J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 6(1):27-32, 2003
Co-encapsulation of two plasmids in chitosan microspheres as a non-viral gene delivery vehicle.
Suna Özbas-Turan1, Cenk Aral1, Levent Kabasakal2, Meral Keyer-Uysal2, and Jülide Akbuga1
Marmara University, Faculty of Pharmacy, Department of Pharmaceutical Biotechnology1 and Pharmacology2, Istanbul, TurkeyReceived 3 April 2002, Revised 16 January 2003, Accepted 3 February 2003
PDF version
Abstract
PURPOSE. The aims of this study are to encapsulate two different plasmid DNAs (pGL2 and pMK3) in the same microsphere structure and to investigate in vivo transfection characteristics of chitosan microspheres. Furthermore, the effect of formulation factors, such as chitosan concentration and plasmid DNA amount on in vitro properties of microspheres were studied. Methods. Double plasmid-loaded chitosan microspheres were prepared by complex coacervation. Release studies were done in phosphate buffered saline at 37°C and released plasmid DNA was determined spectrophotometrically. Integrity of plasmid DNAs was checked by agarose gel electrophoresis. For in vivo transfection studies, microspheres were injected into the muscle of the mice and expression of proteins ( b -galactosidase and luciferase) was measured. Results. High encapsulation efficiency was obtained with chitosan microspheres (90%). The size of particles was about 1.15 - 1.28 m. No dependence was observed between the size and formulation variables (chitosan concentration and the amount of plasmid). After encapsulation process, integrity of two plasmids did not change. Plasmid DNAs were continuously released from chitosan microspheres. Chitosan concentrations and plasmid amounts affected in vitro release properties. After intramuscular injection of double plasmids loaded microspheres into muscle of the mice, co-expression was obtained. High b -galactosidase and luciferase productions were determined with these microspheres after a long post-transfection period (12 weeks). Conclusions. Our results showed that two plasmids could be encapsulated in chitosan microspheres without affecting their structural and functional integrity. Thus, sustained and high protein production was obtained with these microspheres.
Introduction
For successful gene therapy, it is necessary to develop vectors capable of efficiently introducing genetic materials into target cells (1). The most widely investigated non-viral gene delivery systems are liposomes, polycation complexes and microcapsules (2-5). Recently, various carriers for non-viral gene delivery have been developed, among them cationic carriers are widely accepted because of their ability to efficiently condensate plasmid DNA and interact with cell membrane (6, 7). A number of polymers have been investigated as gene carriers, including PLGA [poly(D,L-lactic-co-glycolic acid)] (8), gelatin (9) and chitosan. However, the encapsulation of plasmid DNA in PLGA has been problematic. Incorporation of pDNA is often low (10). Moreover, while developing these microspheres is that the encapsulated plasmid is degraded not only during the encapsulation process but also mainly in the course of the polymer degradation (11).
Chitosan is a non-toxic biodegradable polycationic polymer with low immunogenicity (12-15). It is a good candidate for the gene delivery system, because cationically charged chitosan can be complexed with negatively charged plasmid DNA and promising results with chitosan as a gene delivery carrier have been obtained (4,5,16,17). Chitosan can effectively bind DNA and protect DNA from nuclease degradation (18). On the other hand, sonication and organic solvents are not used for the preparation of chitosan microspheres. This suggests that process is mild enough not to inflict any damage on the DNA. DNA-loaded chitosan microparticles were found stable during the storage (19). In vitro transfection ability of chitosan and derivatives were reported by Erbacher et al., (7). The application of DNA-chitosan nanospheres has advanced in vitro DNA transfection research and data have been accumulating which show their utility for gene delivery (4,16,20).
In general, gene therapy was used against treating monogenic (single-gene) disorders but, it has become clear that many types of diseases including multigenic disorders (such as, coronary hearth disease, Alzheimer's disease, several autoimmune diseases). However, there is no information about multiple plasmid encapsulations.
In this study, an attempt was made to study co-encapsulation possibility of two plasmids in the same delivery vehicle. The pMK3 and pGL2 plasmids were used and a sustained-release delivery system was developed. The in vivo transfection of chitosan microspheres containing multiple plasmids was investigated. Moreover, the effect of formulation variables such as chitosan concentration and the amount of plasmid on in vitro properties of chitosan microspheres were studied.
Materials and Methods
Animals and reagents
Balb/c mice (4-6 weeks, 20 ± 1.55 g) were obtained from Animal House of Marmara University. Luciferase assay kit was obtained from Promega (USA). Chitosan (Mr : ca. 400.000 D, viscosity: ca. 200 mPa in 1% acetic acid at 20°C) was purchased from Fluka (Germany). b -galactosidase, luciferase, Na2 SO4, ONPG (o-nitrophenyl b -D-galactopyranoside), X-Gal and all other chemicals were obtained from Sigma (USA).
Plasmids
Two plasmids containing b -galactosidase and luciferase reporter genes were used in this study. pMK3 plasmid (7213 bp) (ATCC, USA) contains lac Z gene which encoded b -galactosidase. pGL2 plasmid (6046 bp) (Promega, USA) contains of SV40 early promoter and luciferase gene with polyadenylation signal.
Amplification of plasmid DNA
pMK3 and pGL2 plasmids were amplified in the HB101 and JM109 strain of E.Coli respectively and extracted by the alkaline-lysis technique (21) and purified by precipitation with ethanol. Then the precipitate was dissolved and maintained in Tris-EDTA buffer and concentration of DNA was determined spectrophotometrically at 260 nm (Shimadzu, UV-2100S, Japan). The purity of isolated plasmid was confirmed by electrophoresis on a 0.8% w/v agarose gel.
Preparation of DNA-loaded microspheres
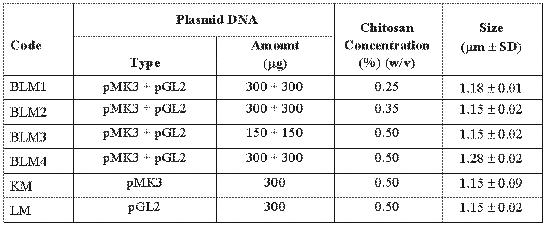
Plasmid loaded chitosan microspheres were prepared by complex coacervation method as previously described (18, 22). Briefly, sodium sulphate solution (20% w/v) containing plasmids were dropped into the chitosan solution (0.25% w/v) and stirred (Ika-Werk, Germany) at 500 rpm for 1 hour. Formed particles were separated by centrifugation (Sigma 30K, Germany) for 10 min. at 12000 rpm. and stored at 4°C after freeze-drying (Lyovac-Leybold GT2, Germany). Size of microspheres was determined by using an ocular micrometer in a light microscope (Olympus BH, Japan) (n= 1000 particles). Encapsulation efficiency of microspheres was estimated by measuring the amount of uncapsulated DNA in the supernatant after centrifugation. Therefore, the amount of DNA encapsulated in the microspheres was calculated by measuring between the total amount of DNA added in the preparation medium and the amount of non-entrapped DNA remaining in the aqueous suspension after coacervation process (23). In order to study the effect of formulation variables (chitosan concentration and plasmid amount) on microsphere properties, different microsphere formulations were prepared (Table 1).
Table 1: Codes and formulations of chitosan microspheres containing plasmid DNA.
Agarose gel electrophoresis of DNA
The purity of plasmid DNAs were investigated by electrophoresis. Plasmid DNA (500 ng equivalent weight of DNA in each lane) was applied into the slots of a 0.8% agarose gel containing tris-boric acid-EDTA (TBE) buffer (pH 8.3) and ethidium bromide (0.5 mg/ml) at constant voltage (80 V) (Horizantal gel apparatus system, ATTO, Japan). DNA was visualized under the UV light (Vilber Lourmat, USA) and the conformation of plasmid DNA was checked using the gel documentation system (Kodak Digital Science, DC 40 Camera and 1D Image Analysis Software, USA).
In vitro release studies
Release of plasmids from chitosan microspheres was determined in phosphate buffered saline (PBS, pH 7.4) at 37 ± 0.5°C and at appropriate time intervals samples were taken and supernatants were separated by centrifugation. The released DNA was measured spectrophotometrically at 260 nm. After each sampling, the microspheres were resuspended in the fresh medium. Corrections due to chitosan were also made during the spectrophotometric measurement therefore empty microspheres were used as a blank. Released samples were checked with agarose gel electrophoresis as described above. For this purpose, released plasmid DNA was precipitated by ethanol and dissolved in TE-buffer prior to electrophoresis.
In vivo transfection
Balb/c mice of both sexes were used in these studies. Each treatment group had six mice (3 male and 3 female). For in vivo transfection experiments, a mixture of BLM 1, 2 and 4 were used and coded as BLM. 100 mg naked or encapsulated plasmid DNAs (suspension in 0.5 ml 0.9% NaCl) were injected into the anterior tibialis muscle of the mice. Saline solution was injected as control. At time intervals (1, 3, 8 and 12 weeks), mice were slightly anaesthetised and biopsy was performed and b-galactosidase and luciferase activities were determined as mentioned below. All animal experiments were conducted in accordance with the "Principles of Laboratory Animal Care" (NIH Publication, 1985).
Determination of b -galactosidase and luciferase expression
The biopsy specimens (1.4 ± 0.13 mg) were washed with 0.9% NaCl solution two times and tissue samples were lysed in PBS by five freezing-thawing cycles after treatment with acetone-toluene (19:1) mixture as described previously (18).
Luciferase gene expression was measured using a luciferase assay kit (Promega, USA) according to manufacturer's instructions by using a luminometer (Berthold, USA).
b-Galactosidase enzyme activity in the muscle of mice was spectrophotometrically assayed at 520 nm using o-nitrophenyl- b-D-galactoside as a substrate of enzyme (18). Moreover total protein amount of the sample was also measured according to Bradford (24).
Histology
The distribution of b-galactosidase expressing cells within the muscle tissue was observed by 5-bromo-4-chloro-3-indolyl- b-D-galactopyranoside (X-gal) histochemical method of Bancroft and Cook (25). Briefly, biopsy specimens were taken and frozen; serial sections were sliced with a cryostat and placed on slides. The slices were fixed with a mixture of paraformaldehyde (2% v/v) and glutaraldehyde (2% v/v) in PBS. Then the specimens were rinsed with PBS and stained with X-gal solution (2 mM MgCl2, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 1 mg/ml X-gal). Blue stained muscle cells were observed for the expression.
Statistical analysis
Differences were compared for significance using either the Mann-Whitney Rank Sum Test or the Student's t-Test ("p" value less than 0.05 was considered significant).
Results and Discussion
Characterization of two plasmids-loaded microspheres
In this study, two different plasmids such as pMK3 and pGL2 were encapsulated into the chitosan microspheres. The mean diameter of microspheres was between 1.15 and 1.28 mm (Table I). Encapsulation efficiency was about 90% (data not shown). High encapsulation was earlier reported for DNA-chitosan nanospheres (16).
After encapsulation process, agarose gel electrophoresis was carried out to assess the integrity of co-encapsulated plasmids. After digestion with PBS, microsphere (BLMs, KM and LM) extracts were separated and supernatants applied on agarose gel and bands were compared with the bands of free plasmids. No change was observed in the electrophoretic mobility of DNA (Figure 1). As seen in the gel photograph, lane A showed the bands similar to that of the plasmids (pMK3 and pGL2) (Lanes D, E and F). This result is in accordance with the results of Leong et al. (16).
Figure 1: Agarose gel electrophoresis of released plasmid DNA from chitosan microspheres.
Lane A : Mixture of pMK3 + pGL2
Lane B : Free pGL2
Lane C : Free pMK3
Lane D : λ DNA/Hind III Marker
Lane E : pMK3-loaded microsphere (KM)
Lane F : pGL2-loaded microsphere (LM)
Lane G : Microsphere containing double plasmids (BLM)
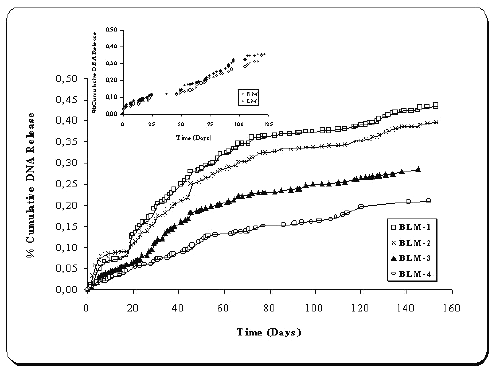
Release profiles of pMK3 and pGL2 loaded microspheres (BLM1-4) are given in Figure 2. In general, very little burst effect was seen in the release profiles of two plasmids-loaded microspheres (Figure 2). Plasmid DNAs were continuously released from chitosan microspheres. When a comparison of four formulations was made, that release patterns of the microspheres (BLM 1-4) are significantly different (p<0.05). The in vitro release of plasmid DNAs from microspheres was dependent on plasmid amount (BLM 3 and 4) and chitosan concentration (BLM 1, 2 and 4) (p< 0.05) (Figure 2).
Figure 2: Release profiles of double plasmid-loaded microspheres (n=5).
Inset : Release profiles KM and LM (single-plasmid loaded chitosan microspheres).
As the amount of plasmids increased, release decreased significantly (p<0.05). The same results were obtained with the microspheres containing different concentration of chitosan. The influence of chitosan concentration on in vitro release characteristics of DNA-loaded chitosan microspheres was reported in our earlier paper (18), so these data are consistent with our previous findings. There is no significant difference between the release patterns of single and double-plasmid loaded microspheres (KM, LM and BLM) (p>0.05).
In vivo transfection studies
DNA-loaded (pMK3 and pGL2) chitosan microspheres were injected into the muscle of the mice and expression of two proteins in the muscle tissue was measured. Findings were compared with the data of naked plasmids and control. Results are given in Figures 3 and 4.
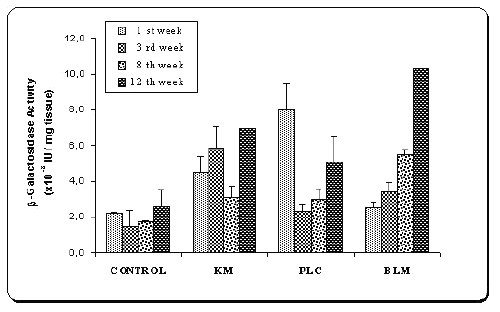
Figure 3: b -Galactosidase activities of tissue samples of the mice after injection of naked plasmid mixture (pMK3 + pGL2) (PLC) or double plasmid loaded microspheres (BLM) or single plasmid loaded microspheres (pMK3) (KM).
Results were compared with saline injections as control.
Values are mean ± SD (n=6).
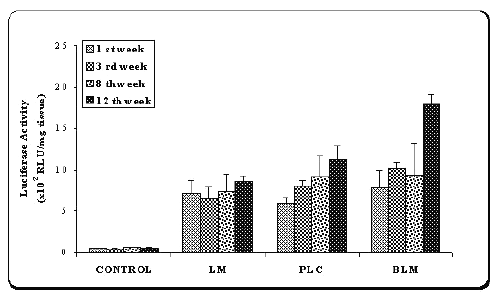
Figure 4: Luciferase activity in the muscle of the mice after injection of naked plasmid mixture (pMK3 + pGL2) (PLC) or double plasmid loaded microspheres (BLM) or single plasmid loaded microspheres (pGL2) (LM).
Results were compared with saline injections as control.
Values are mean ± SD (n=6).
The mixture of naked plasmids (PLC) demonstrated early b -galactosidase expression as shown in Figure 3, such that after 7 days of the transfection, high b -galactosidase level was observed in the samples injected naked plasmid mixture (PLC), but then their protein levels decreased. However injection of two plasmids-loaded microspheres (BLM) produced gradually increasing b -galactosidase activity. As seen in this figure, after a week transfection, expression level of b -galactosidase was even lower than that of naked DNA (p<0.005). When the expressed protein levels of single (KM) and double plasmid loaded microspheres (BLM) were compared during the 8 weeks, higher protein expression was observed with the single-plasmid loaded microspheres (p<0.05). This may be due to plasmid contents of microspheres. However, at the end of the 12th week, encapsulated plasmids (BLM) induced very high protein activity. As it is expected, DNA released from chitosan in a sustained matter and showed gradually increased transfection.
As shown in Figure 4, when the data of luciferase assay compared with control, high luciferase activity was obtained with two plasmids-loaded microspheres (BLM). However, except 12th week after injection, similar protein expression was observed with single (LM) and double (BLM) plasmid-loaded microspheres. A significant difference was observed between the luciferase activity levels of encapsulated (BLM) and naked plasmids (PLC) (p<0.005). On the other hand, after microsphere injection, at 12th week, muscle biopsies were evaluated for tissue distribution of expressed b -galactosidase using X-gal staining.
As seen in Figure 5, blue stained muscle cells confirmed the expression of protein in the muscle of the mice. Briefly, chitosan microspheres containing two different plasmid DNAs showed sustained and longer period of the protein expression. Each plasmid expressed their product continuously. During the first weeks, encapsulated plasmids resulted lower level expression. Similar result was previously reported by Leong et al (16) for chitosan microspheres.
Figure 5: b-Gal staining of the mice tissues after double plasmid-loaded microsphere (BLM Injection The letter "f" indicates muscle fibers and arrows indicated blue stained b-galactosidase expressed muscle fibers.
Various multigenic disorders include coronary artery disease, diabetes mellitus and several neurologic diseases, are the target of current gene therapy studies. Current research is based on use of single gene that coded one of these proteins however, use of two or more genes in the same therapy protocol may be more effective than the other. However, there is no information about the encapsulation of more than one plasmids in the same carrier. However, the cloning the sequences for the two individual gene products into one plasmid can be more expensive and time consuming. Therefore, co-encapsulation of two plasmids into same carriers may be more advantageous.
Two plasmids can be easily encapsulated into chitosan microspheres without affecting their structural and functional integrity. In addition, sustained and high protein expression may be possible with this carrier.
Acknowledgment
This study is partially funded by State Planning Organisation (DPT) of Turkey. The authors also thank to Prof. Dr. Süha Yalçin and Meral Yüksel for using luminometer and Dr. Feriha Ercan for helps in histological studies.
References
Crystal, R.G., Transfer of genes to humans: early lessons and obstacles to success. Science, 270:404, 1995.
Roemer, K. and Friedmann, T., Concept and strategies for human gene therapy. Eur. J. Biochem., 208:211-225, 1992.
Lasic, D.D. and Templeton, N.S., Liposomes in gene delivery. Adv. Drug Delivery Rev., 20:221-266, 1996.
Mao, H,Q., Roy, K., Walsh, S.M., August, J.T. and Leong, K.W., DNA-chitosan nanospheres for gene delivery. Proceed. Int. Symp. Cont. Rel. Bioact. Mater., 23:401-402, 1996.
Lee, K.Y., Kwon, I.C., Kim, Y.H., Jo, W.H. and Jeong, S.Y., Preparation of chitosan self aggregates as a gene delivery system. J. Cont. Rel., 51:213-220, 1998.
Felgner, J.H., Kumar, R., Sridhar, C.N., Wheeler, C.J., Tsai, Y.J., Border, R., Ramsey, P., Martini, M. and Felgner, P.L., Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J. Biol. Chem., 269:2550-2561, 1994.
Erbacher, P., Zou, S., Bethinger, T., Steffan, A.M. and Remy, J.S., Chitosan based vector/DNA complexes for gene delivery: biophysical characteristics and transfection ability. Pharm. Res., 15: 1332-1339, 1998.
Tinsley-Bown, A.M., Fretwell, R., Dowsett, A.B., Davis, S.L. and Farrar, G.H., Formulation of poly(D,L-lactic-co-glycolic acid) microparticles for rapid plasmid DNA delivery. J. Contr. Rel., 66:229-241, 2000.
Truong-Le, V.L., August, J.T. and Leong, K.W., Controlled gene delivery DNA-gelatin nanospheres. Human gene ther., 9(12):1709-1717, 1998.
Wang, D., Robinson, D.R., Kwon, G.S. and Samuel, J., Encapsulation of plasmid DNA in biodegradable poly(D,L-lactic-co-glycolic acid) microspheres as a novel approach for immunogene delivery. J. Contr. Rel., 57:9-18, 1999.
Walter, E., Moelling, K., Pavlovic, J. and Merkle, H.P., Microencapsulation of DNA using poly(D,L-lactic-co-glycolide) stability issues and release characteristics. J. Contr. Rel., 61:361-374, 1999.
Arai, K., Kunumaki, T. and Fujita, T., Toxicity of chitosan. Bull. Tokai Reg. Fish. Lab., 43:89-94, 1968.
Brine, C.J.; Stanford, P.A.; Zikalis, J.P., Advances in chitin and chitosan. Elsevier Applied Sci, London, 1992.
Carrero-Gomez, B. and Duncan, R., Evaluation of the biological properties of soluble chitosan and chitosan microspheres. Int. J. Pharm., 148:131-140, 1997.
Richardson, S., Kolbe, H.V.J. and Duncan, R., Evaluation of highly purified chitosan as a potential gene delivery vector. Proceed. Int. Symp. Contr. Rel. Bioact. Mater., 24:649-650, 1997.
Leong, K.W., Mao, H.Q., Truong-Le, V.L., Roy, K., Walsh, S.M. and August, J.T., DNA-polycation nanospheres as non-viral gene delivery vehicles. J. Cont. Rel., 53:183-193, 1998.
Köping-Höggard, M., Nilsson, M., Edwards, K. and Artursson, P., Chitosan-DNA polyplex: A new efficient, biodegradable gene delivery system. Proceed. Int. Symp. Contr. Rel. Bioact. Mater., 25:368-369, 1998.
Aral, C., Özbaþ-Turan, S., Kabasakal, L., Keyer-Uysal, M. and Akbuða, J., Studies of effective factors of plasmid DNA-loaded chitosan microspheres: I. Plasmid size, chitosan concentration and plasmid addition techniques. STP Pharm. Sci., 10:83-88, 2000.
Akbuða, J., Kabasakal, L. and Özbaþ-Turan, S., Physical and transfectional properties of aged DNA-chitosan microspheres. 10th Annual Meeting of European Society of Gene Therapy, Antibes-France, 2002.
Mao, H.Q., Roy, K., Truong-Le, V., August, J.T. and Leong, K.W., DNA-chitosan nanospheres: Derivazation and storage stability. Proceed. Int. Symp. Control. Rel. Bioact. Mater., 24:671-672, 1997.
Birnboim, H.C., A rapid alkaline extraction method for the isolation of plasmid DNA. Meth. Enzymol, 100:243-255, 1983.
Berthold, A., Cremer, K. and Kreuter J., Preparation and characterization of chitosan microspheres as drug carrier for prednisolone sodium phosphate as model for antiinflammatory drugs. J. Cont. Rel., 39:17-25, 1996.
Mao, H-Q., Roy, K., Troung-Le, V.L., Janes, K.A., Lin, K.Y., Wang, Y., August, J.T. and Leong, K.W., Chitosan-DNA nanoparticles as gene carriers: synthesis characterization and transfection efficiency. J. Contr. Rel., 70:399-421, 2001.
Bradford, M.M., A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72:248-254, 1976.
Bancroft, J.D.; Cook, H.C., Manual of histological techniques and their diagnostic application. Churchill Livingstone, London, 1994.
Corresponding Author: Julide Akbuga, Marmara University, Faculty of Pharmacy, Department of Pharmaceutical Biotechnology, Tibbiye C., No. 49, 81010, Haydarpasa, Istanbul, Turkey. jakbuga@hotmail.com
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps