J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 6(1):1-12, 2003
Experimental estimation of the role of P-Glycoprotein in the pharmacokinetic behaviour of telithromycin, a novel ketolide, in comparison with roxithromycin and other macrolides using the Caco-2 cell model.
Jean I. Pachot1, Roger P. Botham, and Klaus D. Haegele
Department of Drug Metabolism and Pharmacokinetics, Aventis Pharma, Romainville, FranceKinKai Hwang
Department of Drug Metabolism and Pharmacokinetics, Aventis Pharma, Bridgewater, New Jersey, USAReceived 09 October 2002, Revised 25 November 2002, Accepted 18 December 2002
PDF version
Abstract
PURPOSE. The aim of this study was to examine the transport mechanism of telithromycin in comparison with erythromycin, azithromycin, clarithromycin and roxithromycin. METHODS: These antibiotics were examined in Caco-2 cell monolayers in order to demonstrate the potential involvement of P-GP in the absorption process, using verapamil as a P-GP competitor. A model using concentration equilibrium conditions was developed to delineate passive and active permeability components of telithromycin and roxithromycin transport in order to predict absorption in humans. RESULTS: Comparison of telithromycin Papp (AB)/ Papp (BA) ratios with those of the other antibiotics indicated that an efflux pump was involved which limited the transport of the macrolides to a greater extent than that of telithromycin. Modulation of Caco-2 transport of these antibiotics by verapamil and their reciprocal effect upon verapamil transport confirmed the involvement of P-GP and demonstrated that two substrates of P-GP may increase the transport of each other. Under concentration equilibrium conditions, both roxithromycin and telithromycin exhibited high mean Papp values for passive diffusion which extrapolated to 88% and 77% predicted human absorption respectively, if the involvement of P-GP was ignored. Both Km and Vm values suggested that saturation of P-GP by telithromycin may occur at a lower dose level in humans than with roxithromycin (Km= 9.8 mM, Vm= 0.3 mM and Km= 45 mM, Vm= 1.1 mM, respectively). At 4.10-5 M of either telithromycin or roxithromycin the passive flux was respectively 48% and 16% greater than the active efflux. CONCLUSIONS: The high absorption potential of telithromycin combined with the low Km and Vm values and the high dose level suggest that in humans the efflux pump may not limit ketolide absorption and that the interaction with other P-GP substrates may not significantly increase its oral absorption.
Introduction
Multidrug-resistance (MDR), which is a pleotropic expression of cross-resistance to structurally and functionally unrelated drugs represents a major challenge to drug design for optimal absorption, elimination, targeting, tissue distribution and drug-drug interaction (1-8). The MDR phenotype results from expression of an MDR gene which encodes for P-Glycoprotein (P-GP), a 170-Kda plasma membrane protein that belongs to the ATP-binding cassette superfamily (9). P-GP is expressed in several mammalian tissues including the epithelium of the intestinal tract and acts as an ATP-dependent efflux pump for a broad range of compounds (10-12). It has been reported that P-GP expression at intestinal site level is responsible for interpatient variation in oral bioavailability and for the limited oral uptake of drugs which are substrates of the efflux pump (13-16). Since absorption appears to be the key factor affected by this phenomenon, an understanding of the physiological role of P-GP in drug absorption and the underlying post-absorptive events have recently become the focus of a number of studies (9, 17-19).
The Caco-2 model is widely used in the pharmaceutical industry to predict oral absorption in humans of passively transported drugs (20-24). However, due to the fact that the level of expression of P-GP within the Caco-2 cell line is dependent on the culture conditions employed, estimation of human oral absorption from in-vitro permeability data becomes difficult for compounds which are substrates of the efflux pump. This becomes more critical when the considered substrate of P-GP is likely to be co-administered with another drug, which is itself a substrate of P-GP. In this case, anticipation of drug-drug interactions and pharmacokinetic behaviour become important points in the drug development program.
Determination of both the relative affinity of a drug for P-GP as well as the maximal velocity of its efflux rate may provide a method to investigate such drug-drug interactions with regard to absorption potential modulation. Existing models to study P-GP which are based on ATPase activity (25-27) or radioligand-binding assays (28) give an insight into the affinity of a drug for the efflux protein but do not provide information on the functional role of the efflux pump during the transport and may not allow determination of the contribution of P-GP under passive diffusion conditions. Recently a combined approach to evaluate both the passive diffusion and affinity of a substrate for P-GP, which should provide a better understanding of the role of the efflux pump was proposed (29). However, passive permeability of a number of known PG-P substrates is determined solely in the presence of the P-GP inhibitor, verapamil. This assumes that the high affinity of verapamil for PG-P results in complete inhibition of the PG-P driven efflux. In addition, the fact that at least 2 substrate binding sites (30,31) exist for this allosteric protein suggests that interpretation of such radioligand binding assays may not be straightforward.
In this study, the relative contributions of PG-P driven efflux and passive diffusion in the absorption of roxithromycin and telithromycin, which is a novel ketolide with an improved spectrum and level of activity against erythromycin-resistant S. pneumoniae and H. influenzae (32), was investigated. Firstly, the absorptive mechanism was investigated in Caco-2 cell monolayers in order to determine if P-GP was implicated in the transport of the test compounds. Subsequently, an experimental model was developed to quantify both the passive and active permeability components. The impact on the prediction of intestinal absorption in humans was then evaluated.
MATERIALS AND METHODS
Chemicals
Telithromycin, roxithromycin, azithromycin, clarithromycin, erythromycin and verapamil were used throughout the study. The compounds were greater than 96% pure. [3H]-labelled telithromycin and roxithromycin, at a specific activity of 1.31 TBq/mmol and 0.81 TBq/mmol, respectively and greater than 95% purity were synthesised by Aventis, Romainville, France (Figure 1). D-[1-14C]-mannitol (spec. act. 1.11 TBq/mmol) and [3H]- azithromycin, clarithromycin, erythromycin and verapamil (spec. act. 869 GBq/mmol, 821.4 GBq/mmol, 740 GBq/mmol, 3.1 TBq/mmol, respectively) were provided by NEN-DuPont (Les Ulis, France). All other chemicals were at least analytical grade and purchased from Sigma (St-Quentin Fallavier, France).
Figure 1: Structure of telithromycin and roxithromycin and location of the 3H-atom (T) in the radiolabelled compounds.
Caco-2 cell culture
Caco-2 cells, clone TC7, were kindly donated by Dr. A. Zweibaum and Dr. M. Rousset (INSERM, U170, Villejuif, France) at passage 8. Cells, monitored as mycoplasma free by polymerase chain reaction (33), were cultured as described elsewhere (34). Briefly, 3.105 Caco-2 cells were seeded in 25 cm 2 plastic flasks (Costar, Brumath, France) and maintained in a complete medium, containing DMEM supplemented with 20% inactivated foetal calf serum (Boehringer, Meylan, France) and 1% of nonessential amino acids 100 X (Life Technologies, Eragny, France). Cells were incubated at 37°C in a controlled atmosphere at 95% relative humidity with 90% air and 10% CO2. The culture medium was changed every day for 7 days, during which time the cells had reached confluency. For transport studies, cells at passage number 11 to 30 were seeded on 12 mm diameter polycarbonate filter inserts (Transwell®, Costar) at a density of 5.10 5 cells per filter. The cells were allowed to grow and differentiate for 21 to 28 days in the complete medium supplemented with penicillin (100 U/ml) and streptomycin (100 mg/ml). This supplemented medium was changed every day. Transepithelial electrical resistance (TEER) of the monolayers was measured before transport studies, using an epithelial volt-ohmmeter fitted with planar electrodes (Evom-Endhom, WPI, Stevenage, England).
Transport studies across Caco-2 cell monolayers
Caco-2 monolayers, checked for confluency and membrane integrity were used in transport studies with HBSS / HEPES as the transport medium. Donor and receiver HBSS / HEPES solutions were prepared according to the protocol described below. Before the beginning of transport studies, 50-100 ml of the donor solution were withdrawn for analytical assay. Culture medium in both apical and basolateral sides of each well were withdrawn and discarded.
For apical to basolateral (AB) transport studies, the apical side was filled with 0.5 ml of donor solution while the basolateral side was filled with 1.5 ml of receiver medium. Conversely, for basolateral to apical (BA) transport studies, the apical side was filled with 0.5 ml of receiver medium while 1.5 ml of donor medium was added to the basolateral side Replicates of 3 to 6 wells were used in each case.
The plates were maintained in the incubator at 37°C under controlled atmosphere. For transport studies in the AB direction, samples (500 ml) were withdrawn for analytical assay at 20-min intervals for 120 min from the basolateral side and immediately replaced by fresh receiver medium. For transport studies in the BA direction, samples (250 μl) were withdrawn at 20 min intervals for 120 min from the apical side and immediately replaced by fresh receiver medium. At the end of transport studies, 50-100 ml samples were withdrawn from the donor side for analytical assay.
Monolayer integrity was checked again at the end of the experiment by measuring TEER values and mannitol permeability. A greater than 25% decrease in TEER values compared to initial readings and a mannitol permeability greater than 1.10-6 cm/s, were taken as an indicator of loss of monolayer integrity, in which case the results were discarded. Permeability values were calculated as indicated below.
Three sets of experimental conditions were used in these studies:
Concentration gradient conditions which were used to determine transport across Caco-2 cell monolayers either in the AB or BA direction.
Competition studies with verapamil under concentration gradient conditions.
Concentration equilibrium conditions which were used to distinguish passive and active transport.
Concentration gradient conditions: Apical to basolateral and basolateral to apical transport measurement
Transport studies in the AB and BA directions were performed with telithromycin, roxithromycin, erythromycin, azithromycin, clarithromycin and verapamil.
For both roxithromycin and telithromycin, the concentrations ranged from 10-6 M to 10-4 M whereas erythromycin, azithromycin and clarithromycin were used at 5.10-5 M. Finally, for verapamil the concentrations ranged from 10-7 M to 10-4 M.
The unlabelled test compounds were first dissolved in ethanol to give a concentration of 10 -2 M. This solution was diluted in HBSS / HEPES medium, in order to obtain the final desired concentration and then spiked with both the corresponding radioactive compound and [14C] mannitol to give a final donor solution containing 4mCi/ml of [3H]-test compound and 0.4mCi/ml of [14C] mannitol.
To assess the metabolic stability of telithromycin during Caco-2 transport studies, an additional assay was performed in the AB direction using unlabelled compound at 10-4 M.
Competition studies
The interaction of telithromycin and roxithromycin with P-glycoprotein was determined by evaluating the transport of the test compound in both directions (AB and BA) using the same solutions of [3H]-telithromycin, [3H]-roxithromycin, [3H]-erythromycin, [3H]-azithromycin and [3H]-clarithromycin, as described above, to which 10-4 M of cold verapamil was added. The results were compared to those obtained in the standard conditions, i.e. without addition of verapamil to the donor solutions.
Furthermore, to investigate the reciprocity of competition with P-GP, the unidirectional transport of [3H]-verapamil (4 mCi/ml) across Caco-2 monolayers was followed in both AB and BA directions at a final concentration of 10-6 M in combination with either cold telithromycin or roxithromycin at both 10-6 M and 10-4 M.
Transport studies in concentration equilibrium conditions: passive and active efflux determination
Transport studies of both telithromycin and roxithromycin were carried out under concentration equilibrium conditions in order to evaluate active transport independently of passive transport. For this purpose, a special protocol was designed in which the test compound was present in both apical and basolateral sides at the same concentration, ranging from 10-6 M to 5.10-5 M. However, the donor solution was spiked with 4 mCi/ml of [3H]]- compound and 0.4 mCi/ml of [14C] mannitol.
Sample analysis
Samples of [3H]-telithromycin, [3H]-roxithromycin, [3H]-erythromycin, [3H]-azithromycin, [3H]-clarithromycin, [3H]-verapamil and [14C]-Mannitol were assayed by dual labelled b-scintillation counting with quench correction (LKB Wallac 1213, Broma, Sweden). For assessment of the metabolic stability of telethromycin during transport studies at 10-4 M, samples were assayed by LC/APCI/MS.
Apical to basolateral and basolateral to apical transport measurement
Unidirectional flux J (absorptive JA→B and secretory JB→A) for the test compound and permeability (Papp ) values for both the test compound and [14C] mannitol were calculated as described below.
The radioactive concentration (nmol eq/ml) of [3H]-telithromycin, [3H]-roxithromycin, [3H]-erythromycin, [3H]-azithromycin, [3H]-clarithromycin and [3H]-verapamil were determined at t0 (time zero) by measuring the mean radioactivity in 1 ml of donor solution. The concentration of radioactivity in the receiver side (Cs0 ) was calculated as follows:
(1)
Where DPM receiver is the radioactivity in 1 ml of receiver sample. 0 is the concentration of [3H]-telithromycin, [3H]-roxithromycin, [3H]-erythromycin, [3H]-azithromycin, [3H]-clarithromycin or [3H]-verapamil used in the donor medium. Mean DPMdonor is the mean value of counts in 2 aliquots of the donor samples at t0.
The cumulative amount of test compound (Q in eq.nmol) transported across Caco-2 cell monolayers between time zero to time i was calculated. For this purpose, radioactive concentrations in the receiver side were corrected for dilution produced during transport studies as indicated in the following equation:
(2)
Where, Csi is the radioactive concentration at time i. Vp, is the volume of samples and Vt is the total volume in the receiver side.
Unidirectional flux values (J nmol/ cm2 .h), were calculated according to the following equation:
(3)
Where, DQ represents the amount of compound accumulated in the receiver fluid during the time interval Dt and A, exposed area of monolayers (1.13 cm2 ).
Apparent permeability (Papp in cm/s) of each test compound was derived from the fluxes according to the equation:
(4)
Where C0 is the initial concentration in the donor medium.
The ratio between the permeability values obtained in transport studies in the (AB) and (BA) directions was calculated according to the following equation:
(5)
To achieve this calculation, the wells were paired in a random manner.
Passive and active efflux transport determination
The passive and active transport of telithromycin and roxithromycin were determined in concentration equilibrium conditions according to the methodology described below.
Unidirectional flux values (absorptive JA→B and secretory JB→A) were calculated according to equation 3. Assuming that an active efflux affected transport of the test compound, the active efflux was calculated for each concentration according to the following equations:
(6)
(7)
The net flux (Jnet ), can be written as follows:
(8)
Since passive diffusion from the apical to basolateral side is equal to that in the opposite direction, the active efflux for each concentration tested is then deduced according to the following equation:
(9)
To achieve calculations for equation 9, the wells were paired in order to determine the standard errors about the means (sem).
Finally, flux for passive diffusion of the compounds and its corresponding permeability were calculated using the same pairs of wells as indicated above according to the following equations:
(10)
(11)
Where Ci is the concentration of test compound in the apical side.
Apparent affinity for P-glycoprotein and maximal velocity determination
For telithromycin and roxithromycin, the calculated active efflux JB→AActive of each compound was plotted against the concentration. Assuming that P-GP acts as an enzyme which obeys Michaelis-Menten kinetics, the plots were fitted with a non linear model using Deltagraph software as indicated in the following equation:
(12)
Where, J0 is the initial flux (nmol/ cm2. hr) of the active transport, Jm = Vm (nmol/ cm2.hr) which is the maximal velocity of the active transport, Km is the apparent affinity for the efflux pump (nmol/l) and C0 , is the concentration used in the donor medium.
The apparent affinity and maximal velocity Vm were then obtained from the best non-linear fit.
Estimation of maximal fraction absorbed in humans after oral administration
The fraction absorbed (fa) in humans after oral administration of telithromycin and roxithromycin was estimated using calculated passive permeability values and assuming that dissolution in-vivo and the administered dose level were not rate-limiting steps for intestinal absorption. The calculation was based on (i) Papp data obtained in Caco-2 transport studies performed in the laboratory with 29 reference compounds which are transported passively, (ii) the percentage absorbed in humans following oral administration of the same 29 compounds. These data were plotted as a standard calibration curve as described earlier (35):
(13)
The non-linear fit of the calibration curve indicated that at p=0.05 A= -5.664 and B = -26.018.
fa values were calculated using the mean passive Papp values at different concentrations calculated under concentration equilibrium conditions. Assuming that P-GP is not expressed at all in humans, such calculated fa values determine the maximal fraction absorbed in humans irrespective of the active efflux.
Statistical analysis
The means and standard error of the means were calculated by means of Excel Software. The means and the standard error of the means were expressed to 2 significant figures for the fluxes and permeability values.
Results
Membrane integrity control
Caco-2 cell monolayers exhibited TEER values greater than 180 W.cm2 prior to transport studies. At the end of these studies no decrease in TEER values was observed for any of the compounds in any of the experimental conditions. Permeability values obtained for Mannitol during transport studies were lower than 1.10-6 cm.s-1 irrespective of the experimental conditions and test compound used (data not shown).
Transport parameters in concentration gradient conditions
Transport studies designed to assess the metabolic stability of unlabeled telithromycin (10-4 M) indicated that the compound was stable. At the end of the 2 hour assay the sum of the Apical and Basolateral concentrations of telithromycin was found to be equivalent to the initial concentration of parent telithromycin in the donor solution. No known metabolites of telithromycin were identified in either the apical or basolateral solutions. That is, total recovery of parent drug was observed.
Transport studies using telithromycin at concentrations ranging from 10-6 M to 10-4 M across Caco-2 cell monolayers indicated that their was no correlation between either the absorptive or secretory fluxes and the concentration of the compound on the donor side (Figure 2).
Figure 2: Mean apical to basolateral (A) and basolateral to apical (B) flux values of telithromycin and roxithromycin across Caco-2 cell monolayers as a function of the concentration in the donor medium in the absence or in the presence of 10-4 M of verapamil and in concentration gradient conditions.
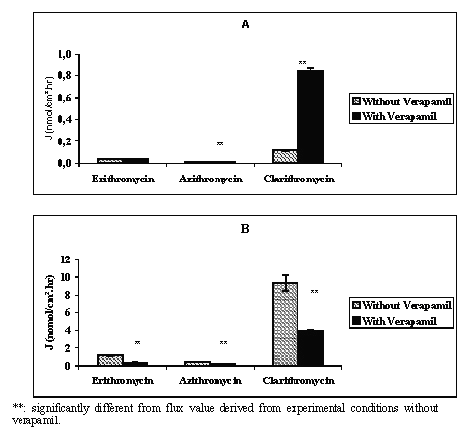
At 10-6 M the mean flux values measured in AB direction were 0.001 ± 0.0001 and 0.003 ± 0.001 nmol/cm2.hr while those measured in the BA direction were 0.056 ± 0.002 and 0.079 ± 0.007 nmol/cm2.hr respectively for telithromycin and roxithromycin. At 5.10-5 M the mean absorptive flux values were 0.14 ± 0.01, 0.023 ± 0.0088, 0.038 ± 0.001, 0.008 ± 0.001, and 0.118 ± 0.006 nmol/cm2.hr respectively for telithromycin, roxithromycin, erythromycin, azithromycin and clarithromycin, while those measured in the BA direction were respectively 18, 153, 30, 54 and 4,400 fold greater (Figures 2, 3).
Figure 3: Mean apical to basolateral (A) and basolateral to apical (B) flux values of erythromycin, azithromycin and clarithromycin across Caco-2 cell monolayers in the absence or in the presence of 10-4 M of verapamil and in concentration gradient conditions (N = 3 to 4).
For each compound tested, the measured flux value in the BA direction was significantly higher than that measured in the AB direction (p<0.01). As a result, the permeability Papp ratios were 19, 70, 30, 67 and 80 respectively for telithromycin, roxithromycin, erythromycin, azithromycin and clarithromycin (Table 1). When the concentration in the donor medium was increased from 10-6 M to 10-4 M it resulted in an increase in the measured flux values in the AB direction of 260 and 52 fold while those measured in the BA direction increased 64 and 90 fold respectively for telithromycin and roxithromycin. For each concentration tested, the flux value in the BA direction was significantly higher than that in the AB direction (p<0.01), and the calculated Papp ratios ranged from 13 to 99 for telithromycin and from 46 to 70 for roxithromycin (Table 1).
Table 1: Mean value of the ratio Papp (BA) / Papp (AB) obtained in transport studies across Caco-2 cell monolayers under standard conditions with or without 10-4 M verapamil.
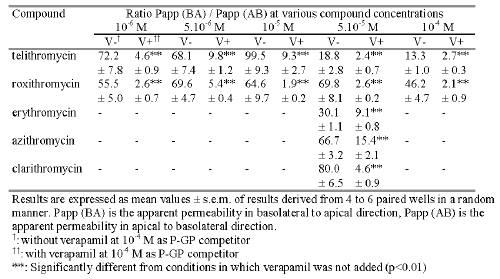
In the presence of 10-4 M verapamil the results indicated that except for erythromycin, compared to data obtained without the P-GP inhibitor, the absorptive flux values of the antibiotics increased while those of secretion decreased with a significant level of p<0.01 (Figure 2 and Figure 3). The addition of 10-4 M verapamil in combination with 10-6 M telithromycin or roxithromycin increased significantly the mean flux values in the AB direction 4.0 and 1.7 fold and decreased significantly those in the BA direction 2.4 and 6.1 fold respectively (p<0.01). At 5.10-5 M, the measured flux values in the AB direction were increased 2.4, 9.0, 1.8 and 7.2 fold respectively for telithromycin, roxithromycin, azithromycin and clarithromycin (p<0.01), while that of erythromycin remained unchanged. In the BA direction, flux values were significantly decreased for all the antibiotics by a factor ranging from 2.2 to 6.4 (p<0.01). However, the calculated P app ratios were significantly less (p<0.01) than those observed without verapamil and ranged from 9.8 to 2.7 for telithromycin and 5.4 to 1.9 for roxithromycin depending on the concentration used (Table 1). Verapamil in combination with one of the antibiotics at a concentration of 5.10-5 M induced a significant decrease in the calculated Papp ratio by a factor of 7.8, 26.8, 3.3, 4.3, and 17.4 respectively for telithromycin, roxithromycin, erythromycin, azithromycin and clarithromycin (p<0.01).
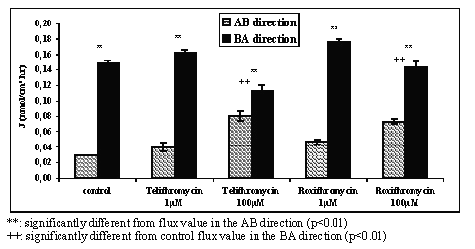
The reciprocal effect of roxithromycin or telithromycin upon the transport of verapamil (10 -6 M) was concentration dependent (Figure 4). The value for verapamil flux in the AB direction increased 1.3 and 1.6 fold in the presence of 10-6 M telithromycin or roxithromycin respectively with a significance level of p<0.01. At a concentration of 10 4 M telithromycin or roxithromycin the AB flux values of verapamil were 2.7 and 2.4 fold greater respectively with a significance level of p<0.001. In the BA direction a significant decrease in verapamil flux values was only observed with the antibiotics at a concentration of 10-4 M.
Figure 4: Mean apical to basolateral (AB) and basolateral to apical (BA) flux values of verapamil across Caco-2 cell monolayers in the absence (control) or in the presence of 10-4 M and 10-6 M of either telithromycin or roxithromycin in the donor solution and in concentration gradient conditions (N=3 to 4).
Apical to basolateral (AB) and basolateral to apical (BA) transport under concentration equilibrium conditions
Under these conditions, transport studies with telithromycin and roxithromycin at concentrations ranging from 10-6 M to 4.10-5 M indicated that the absorptive and secretive fluxes were not linearly correlated with the donor concentration and that transport in the BA direction was considerably greater than that observed in the AB direction (Figure 5).
Figure 5: Mean apical to basolateral (AB) and basolateral to apical (BA) flux values of telithromycin and roxithromycin across Caco-2 cell monolayers as a function of the concentration in the donor medium and in concentration equilibrium conditions (N= 3).
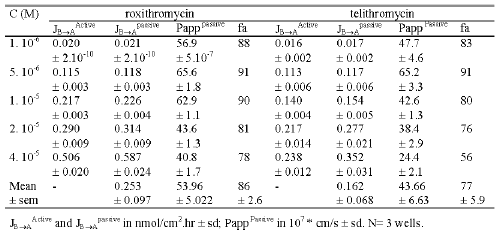
At 10-6 M the mean flux values for telithromycin were 0.001 ± 0.0001 and 0.033 ± 0.003 nmol/cm2 .hr in the AB and BA direction, respectively while those measured at a concentration of 4.10-5 M were respectively 114 fold and 18 fold greater. The mean Papp value in the AB direction significantly increased (p<0.01) from 2.9 10-7 ± 0.2 10-7 at 10-6 M to 7.9 10-7 ± 1.4 10-7 cm/s at 4.10-5 M, while the mean Papp value in the BA direction significantly decreased from 99.4 10-7 ± 2.4 10-7 cm/s at 10-6 M to 41.0 10-7 ± 2.6 10-7 cm/s at 4.10-5 M (Table 2).
Table 2: Passive flux, active efflux, passive permeability and extrapolated maximal absorption in humans of telithromycin and roxithromycin calculated in transport studies across Caco-2 monolayers in concentration equilibrium conditions.
Similar results were obtained for roxithromycin. At 10-6 M the mean flux values were 0.001 ± 0.0001 and 0.040 ± 0.0001 nmol/cm2 .hr respectively in the AB and BA directions while those measured at 4.10-4 M were respectively 81 fold and 27 fold greater (Figure 5). The mean Papp value in the AB direction increased from 3.2 10-7 ± 0.1 10-7 cm/s at 10-6 M to 5.6 10-7 ± 0.3 10-7 cm/s at 4.10-5 M, while the Papp value in the BA direction decreased from 119.3 10-7 ± 3.7 10-7 at 10-6 M to 75.9 10-7 ± 3.1 1-7 cm/s at 4.10-5 M (Table 2).
Active and passive transport determination
Under concentration equilibrium conditions, the active efflux, passive flux and passive permeability values were calculated for each concentration used in the experiment. Telithromycin efflux increased as the concentration of the compound increased until a plateau was reached at 2.10-5 M with a value of 0.2 nmol/ cm2 .hr. Conversely, roxithromycin efflux did not appear to plateau, even at 4.10-5 M where the efflux was about 0.5 nmol/ cm2 .hr. For both roxithromycin and telethromycin, calculated passive flux indicated that at concentrations equal to or lower than 1.10-5 M, the values were similar to those obtained for the active efflux (Table 2). Roxithromycin passive flux values were 8% and 16% greater than those for active efflux at 2.10-5 M and 4.10-5 M respectively, while for telithromycin at the same concentrations, passive flux values were 28% and 48% greater than active efflux values respectively.
Over the range of concentrations used the calculated permeability values for passive diffusion varied from 40.8 10-7 cm/s to 65.6 10- 7 cm.s -1 for roxithromycin and 24.4 10 7 cm/s to 65.2 10-7 cm/s for telithromycin.
Apparent affinity for P-Glycoprotein and maximal velocity determination
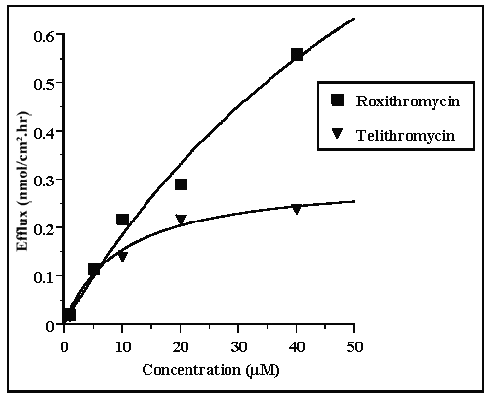
Plots of active efflux versus concentration of the compounds are shown in Figure 6. The best fit plots indicated that Km values were 45.4 and 9.8 mM respectively for roxithromycin and telithromycin and that Vm values were 1.1 and 0.3 nmol/ cm 2 .hr respectively.
Figure 6: Michaelis-Menten plot of the active BA efflux values of telithromycin and roxithromycin across Caco-2 cell monolayers in concentration equilibrium conditions.
Estimation of maximal fraction absorbed in humans
Percent absorption in humans after oral administration of either roxithromycin or telithromycin was calculated from passive permeability values obtained in concentration equilibrium conditions. The percent absorption in humans ranged from 78% to 91% for roxithromycin and 56% to 91% for telithromycin, depending on the calculated permeability values obtained at different concentrations of the relevant antibiotic, while the mean percent absorption in humans was 86% and 77% respectively.
DISCUSSION
TEER values remained unchanged during transport studies and irrespective of both the concentration of antibiotics and the experimental conditions used, the permeability of mannitol was always less than 1.10-6 cm/s. These results demonstrate that no adverse alteration of the monolayers occurred.
Results with unlabeled telithromycin (10-4 M) showed that there was no significant metabolism of the compound during the 2 hour transport studies. Thus, it is assumed that the apparent permeability determined in transport studies with 3 H-telithromycin reflects the permeability of unchanged telithromycin. Roxithromycin metabolic stability was not determined during transport studies. Nevertheless, metabolic stability of both roxithromycin and telithromycin during intestinal transport needs to be confirmed in-vivo to assess the impact on oral absorption.
Under concentration gradient conditions, flux values obtained in both AB and BA directions as well as the Papp ratio values indicated that an active efflux mechanism could be involved in the transport of telithromycin, roxithromycin, erythromycin, azithromycin and clarithromycin (Figures 2, 3 and Table 1).
The decrease in Papp ratio values as a function of concentration was more pronounced for telithromycin than for roxithromycin suggesting that the efflux pump became saturated at lower concentrations of telithromycin than roxithromycin (Table 1). Comparison of the Papp ratio for telithromycin with those obtained for erythromycin, azithromycin and clarithromycin at 5.10-5 M, indicated that active secretion by the efflux pump, in comparison with passive permeability, was more pronounced for the latter antibiotics than for telithromycin. As a consequence of the involvement of an efflux pump in telithromycin transport, a non linear relationship between Cmax and low dose levels may be expected in humans following oral administration.
Addition of verapamil (10-4 M) to the donor solutions of telithromycin and roxithromycin resulted in both a significant increase in their flux values in the AB direction and a decrease in flux values in the BA direction whatever the concentration of antibiotics (Figure 2). Similar results were obtained with erythromycin, azithromycin and clarithromycin at 5.10-5 M in the presence of verapamil (Figure 3). Modulation of the transport of these antibiotics by verapamil suggested that P-GP was involved in the transport process. Previous in vitro studies with Caco-2 monolayers clearly demonstrated that the transport of telithromycin was modulated by cisapride whilst transport of cisapride was modulated by telithromycin. (data not shown). In addition, the amount of telithromycin found in the mesenteric vein following perfusion of the rat jejunum with a solution containing 10-4 M telithromycin, in the presence of either verapamil or ketoconazole (2x10-4M), increased 1.5 and 1.7 fold respectively compared to perfusion of telithromycin alone (data not shown). These results further support the idea that transepithelial transport of telithromycin is P-GP dependent. Other studies have demonstrated that erythromycin, clarithromycin and azithromycin are substrates of P-GP (36-38).
The values of the Papp ratio for both telithromycin and roxithromycin were more than six fold lower in the presence of verapamil than in the absence of verapamil. However, irrespective of the concentration of antibiotics in the donor solution, verapamil (10-4 M) was unable to completely inhibit the P-GP efflux pump. These results suggest that verapamil cannot be used as a P-GP competitor to study passive permeation of drugs in complete absence of the active efflux as reported in an earlier study (29). Addition of verapamil induced a variable decrease in the values of the Papp ratio for erythromycin, azithromycin, telithromycin, clarithromycin and roxithromycin, at a concentration of 5.10-5 M. However, verapamil modulated the transport of erythromycin in the BA direction but failed to influence its transport in the AB direction. The reason for the lack of effect of verapamil on erythromycin transport from the apical to the basolateral side is unknown. Modulation of the Papp ratio of the antibiotics by verapamil suggests that the greatest decrease in the ratio value was indicative of the lowest relative apparent affinity for P-GP. Thus, the rank order of apparent affinity for P-GP should be the following, roxithromycin < clarithromycin < telithromycin < erythromycin < azithromycin.
The results of the studies to assess whether there was a reciprocal effect on verapamil transport by telithromycin or roxithromycin showed that in the presence of the antibiotics, an increase in AB transport of verapamil occurred (Figure 4). These results suggest that co-administration to humans of two drugs which are substrates of P-GP may produce an increase in the oral absorption of both of these drugs.
Since P-GP expression in the Caco-2 cell line is known to be dependent on culture conditions (39), the prediction of intestinal absorption in humans from Papp values of compounds which interact with this efflux transporter may be underestimated. In order to evaluate the apparent affinity for P-GP in a functional model, transport studies have been performed under concentration gradient conditions using cell monolayers (40, 41). However, in such experimental models P-GP driven efflux may be overestimated due to the existence of a chemical driving force. Hunter et al., (3) used a protocol in which the unlabelled compound was maintained in concentration equilibrium conditions and the same compound as radiolabelled tracer was introduced either into the apical or basolateral side. The apparent affinity for P-GP was derived from the net efflux of the tracer (Jnet = JB→A - JA→B) as a function of the concentration of the cold material. In our conditions active efflux was calculated assuming that (i) no driving force was present since concentration equilibrium conditions were respected, (ii) the contribution of the radiolabelled compound at tracer concentration in the donor side was negligible and did not affect the concentration equilibrium, (iii) the efflux was due solely to P-GP (iv) the active efflux was equal to half of the net flux of the radiolabelled tracer compound since in both AB and BA transport only the radiolabelled compound was monitored. In fact, both JA→B and JB→A are the macroscopic reflection of the algebraic sum of both passive flux and active efflux vectors of the radiolabelled tracer compound which, in contrast to the unlabelled compound, is present in concentration gradient conditions. In such conditions, delineation between passive and active efflux in Caco-2 transport studies is possible and may give a better insight into the impact of P-GP in humans by comparison with the passive flux component.
Non linear regression analysis, following a Michaelis-Menten equation plot indicated that apparent Km values for P-GP were 4.5 10-5 M and 1.10-5 M, respectively for roxithromycin and telithromycin. In addition, Vm values indicated that the maximum rate of efflux for telithromycin was about 3.5 fold lower than that for roxithromycin (1.1 and 0.30 nmole/cm2.h respectively). These results were in agreement with those obtained under concentration conditions and in competition studies in which the Papp ratios suggested that telithromycin had a higher apparent affinity for P-GP than roxithromycin. On the other hand, comparison of active efflux and passive flux values for roxithromycin indicated that these values were of the same order of magnitude at concentrations lower than 2.10-5 M. Conversely, at high telithromycin concentrations the passive flux was 48% greater than the active efflux which suggested that there was a relatively lower influence of P-GP induced efflux in telithromycin absorption than in roxithromycin absorption (Table 2).
Telithromycin is administered to humans daily at a dose of 800g and the aqueous solubility of telithromycin in buffer at pH 2.2 and 7 are 0.24 M and 3.2x10-2 M respectively. Thus, oral administration of 800 g (» 1 mole) of telithromycin should give rise to concentrations of telithromycin in the stomach (250 ml) and intestinal fluids (e.g. 1 liter) of around 0.24 M and 3.2x10-2 M respectively. In our Caco-2 transport studies, in which the concentration of telithromycin ranged from 10-6 M to 10-4 M, saturation of the efflux pump appeared to occur at 20 mM telithromycin. Therefore, following administration of telithromycin at high dose levels, in which P-GP is likely to be saturated, it is expected that active efflux should be of minor importance in oral absorption of this ketolide. Similarly, for roxithromycin one may expect a concentration of 1.2x10-4 M in intestinal fluid after oral administration of a 150 mg dose.
Analysis of passive diffusion data indicated that both roxithromycin and telithromycin exhibited intrinsically high mean Papp values extrapolating to 88% and 77% predicted absorption in humans, respectively irrespective of the involvement of P-GP and the rate of in-vivo dissolution of the drugs. This is in agreement with the in-vivo data obtained in humans, where absolute bioavailability of telithromycin is estimated to be 57%.
The results imply that, as a consequence of the high passive permeability of telithromycin, its high oral dose level together with its apparent high affinity for P-GP and low Vm value, the efflux pump may not limit the oral absorption of this ketolide in humans and its interaction with other drugs mediated by P-GP, may not result in a significant increase in its intestinal uptake. The strategy employed here to delineate between the contribution of passive permeability and active efflux in order to predict oral absorption of drugs subjected to a P-GP-efflux mechanism requires confirmation through the study of further compounds.
References
Chin J.E., Soffir R., Noonan K.E., Choi K., and Roninson I.B., Structure and expression of the human MDR (P-Glycoprotein) gene family. Mol. Cell. Biol., 9: 3808-3820, 1989.
Oudard S., Thierry A., Jorgensen T.J., and Rahman A., Sensitization of multidrug-resistance colon cancer cells to doxorubicin encapsulated in liposomes. Cancer Chemother. Pharmacol. 28: 259-265, 1991.
Hunter J., Hirst B.H., and Simmons N.L., Drug absorption limited by P-glycoprotein-mediated secretoy drug transport in human intestinal epithelial Caco-2 cell layers. Pharm. Res., 10: 743-749, 1993.
Schinkel A.H., Wagenaar E., Van Deemter L., Mol C.A., and Borst P., Absence of the mdr1a P-glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, dogoxin, and cyclosporin A. J. Clin. Invest. 96: 1698-16705, 1995.
Sparreboom A., Van Asperen J., Mayer U., Schinkel A.H., Smit J.W., Meijer D.K.F., Borst P., Nooijen W.J., Beijnen J.H., and Van Tellingen O., Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. Proc. Natl. Acad. Sci. 94: 2031-2035, 1997.
Wakasugi, H., Yano, I., Ito T., Hashida, T., Futami, T., Nohara, R., Sasayama, S., and Inui, K., Effect of clarithromycin on renal excretion of digoxin: interaction with P-glycoprotein. Clin. Pharmacol. Ther. 64: 123-128, 1998.
Zhang Y., Hsieh Y., Izumi T., Lin E.T., and Benet L.Z., Effects of ketoconazole on the intestinal metabolism, transport and oral bioavailability of K02, a novel Vinylsulfone peptidomimetic cysteine protease inhibitor and a P450 3A, P-glycoprotein dual substrate, in male Sprague-Dawley rats., J. Pharmacol. Exp. Ther. 287: 246-252, 1998.
Yu D.K., The contribution of P-glycoprotein to pharmacokinetic drug-drug interaction., 39: 1203-1211, 1999.
Hsing S., Gatmaitan Z., and Arias I.M., The function of Gp170, the multidrug-resistance gene product, in the brush border of rat intestinal mucosa. Gastroenterology, 102: 879-885, 1992.
Pavelic Z.P., Reising J., Pavelic L., Kelley D.J., Stambrook P.J., and Gluckman J.L., Detection of P-glycoprotein with four monoclonal antibodies in normal and tumor tissues. Arch. Otolaryngol. Head Neck Surg. 119: 753-757, 1993.
Terao T., Hisanaga E., Sai Y., Tamai I., and Tsuji A., Active secretion of drugs from the small intestinal epithelium in rats bu P-glycoprotein functioning as an absorption barrier. J. Pharm. Pharmacol., 48: 1083-1089, 1996.
Makhey V.D., Guo A., Norris D.A., Hu P., Yan J., and Sinko P.J., Characterization of the regional intestinal kinetics of drug efflux in rat and human intestine and in Caco-2 cells. Pharm. Res. 15: 1160-1167, 1998.
Saitoh H. and Aungst B.J., Possible involvement of multiple P-glycoprotein-mediated efflux systems in the transport of verapamil and other organic cations across rat intestine. Phram. Res. 12: 1304-1310, 1995.
Lown K.S., Mayo R.R., Leichtman A.B., Hsiao H.L., Turgeon D.K., Schmiedlin-Ren P., Brown M.B., Guo W., Rossi S.J., Benet L.Z., and Watkins P.B., Role of intestinal P-glycoprotein (mdr1) in interpatient variation in the oral bioavailability of cyclosporine. Clin. Pharmacol. Ther. 62: 248-260, 1997.
Kim, R.B., Fromm, M.F., Wandel, B.L., Wood, A.J.J., Roden D.M., and Wilkinson G.R., The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J. Clin. Invest., 101: 289-294, 1998.
Van Asperen, J., Van Tellingen, O., Schinkel, A.H., and Beijnen, J.H., Comparative pharmacokinetics of vinblastine after a 96-hour continuous infusion in wild-type mice and mice lacking mdr1a P-glycoprotein. J. Pharmacol. Exp. Ther., 289: 329-333, 1999.
Emi, Y., Tsunashima, D., Ogawara, K-I., Higaki, K., and Kimura T., Role of P-glycoprotein as a secretory mechanism in quinidine absorption from rat small intestine. J. Phram. Sci. 87: 295-299, 1997.
Van Asperen, J., Van Tellingen, O., Schinkel, A.H., and Beijnen, J.H., The pharmacological role of P-glycoprotein in the intestinal epithelium. Pharm. Res. 37: 429-435, 1998.
Ito, K., Kusuhara, H., Sugiyama, Y., Effects of Cyp3A4 and P-glycoprotein on oral drug absorption-theoretical approach. Pharm. Res., 16: 225-231, 1999.
Kim, D.-C., Burton, P.S., and Borchardt, R.T., A correlation between the permeability characteristics of a series of peptides using an in vitro cell culture model (Caco-2) and those using an in situ perfused rat ileum model of the intestinal mucosa. Pharm. Res., 10: 1710-1714, 1993.
Artursson, P., Palm, K., and Luthman, K., Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 22: 67-84, 1996.
Bayley, C.A., Bryla, P., and Malick, A.W., The use of the intestinal epithelial cell culture model, Caco-2, in pharmaceutical development. Adv. Drug Deliv. Rev., 22: 85-103, 1996.
Gan, L.-S. L. and Thakker, D.R., Applications of the Caco-2 model in the design and development of orally active drugs : elucidation of biochemical and physical barriers posed by the intestinal epithelium. Adv. Drug Deliv. Rev., 23: 77-98, 1997.
Yee, S., In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal absorption in man - fact or myth. Pharm. Res., 14: 763-766, 1997.
Garrigos, M., Belehradek J.Jr., Mir, L.M., and Orlowski S., Absence of cooperativity for MgATP and verapamil effects on the ATPase activity of P-glycoprotein containing membrane vesicules. Biochem. Bioph. Res. Com., 196: 1034-1041, 1993.
Orlowsky, S., Mir, L.M., Belehradek, J., and Garrigos, M., Effects of steroids and verapamil on P-glycoprotein ATPase activity: progesterone, desoxycorticosterone, corticosterone and verapamil are mutually non exclusive modulators. Biochem. J. 317: 515-522, 1996.
Orlowsky, S., Valente, D., Garrigos, M., and Ezan, E., Bromocriptine modulates P-glycoprotein function. Biochem. Bioph. Res. Com., 244: 481-488, 1998.
Döppenschmitt, S., Spahn-Langguth, H., Regårdh, C.G., and Langguth P., Radioligand-binding assay employing P-glycoprotein overexpression cells: Testing drug affinities to the secretory intestinal multidrug transporter. Pharm. Res., 15: 1001-1006, 1998.
Döppenschmitt, S., Spahn-Langguth, H., Regårdh, C.G., and Langguth P., Role of P-glycoprotein-mediated secretion in absorptive drug permeability: an approach using passive membrane permeability and affinity to P-glycoprotein. J. Pharm. Sci., 88: 1067-1072, 1999.
Garrigos, M., Mir, L.M., and Orlowski S., Competitive and non-competitive inhibition of the multidrug-resistance-associated P-glycoprotein ATPase. Further experimental evidence for a multisite model. Eur. J. Biochem., 244 : 664-673, 1997.
Pascaud, C., Garrigos, M., Orlowski, S., Multidrug resistance transporter P-glycoprotein has distinct but interacting binding sites for cytotoxic drugs and reversing agents. Biochem. J., 333: 351-358, 1998.
Denis, A., Agouridas, C., Auger, J.M., Benedetti, Y., Bonnefoy, A., Bretin, F., Chantot, J.F., Dussarat, A., Fromentin, C., D’Ambrières, S.G., Lachaud, S., Laurin, P., Le Martret, O., Loyau, V., Tessot, N., Pejac, J-M. Perron, S., Synthesis and antibacterial activity of HMR 3647 a new ketolide highly potent against erythromycin-resistant and susceptible pathogens. Bioorg Med Chem Lett., 9: 3075-3080, 1999.
Rawadi, G., Lecaque, D., Pirot, D., and Roman-Roman, S., Detection and identification of mycoplasma contamination in cell cultures by polymerase chain reaction. Meth. Molec. & Cell. Biol. 4: 147-156, 1993.
Boisset, M., Botham, R.P., Haegele, D.K., Lenfant, B., Pachot, J.I., Absorption of angiotensin II antagonists in Ussing chambers, Caco-2, perfused jejunum loop and in vivo: Importance of drug ionization in the in vitro prediction of in vivo absorption. E. J. Pharm. Sci, 10: 215-224, 2000.
Pontier C., Pachot J., Botham R., Lenfant B., and Arnaud P., HT29-MTX and Caco-2/TC7 monolayers as predictive models for human intestinal absorption: Role of the mucus layer. J. Pharm. Sci. 90: 1608-1619, 2001.
Takano M., Hasegawa R., Fukuda T., Yumoto R., Nagai J., and Murakami T., Interaction with P-glycoprotein and transport of erythromycin, midazolam and ketoconazole in Caco-2 cells. Eur. J. Pharmacol. 358: 289-294, 1998.
[37] Saito H., Fukasawa Y., Otsubo Y., Yamada K., Sezaki H., and Yamashita S., Carrier-mediated transport of macrolide antibacterial agents across Caco-2 cell molnolayers. Pharm. Res. 17: 761-765, 2000.
He L., and Liu G.Q., Effects of various principles from Chinese herbal medicine on rhodamine123 accumulation in brain capillary endothelial cells. Acta Pharmacol. Sin., 2002, 23: 591-596, 2002.
Anderle P., Niederer E., Rubas W., Hilgendorf C., Spahn-Langguth H., Wunderli-Allenspach H., Merkle H.P., and Langguth P., P-glycoprotein (P-GP) mediated efflux in Caco-2 monolayers: the influence of culturing conditions and drug exposure on P-GP expression levels. J. Pharm. Sci., 87: 757-762, 1998.
Zhang Y., and Benet L.Z., Characterization of P-glycoprotein mediated transport of K02, a novel vinylsulfone peptidomimetic cysteine protease inhibitor, across MDR1-MDCK and Caco-2 cell monolayers. Pharm. Res. 15: 1520-1524, 1998.
Walle, U.K., and Walle, T., Taxol transport by human intestinal epithelial Caco-2 cells. J. Pharmacol. Exp. Ther. 26: 343-346, 1998.
Corresponding Author: Jean I. Pachot, Aventis, 102 route de Noisy, 93235 Romainville Cedex, France. jean.pachot@aventis.com
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps