 73: 399-401, 2002.
Dhawan, K., Kumar, S. and Sharma, A., Anti-asthmatic activity evaluation of methanol extract of leaves of Passiflora incarnata. Phytotherapy Research, Manuscript No. PhyRes/2001/ 168 (in press): 2003.
Dhawan, K., Malik, J., Kumar, S. and Sharma, A., Aphrodisiac activity of methanol extract of leaves of Passiflora incarnata in mice. Phytotherapy Research, PhyRes/2001/000108 (in press): 2003.
Dhawan, K., Kumar, S. and Sharma, A., Antianxiety studies on extracts of Passiflora incarnata Linneaus. Journal of Ethnopharmacology, 78: 165-170, 2001.
Dhawan, K., Kumar, S. and Sharma, A ., Passiflora incarnata Linn.: A Promising Anxiolytic and Sedative - Isolation of a benzoflavone moiety as the bioactive agent. Presentation at the 42nd Annual Meeting of the American Society of Pharmacognosy, July 14-18, 2001, at Oaxaca, Mexico., Published in the Abstract Book as Oral Presentation - 29, 2001.
Dhawan, K., Kumar, S. and Sharma, A., Attenuation of drug/substance dependence by a novel tri-substituted benzoflavone moiety from Passiflora incarnata Linneaus. New Brunswick, New Jersey: 43rd Annual Meeting of the American Society of Pharmacognosy and 3rd Monroe Wall Symposium, July 28 – 31, 2002. Published in the Abstract Book as Oral Presentation -16, 2002.
Dhawan, K., Kumar, S. and Sharma, A., Reversal of tolerance and dependence of morphine by Passiflora incarnata Linneaus- A traditional medicine to combat morphine addiction. Pharmaceutical Biology (International Journal of Pharmacognosy), 40: 576-580, 2002.
Dhawan, K., Kumar, S. and Sharma, A., Reversal of cannabinoids (D9-THC ) by the benzoflavone moiety from methanol extract of Passiflora incarnata Linneaus in mice: a possible therapy for marihuana addiction. Journal of Pharmacy and Pharmacology, 54: 875-881, 2002.
Dhawan, K., Kumar, S. and Sharma, A., Nicotine reversal effects of the benzoflavone moiety from Passiflora incarnata Linneaus in mice. Addiction Biology, 7: 435-442, 2002.
Dhawan, K., Kumar, S. and Sharma, A., Suppression of alcohol-cessation-oriented hyper-anxiety by the benzoflavone moiety of Passiflora incarnata Linneaus in mice. Journal of Ethnopharmacology, 81: 239-244, 2002.
Dhawan, K., Kumar, S. and Sharma, A., Beneficial Effects of Chrysin and Benzoflavone on Virility in 2- Year-Old Male Rats. Journal of Medicinal Food, 5: 43-47, 2002.
Dhawan, K., and Sharma, A., Prevention of chronic alcohol and nicotine-induced azospermia, sterility and decreased libido, by a novel tri-substituted benzoflavone moiety from Passiflora incarnata Linneaus in healthy male rats. Life Sciences, 71: 3059-3069: 2002.
Dhawan, K. and Sharma, A., Attenuation of chronic D9 THC-induced decline in sexuality in male rats by a novel benzoflavone moiety of Passiflora incarnata Linn. British Journal of Pharmacology, 138: 117-120, 2003.
Vogel, H. G. and Vogel, W. H., Drug Discovery and Evaluation, Pharmacological Assays. Springer-Verlag, Germany, Heidelberg: 1997.
Kulkarni, S.K. and Sharma, A., Reversal of diazepam withdrawal induced hyperactivity in mice by BR 16-A (Mentat), a herbal preparation. Indian Journal of Experimental Biology, 32: 886-888, 1994.
Farnsworth, N. R., Biological and Phytochemical Screening of Plants. Journal of Pharmaceutical Sciences, 55: 225-286, 1966.
Kulkarni, S.K. and Reddy, D.S., Animal Behavioral Models for Testing Antianxiety Agents. Methods and Findings in Experimental and Clinical Pharmacology, 18: 219-240, 1996.
Bolton, S., Pharmaceutical Statistics, Practical and Clinical Applications. Marcel Dekker, Inc., 270, Madison Avenue, NY, the United States of America, New York: 516-521, 1990.
Woods, J.H., Katz, J. L. and Winger, G., Abuse liability of benzodiazepines. Pharmacology Reviews, 39: 251-419, 1987.
Madden, J.S., Psychiatric advances in the understanding and treatment of alcohol dependence. Alcohol and Alcoholism, 19: 339-353, 1984.
Roelofs, S.M., Hyperventilation, anxiety, craving for alcohol: a subacute alcohol withdrawal syndrome. Alcohol, 2: 501-505, 1985.
Boerngen-Lacerda, R. and Souza-Formigoni, M.L., Does the increase in locomotion induced by ethanol indicate its stimulant or anxiolytic properties? Pharmacology Biochemistry and Behavior, 67: 225-232, 2000.
Dawson, G. R., Crawford, S. P., Collinson, N., Iversen, S. D. and Tricklebank, M. D., Evidence that the anxiolytic-like effects of chlordiazepoxide on the elevated plus-maze are confounded by increases in locomotor activity. Psychopharmacology (Berlin), 118: 316-323, 1995.
Reisine, T. and Pasternak, G., Opiod analgesics and antagonists. In: Hardmann, J.G., Limbird, L.E., Molinoff, P.B., Ruddon, R.W. and Gilman, A.G., (eds). The Pharmacological Basis of Therapeutics. MacGraw Hill, New York: 521-556, 1996.
Yamamoto, Y., Isoyama, E., Sofikitis, N. and Miyagawa, I, Effects of smoking on testicular function and fertilizing potential in rats. Urology Research, 26: 45-48, 1998.
Tentler, J.J., LaPaglia, N., Steiner, J., Williams, D., Castelli, M., Kelley, M.R., Emanuele, N.V. and Emanuele, M.A., Ethanol, growth hormone and testosterone in peripubertal rats. Journal of Endocrinology, 152: 477-487, 1997.
Block, R.I., Farinpour, R. and Schlechte, J.A., Effects of chronic marijuana use on testosterone, luteinizing hormone, follicle stimulating hormone, prolactin and cortisol in men and women. Drug Alcohol Dependence, 28: 121-128, 1991.
Dong, E., Matsumoto, K. and Watanabe, H., Diazepam binding inhibitor reduces testosterone and estradiol levels in vivo. Life Science, 70: 1317-1323, 2002.
Sanbuissho, A., Terada, S., Suzuki, K., Masuda, N., Teranishi, M. and Masuda, H., Male reproductive toxicity study of nitrazepam in rats. Journal of Toxicology Science, 20: 319-328, 1995.
Leung, M., Drug-Induced sexual dysfuntion: assessment and management in men and women. Pharmacy Connects, Copyright © Rogers Media, Toronto: Web site http:// www.pharmacyconnects.com/pharprac/contact.html, through e-mail: pharmacypractice@mhpublishing.com, 1998.
Moerck, H.J. and Majelung, G., Gynaecomastia and diazepam abuse. Lancet, : 1344-1345, 1979.
Chopra, I.C., Drug Addiction. Indian Journal of Pharmacology, 3: 43-50, 1971.
Kishi, K., Kanamori, S., Maruyama, T., Sasaki, K., Hara, K., Kawai, M. and Ikeuchi, K., Potential parameters of male reproductive toxicity: reproductive performance, histopathology and sperm evaluation in SD rats given nitrazepam. Journal of Toxicology Science, 20: 329-339, 1995.
Calvo, D.J., Campos, M.B., Calandra, R.S., Medina, J.H. and Ritta, M.N., Effect of long term diazepam administration on testicular benzodiazepine receptors and steroidogenesis. Life Science, 49: 519-525, 1991.
Gavish, M., Hormonal regulation of peripheral-type benzodiazepine receptors. Journal of Steroid Biochemistry and Molecular Biology, 53: 57-59, 1995.
Frye, C.A., The role of neurosteroids and non-genomic effects of progestins and androgens in mediating sexual receptivity of rodents. Brain Research Reviews, 37: 201-222, 2001.
Primus, R.J. and Kellogg, C.K., Developmental influence of gonadal function on the anxiolytic effect of diazepam on environment-related social interaction in the male rat. Behavior Pharmacology, 1: 437-446, 1990.
Cooper, M. A. and Ritchie, E.C., Testosterone replacement therapy for anxiety. American Journal of Psychiatry, 157: 1884, 2000.
(58) Roberts, A.J. and Koob, G.F., The Neurobiology of Addiction: An Overview. Alcohol Health & Research World, 21: 101-106, 1997.
Reddy, D. S. and Kulkarni, S. K ., Differential anxiolytic effects of neurosteroids in the mirrored chamber behavior test in mice. Brain Research, 752: 61-71, 1997.
Reilly, M. T., Crabbe, J.C., Rustay, N.R. and Finn, D.A., Acute neuroactive steroid withdrawal in withdrawal seizure-prone and withdrawal seizure-resistant mice. Pharmacology Biochemistry and Behavior, 67: 709-717, 2000.
Lambert, J.J., Belelli, D., Hill-Venning, C. and Peters, J.A., Neurosteroids and GABA-A receptor function. Trends in Pharmacological Science, 16: 295-303, 1995.
Majewska, M.D., Neurosteroids: endogenous bimodal modulators of the GABA-A receptor mechanism of action and physiological significance. Progresses in Neurobiology, 38: 379-395, 1992.
Gee, K.W., McCauley, L.D. and Lan, N.C., A putative receptor for neurosteroids on the GABA-A receptor complex: the pharmacological properties and therapeutic potential of epalons. Critical Reviews in Neurobiology, 9: 207-227, 1995.
Kao, Y.C., Zhou, C., Sherman, M., Laughton, C.A. and Chen, S., Molecular basis of the inhibition of human aromatase (estrogen synthetase) by flavone and isoflavone phytoestrogens: A site-directed mutagenesis study. Environmental Health Perspective, 106: 85-92, 1998.
Kellis, J.T. and Vickery, L.E., Inhibition of estrogen synthetase (aromatase) by flavones. Science, 225: 1032-1033, 1984.
Lee, H.S., Jin, C., Park, J. and Kim, D.H., Involvement of P-4503A4 in the metabolism of 7,8-benzoflavone by human liver microsomes. Xenobiotica, 24: 1053-1062, 1994.
Guengerich, F.P., CYTOCHROME P-450 3A4: Regulation and Role in Drug Metabolism. Annual Reviews in Pharmacology and Toxicology, 39: 1-17, 1999.
Lee, H.S., Jin, C., Park, J. and Kim, D.H., Modulation of cytochrome P450 activities by 7,8-benzoflavone and its metabolites. Biochemistry and Molecular Biology International, 34: 483-491, 1994.
Robertson, K.M., Donnell, L.O., Jones, M.E., Meachem, S.J., Boon, W.C., Fisher, C.R., Graves, K.H., McLachlan, R.I. and Simpson, E.R., Impairment of spermatogenesis in mice lacking a functional aromatase (cyp 19) gene. Developmental Biology, 96: 7986-7991, 1999.
Sharpe, R. M., Natural and anthropogenic environmental oestrogens: the scientific basis for risk assessment - Environmental oestrogens and male infertility. Pure & Applied Chemistry, 70: 1685-1701, 1998.
Lam, M., Andropause. through www.DrLam.com and dr@DrLam.com, 2001.
Crémoux, P., Aromatase inhibitors: pharmacological aspects. Bulletin du Cancer, 87: 23-30, 2000.
Chen, S., Kao, Y.C. and Laughton, C. A., Binding characteristics of aromatase inhibitors and phytoestrogens to human aromatase. Journal of Steroid Biochemistry and Molecular Biology, 61: 107-115, 1997.
Cohen, R., Estrogen - The Unrecognized Male Hormone. International Antiaging Systems, Les Autelets Suite A, Sark GY9 0SF, Channel Islands, Great Britain: through http://www.smart-drugs.com, 2001.
Merken, H. M. and Beecher, G. R., Finessing the Flavonoids: A part of Human Nutrition, an ARS National Program. U. S. Government Printing Office, Beltsville, MD: 2001.
Lader, M., Treatment of anxiety. British Medical Journal, 309: 321-324, 1994.
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps