J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 6(2):274-291, 2003
Comparative evaluation of plastic, hydrophobic and hydrophilic polymers as matrices for controlled-release drug delivery.
Md. Selim Reza1, Mohiuddin Abdul Quadir, Syed Shabbir Haider
Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, University of Dhaka, Dhaka-1000, BangladeshReceived 3 June 2003, Revised 15 June 2003, Accepted 5 August 2003
PDF version
Abstract
Purpose: The present study was undertaken to investigate the effect of plastic, hydrophilic and hydrophobic types of polymers and their content level on the release profile of drug from matrix systems. As the physico-chemical nature of the active ingredients influence the drug retarding ability of these polymers, three different drugs were used to evaluate their comparative release characteristics in similar matrices. METHODS: Matrix tablets of theophylline, diclofenac sodium and diltiazem HCl using Kollidon SR, Carnauba wax and Hydroxypropyl methylcellulose (HPMC-15cps) were prepared separately by direct compression process. The USP Basket method was selected to perform the dissolution test carried out in 250 ml 0.1N HCl for first two hours and 1000 ml phosphate buffer of pH 6.8 for ten hours. RESULTS: Statistically significant differences were found among the drug release profile from different classes of polymeric matrices. The release kinetics was found to be governed by the type and content of polymer in the matrix system. Higher polymeric content (75%) in the matrix decreased the release rate of drug because of increased tortuosity and decreased porosity. At lower polymeric level (25%), the rate and extent of drug release was elevated. Carnauba wax was found to cause the strongest retardation of drug. On the otherhand, highest drug release was from HPMC matrices while Kollidon SR gave an intermediate release profile between these two polymers. Release rate was also found to be the function of physico-chemical nature of drug molecule. Theophylline and diltiazem HCl, being soluble in nature, released faster than diclofenac sodium from all matrix systems. The release mechanism was explored and explained with biexponential equation. Release profile showed a tendency to follow zero-order kinetics from HPMC matrix systems whereas Fickian (Case I) transport was predominant mechanism of drug release from Kollidon SR matrix system. The mean dissolution time (MDT) was calculated for all the formulations and the highest MDT value was obtained with Carnauba wax for all the drugs under investigation. CONCLUSIONS: The results generated in this study showed that the profile and kinetics of drug release were functions of polymer type, polymer level and physico-chemical nature of drug. A controlled plasma level profile of drug can be obtained by judicious combination of polymers and modulation of polymer content in the matrix system.
Introduction
In the last two decades, sustained-release dosage forms have made significant progress in terms of clinical efficacy and patient compliance (1). Preparation of drug-embedded matrix tablet that involves the direct compression of a blend of drug, retardant material and additives is one of the least complicated approaches for delivering drug in a temporal pattern into the systemic circulation. The matrix system is commonly used for manufacturing sustained-release dosage forms because it makes such manufacturing easy (2). A wide array of polymers has been employed as drug retarding agents each of which presents a different approach to the matrix concept. Polymers forming insoluble or skeleton matrices constitute the first category of retarding materials, also classed as plastic matrix systems. The second class represents hydrophobic and water-insoluble materials, which are potentially erodable, while the third group includes polymers those form hydrophilic matrices.
Plastic matrix systems, due to their chemical inertness and drug embedding ability, have been widely used for sustaining the release of drug. Liquid penetration into the matrix is the rate-limiting step in such systems unless channeling agents are used. The hydrophobic and waxy materials, on the other hand, are potentially erodable and control the release of drug through pore diffusion and erosion (3). Polymers belonging to hydrophilic matrix systems, when exposed to an aqueous medium, does not disintegrate, but immediately after hydration develops a highly viscous gelatinous surface barrier which controls the drug release from and the liquid penetration into the centre of the matrix system (4).
The objective of this work is to evaluate the comparative efficiency of abovementioned classes of polymers on sustaining the release of active ingredients having different physical and chemical properties. Kollidon SR (Polyvinyl acetate and polyvinyl pyrrolidone based matrix forming agent), Carnauba wax and Hydroxypropyl methyl cellulose (HPMC-15cps) have been selected as the representatives of plastic, hydrophobic and hydrophilic matrix systems respectively. Three drugs e.g. theophylline as a soluble neutral drug, diclofenac sodium as an acidic drug with pH-dependant solubility and diltiazem HCl as basic drug of acidic salt were used. Present study is aimed to evaluate the influence of polymer content and polymer type on the release profile of drug as well as to establish a relationship between drug retaining efficacy of the polymer and physico-chemical nature of the drug.
The use of HPMC-15 cps as matrix material in direct compression process has been reported previously (5,6). HPMC is the excipient chosen by most formulators for the preparation of hydrophilic matrix system most probably due to its claim as a fast gel formation to control initial release, and formation of strong, viscous gel to control drug release (7). While HPMC could potentially retard (and therefore control) the release of a soluble drug, it could also facilitate the release of relatively insoluble drug (e.g. hydrochlorthiazide). In the latter case, insolubility of drug molecule would be the main deterrent in the release and HPMC's solubulizing effect would facilitate release. The net result is controlled drug delivery for a prolonged period of time (8) The use of Kollidon SR as a plastic material in direct compression process to formulate sustained release dosage form has also been reported (9,10). Kollidon SR is particularly suitable for the manufacture of pH-independent sustained release matrix tablets. It contains no ionic group, which render the polymer inert to the drug molecule (11). Carnauba wax, due to its ease and safety of application, has also been used as rate retarding polymer (12) and extensively studied by different investigators (13,14). All the three drugs chosen in this study are proven candidates to be formulated in sustained release dosage form and have been subjected to thorough investigation for their candidature (15,16,17). The release mechanism of drug from the matrix systems has been explored and explained with the help of exponential model.
Experimental
Materials
Theophylline and diclofenac sodium were kind gifts from Square Pharmaceuticals Bangladesh Limited. Ditiazem HCl was generously donated by Drug International Ltd. Kollidon-SR and Ludipress were used as received from BASF Bangladesh Limited. Ludipress is neutral filler composed of lactose, Kollidon 30 (Polyvinylpyrrolidone) and Kollidon CL (Cross-linked Polyvinylpyrrolidone). Hydroxypropyl methylcellulose-15cps was from Shin-Etsu Chemical Co.Ltd.Japan and carnauba wax was from Koster Keunen Inc. USA. Aerosil (Silicon di oxide) and Magnesium Stearate were procured from Hanau Chemicals Limited, Japan. Monobasic potassium phosphate, sodium hydroxide and Hydrochloric acid were purchased from BDH, UK.
Methodology
Preparation of matrix tablets
The active ingredient, release retardants, filler, lubricant and flow promoters were blended together by dry mixing and made into tablets by direct compression at a fixed compression force. The formulations of the tablets with their codes are listed in Table 1.
Table 1: Composition (in mg) of 100 mg drug loaded matrix tablets.
The characteristic of these formulations is that, the amount of matrix forming polymers decreases gradually for each set of formulation and the reduced amount of matrix forming polymer was replaced by Ludipress. In all cases, the amount of the active ingredient is 100 mg and the total weight of the tablet is 406 mg. Properly weighed matrix-forming polymers, with or without Ludipress, magnesium stearate, aerosil and the active ingredient were blended in a laboratory mixture for 10 minutes. Particular attention has been given to ensure thorough mixing and phase homogenization. The appropriate amounts of the mixture were then compressed using a Perkin-Elmer laboratory hydraulic press equipped with a 13 mm flat faced punch and die set. The compression force and compression time were 5 ton and 30 seconds respectively. Before compression, the surfaces of the die and punch were lubricated with magnesium stearate. All the preparations were stored in airtight containers at room temperature for further study. This method of tablet production has previously been described by several authors (18, 19) that provided reproducible experimental results in terms of in vitro release. However, this process of tablet manufacturing differs from practical condition to a large extent and does not consider some critical tabletting parameters such as porosity, tablet hardness and versatility of process conditions. Data generated from such systems require sufficient scaling up and should not be directly extrapolated to commercially prepared controlled release tablets.
Dissolution studies: In vitro drug release studies from the prepared matrix tablets were conducted for a period of 12 hours using a six station USP XXII type 1 apparatus at 37 ± 0.5° C and 50 rpm speed. The dissolution studies were carried out in triplicate for 12 hours (initial 2 hours with simulated gastric fluid and rest 10 hours in phosphate buffer of pH 6.8) under sink condition. At every 1-hour interval samples of 10 ml were withdrawn from the dissolution medium and replaced with fresh medium to maintain the volume constant. After filtration and appropriate dilution, the sample solution was analyzed at 271 nm for theophylline, 277 nm for diclofenac sodium and 238 nm for diltiazem HCl by a UV spectrophotometer (Shimadzu, Japan). The amounts of drug present in the samples were calculated with the help of appropriate calibration curves constructed from reference standards. Drug dissolved at specified time periods was plotted as percent release versus time (hours) curve.
Data treatment
Different kinetic equations (zero-order, first-order and Higuchi's equation) were applied to interpret the release rate from matrix system. The best fit with higher correlation (r2 > 0.98) was found with the Higuchi's equation for all the formulations. Two factors, however, diminish the applicability of Higuchi's equation to matrix systems. This model fails to allow for the influence of swelling of the matrix (upon hydration) and gradual erosion of the matrix. Therefore, the dissolution data were also fitted according to the well-known exponential Eq. (1), which is often used to describe the drug release behaviour from polymeric systems:
Mt/Mµ=ktn (1)
Where Mt/Ma is the fractional (0.1-0.7) drug release at time t; k is a constant incorporating the properties of the macromolecular polymeric systems and the drug and n is a kinetic constant which depends on and is used to characterize the transport mechanism. The value of n for a tablet, n = 0.45 for Fickian (Case I) release, >0.45 but <0.89 for non-Fickian (Anomalous) release and 0.89 for Case II (Zero order) release and >0.89 for super case II type of release (20). Case II transport generally refers to the dissolution of the polymeric matrix due to the relaxation of the polymer chain and anomalous transport (Non Fickian) refers to the summation of both diffusion and dissolution controlled drug release. From the above equation the n values for different formulations have been calculated to identify the drug release mechanism. Talukder et al applied this equation to evaluate the drug release mechanism from xanthan gum matrix tablets (4). Shato et al used this equation for wax matrix granules whereas Hakim et al applied for kollidon SR matrix system (21,22). Due to the differences in drug release kinetics and test conditions, the constant k, though is one of the measures of release rate, should not be used for comparison. Therefore, to characterize the drug release rate in different experimental conditions, mean dissolution time (MDT) was calculated from dissolution data according to Mockel and Lippold using the following equation (23):
MDT=(n/n+1).k-1/n (2)
Statistics
To compare the means of all release data and to assess statistical significance between them, either single-factor analysis of variance (ANOVA) or an unpaired two-tailed t-test was carried out at 5% significance level.
Results and Discussion
Effect of physico-chemical property of drug on release rate
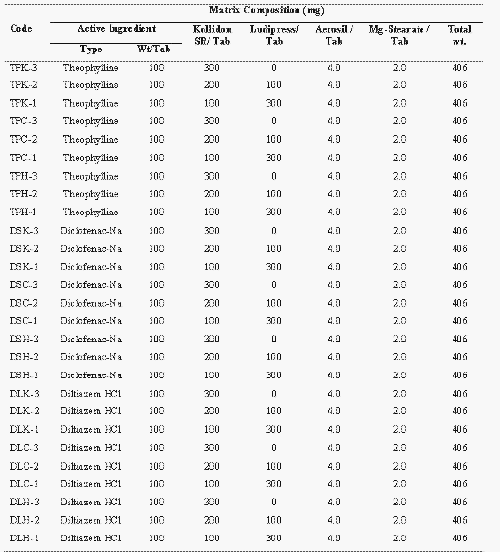
The effect of physico-chemical property of drug molecule on release retarding ability of Kollidon SR, Carnauba wax and HPMC at 75%, 50% and 25% polymeric content is illustrated in Fig.1-3 respectively. Release rates ( % drug released / hr 1/2 ) of theophylline, diclofenac sodium and dilitiazem HCl were found to be significantly different from each specific class of polymer (P<0.0001, Single factor ANOVA). This indicates that the release-retarding efficiency of the polymers critically depends on the physico-chemical nature of the drug molecule. Figure 1 (a-c) shows that, at any particular polymeric level, Kollidon SR can exert the highest drug retarding effect on diclofenac sodium.
Figure 1: Mean (± s.d.) percent of drug release from plastic matrix tablets containing (a) 300 mg (b) 200 mg and (c) 100 mg Kollidon SR in dissolution study at pH 1.2 and 6.8 (n=3).
TP: Theophylline, DS: Diclofenac sodium and DL: Diltiazem HCl.
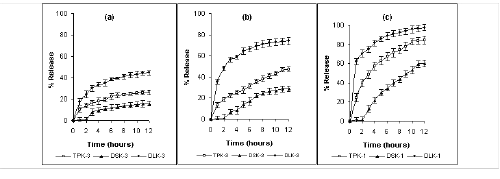
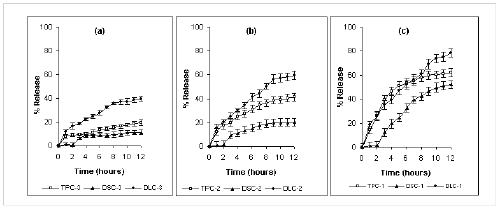
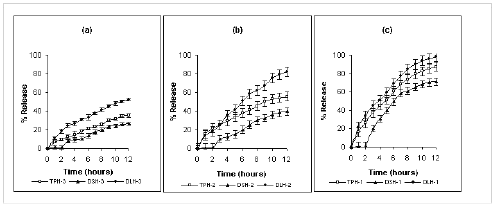
Only 16% and 60% of diclofenac sodium was released after 12 hours from formulations containing 75% and 25% kollidon SR respectively. Furthermore, an insignificant amount of diclofenac sodium was released in the first two hours of dissolution period. Release of diltiazem HCl was highest from Kollidon SR matrix tablets. Formulation DLK-3 and DLK-1 containing 75% and 25% of Kollidon SR released 45% and 98 % of diltiazem HCl respectively after 12 hours. However a burst release of diltiazem HCl was observed with formulation DLK-1 and DLK-2. About 60% of drug was released from DLK-1 within the first hour of dissolution period. Kollidon SR exerted a more controlled effect on the release of theophylline, e.g., 26%, 48% and 85% of theophylline was released after 12 hours from formulation TPK-3, TPK-2 and TPK-1 containing 75, 50 and 25% of Kollidon SR respectively. Neither a lag phase nor a burst release was observed with theophylline. Carnauba wax imparted stronger retardation over drug release than that of HPMC. Although the drug release rates from Carnauba wax and HPMC matrices were significantly different (P <0.0001, unpaired t-test), diclofenac sodium exhibited slowest release from both classes of matrices while diltiazem HCl showed the fastest rate of release irrespective of polymeric content (Figure 2 and 3).
Figure 2: Mean (± s.d.) percent of drug release from plastic matrix tablets containing (a) 300 mg (b) 200 mg and (c) 100 mg Carnauba wax in dissolution study at pH 1.2 and 6.8 (n=3).
TP: Theophylline, DS: Diclofenac sodium and DL: Diltiazem HCl.
Figure 3: Mean (± s.d.) percent of drug release from plastic matrix tablets containing (a) 300 mg (b) 200 mg and (c) 100 mg HPMC-15 cps in dissolution study at pH 1.2 and 6.8 (n=3).
TP: Theophylline, DS: Diclofenac sodium and DL: Diltiazem HCl.
This disparity in release rate of different classes of drugs can be attributed to the differences in their physical and chemical properties particularly on the solubility profile (24). Drug particles present in the surface of the matrix is initially released into the surrounding media generating many pores and cracks which facilitates further release of drug. The solubility of diclofenac sodium is 0.187% w/v at pH 6.8 (25). The lower solubility of diclofenac sodium at pH 1.2 limits the initial release of surface drugs as well as the formation of channels within the matrix. Consequently, the overall release of diclofenac sodium is decreased. The initial lag period of 2 hours is due to the acidic nature of diclofenac sodium. The drug, being a weak acid (pka 4.0) is practically insoluble in acidic solution but dissolves readily in intestinal fluid and water (26). Similar result was also reported by Billa et al (27).
Diltiazem HCl is an acidic salt of basic drug having a pKa value of 7.7 and the molecule is freely soluble in water. Alderman reported that, the release kinetics of hydrosoluble drugs is mainly governed by diffusion from hydrophilic matrices (28). Diltiazem HCl present in the surface of kollidon SR matrix tablet rapidly leaves the matrix system because of its basic nature. The burst effect observed with diltiazem HCl from formulations DLK-2 and 3 can be attributed to rapid ionization and higher solubility of diltiazem in acidic medium as well as the non-swellable property of Kollidon SR. Release of theophylline from Kollidon SR matrix, on the other hand, was found to be higher than that of diclofenac sodium. The fact is attributable to the higher pka value (pka 8.6) and solubility profile of theophylline in both type of dissolution medium with pH value of 1.2 and 6.8. It has been reported that, theophylline shows the solubility of 12.76 mg ml-1 in acidic medium while 9.03 mg ml-1 in basic medium (29). Again, in dissolution medium with acidic pH, theophylline release is less than that of dilitiazem HCl in spite of higher pka value of theophylline. The observation can be explained in the way that, diltiazem HCl, being a salt of weak base was rapidly ionized and subsequently get solvated than anhydrous form of theophylline.
Effect of Polymeric content on the release profile of drugs
The effect of polymer content on drug-release as a function of time was found to be significantly different (P <0.0001, single factor ANOVA) for a specific set of drug and polymer irrespective of their chemical nature. Comparing the corresponding release profile for a particular drug and polymer system from Figure 1-3, it can be observed that, for all the drugs under investigation, drug release is inversely proportional to the level of rate retarding polymer present in the matrix system, i.e. the rate and extent of drug release increases with decrease in total polymeric content of the matrix. It is observed that, for kollidon SR matrix system, 26%, 48% and 85% of theophylline was released from formulation TPK-3, TPK-2 and TPK-1 containing 75, 50 and 25 % of polymer. On the other hand 16%, 29% and 60% of diclofenac sodium and 45 %, 74 % and 97 % of diltiazem HCl was released from formulations containing 75, 50 and 25 % of kollidon SR respectively. Although the release rates were different, similar trend was found when Carnauba wax and HPMC-15 cps were used as the matrix-forming polymer. A linear relationship exist between the polymer content and rate of drug release irrespective of physico-chemical nature of drug and polymer as characterized by higher values of correlation-coefficient (r2 > 0.98) illustrated in Figure 4a-c.
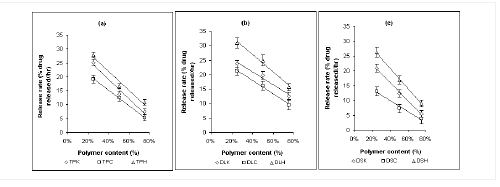
Figure 4: Effect of polymer level on Higuchi release rate (% drug released / time1/2) of (a)
Theophylline (b) Diclofenac sodium and (c) Diltiazem HCl in dissolution study at pH 6.8 (n=3).
TPK: Theophylline-Kollidon SR system, TPC: Theophylline-Carnauba wax system, TPH: Theophylline-HPMC system, DSK: Diclofenac sodium-Kollidon SR system, DSC: Diclofenac sodium-Carnauba wax system, DSH: Diclofenac sodium-HPMC system; DLK: Diltiazem HCl-Kollidon SR system, DLC: Diltiazem HCl-Carnauba wax system and DLH: Diltiazem HCl-HPMC system.
The rate of drug release was calculated from the slope of the Higuchi curve expressed as % drug released / hr1/2. Such increase in the polymer content results in a decrease in the drug release rate due to a decrease in the total porosity of the matrices (initial porosity plus porosity due to the dissolution of the drug) (30). Again, the reduced amount of drug-retarding polymer was replaced by ludipress, which contains lactose and kollidon CL (7). Lactose caused a decrease in the tortuosity of the diffusion path of the drug and Kollidon CL, by its swelling effect, weakened the matrix integrity (11). These two factors can be ascribed for the higher release rate of drug with formulations containing lower percentage of polymers. Analogous result was reported with previous investigations (6,30,31).
Effect of polymer type on the release profile of drugs
The class and nature of the matrix forming polymers influenced the release profile of active ingredient. Types of polymers used to prepare the matrix were also found to impart differential effect on matrix disintegration. Carnauba wax based matrix tablets did not show any disintegration after 12-hours of dissolution period irrespective of polymer content. Matrix tablets containing 75% and 50% of Kollidon SR and HPMC did not disintegrate after 12 hours indicating the formation of true matrices. On the other hand, formulations containing 25% Kollidon SR disintegrated after 10 hours while matrix system loaded with 25% HPMC disintegrated after 8 hours leaving a gell-like mass. Disintegration time was found to be independent of physico-chemical property of the drug. Proportion of ludipress in the matrix and chemical nature of the rate-retarding agent imparted the governing effect on matrix disintegration. The effect of polymer type on drug-release as a function of time can be observed by comparing the corresponding release profile of a particular drug from any matrix system at a particular polymeric level in Figure1-3. The overall rate of release of a particular drug at a specific polymeric content was again found to be significantly different for different polymers (P<0.0001, single factor ANOVA). Irrespective of chemical nature of the drugs, it was found that, the release is highest from HPMC-15 cps matrix whereas lowest drug release was found with Carnauba wax system. At 75% polymeric content, 40%, 45% and 52% of diltiazem HCl was released from Carnauba wax, Kollidon SR and HPMC-15 cps matrix system after 12 hour of dissolution period. On the other hand, at 25 % polymeric load, 79, 97 and 99 % of the same drug was released from the abovementioned matrix systems respectively after 12-hour . Analogous results were obtained with diltiazem HCl and theophylline though the release rates were significantly different for either of the cases ( P < 0.0001, single factor ANOVA). The fact can be reasoned on the basis of polymeric nature and the mechanism by which the polymers release drug in the surrounding medium. For all the drugs, Carnauba wax imparted the highest retarding effect and extensively delayed drug release. This finding is in accordance with the work carried out by Dakkuri et al and Quadir et al (12,32). Carnauba wax is extremely hydrophobic in nature with lower wettebility. Total release of drug from such matrix system is not possible since a certain fraction of dose is coated with impermeable wax film. It is also postulated that, in the absence of additives, drug release is prolonged and non-linear from wax matrix systems (3). Since our formulation contain no channeling agents, formation of pores and cracks did not occur to facilitate drug release and the impervious hydrophobic matrix of Carnauba wax decreased drug release . The extent of drug release from Kollidon SR was found to be higher than Carnauba wax system. Except the formulation DLK-1 and 2 (which showed burst release in first hour), all the formulations prepared with Kollidon SR released the corresponding active ingredient in a sustained fashion for 12 hour. The higher drug release rate of Kollidon SR matrix tablet as compared to drug release from Carnauba wax matrix may be attributed to dissolution of Polyvinylpyrrolidone (PVP) molecules which are components of Kollidon SR, creating pores and channels and thus facilitating solvent front penetration and elevation of drug release. Analogous result was reported by Sugthongjeen et al with pectin matrix system (33). Polyvinyl acetate (PVA) itself, although not soluble in water, does not impede diffusion of drug from the matrix system and causes a higher proportion of drug to be released than carnauba wax matrix. The presence of burst release in formulation DLK-1 and 2 can be ascribed to low hydration and water uptake profile of Kollidon SR. It is important to state that, this initial rapid release of drug from the matrix system is often therapeutically undesirable because the total amount of drug released is remarkably influenced by this initial control of release from dosage form. If the polymer does not hydrate quickly, there is a chance of a large portion of drug to be released during the first initial phase of release profile (4).
The rate and extent of drug release was found highest with HPMC-15 cps polymeric systems. Similar results were obtained by Nokhodchi et al (31). Additionally, HPMC also imparted a more controlled influence on the release pattern of all drugs with reduction and/ or elimination of the tendency of burst release which was evident in formulation DLK-1 and TPK-1. The fact can be attributed to the hydrophilic nature of HPMC. When exposed to the dissolution medium, the solvent penetrates into the free spaces between macromolecular chains of HPMC. After solvation of the polymer chains, the dimensions of the polymer molecule increase due to the polymer relaxation by the stress of the penetrated solvent. This phenomenon is defined as swelling and it is characterized by the formation of a gel-like network surrounding the tablet (28). This swelling and hydration property of HPMC causes an immediate formation of a surface barrier around the matrix tablet that eliminates the burst release. The higher percentage of drug release at the end of 12 hour-dissolution period can be attributed to the erosion of the matrix which takes place after complete hydration of outer layer. In this phase, the completely hydrated gel-layer start to disperse due to an attrition process which furthermore allows the penetration of liquid to continue until the tablet completely disperses or disappears.
Release kinetics
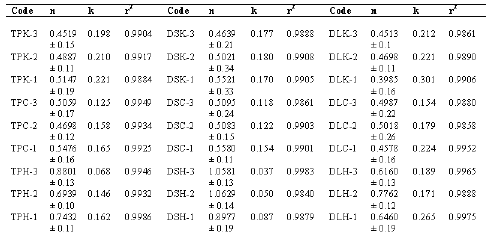
The values of release exponent (n), kinetic rate constant (k) and mean dissolution time (MDT) as calculated from Eq. 1 and Eq.2 are presented in Table 2.
Table 2: Value of the release exponent with standard deviation (n ± s.d.), kinetic constant (k) and correlation coefficient (r2).
As observed from the table, the value of correlation co-efficient (r2) for all the formulations were high enough to evaluate the drug dissolution behaviour by Eq.1. The value of the release exponent (n) was found to be a function of polymer used and the physico-chemical property of the drug molecule itself. At 75% polymeric content theophylline and diclofenac sodium loaded Kollidon SR tablets, by their very nature of releasing drug by pore-diffusion, demonstrated drug release mechanism by Fickian (Case I) transport as observed from their n values. Reducing the Kollidon SR level in formulations containing theophylline and diclofenac sodium showed a significant deviation from Fickian or diffusional transport. This can be attributed to the presence of higher proportion of ludipress that contains a swellable component i.e. Kollidon CL (11). Diltiazem HCl-Kollidon SR system released drug by Fickian (Case I) mechanism at 75% and 50% polymeric level. However at 25% Kollidon SR content, such matrix system showed a significant deviation from diffusional transport mechanism. Though, higher molecular weight of diltiazem HCl (450.99) can be held responsible for its lower diffusivity (24) and consequent deviation from Fickian mechanism, this may not be the single operative factor in this case. Reduced polymer content with respect to active ingredient, failure of the matrix material to form a non-erodable frame work, presence of swellable component (i.e. Kollidon CL in Ludipress) at relatively higher proportion may be considered for such deviation. In general, solubility of drug molecule itself crucially governs the rate and extent of diffusional release. For diffusion to occur, the first step is wetting of drug by water, followed by its dissolution so that the drug molecule is available in molecular form to diffuse out of the matrix. Hence, the net release rate observed is a cumulative effect of drug's solubility ( influenced by its structure, molecular weight and pk a ), polymer property (hydrophilicity / lipophilicity, molecular weight, tortuosity) and the relative ratio of drug and polymer in the tablet.
Kinetic analysis of Carnauba wax matrices yielded an aberrant value of release exponent (n) irrespective of physico-chemical nature of the drug and no clear inference could be made regarding the kinetics of drug release from such matrices. The mechanism of drug release from wax matrices has been a matter of controversy since wax-systems tend to be crude and more heterogeneous than other classes of polymeric systems (34). In some cases, it has been reported that the mechanism of release from wax matrices involves the leaching of drug by the eluting medium. Fluid enters through the cracks and pores of the matrix with diffusion of drug through the matrix being insignificant (35,36). Others have reported that release from a typical wax matrix is diffusion-controlled and is best described by Higuchi's t 1/2 model (37,38,39,40).
Incorporation of HPMC-15 cps as the matrix forming agent, increased the value of n, indicating the tendency of drug release kinetics nearer to zero-order or case II transport rather than Fickian (Case I) mechanism. This phenomenon can generally be attributed to structural changes induced in the polymer by the penetrant (41). HPMC-15 cps, being a class of hydrophilic matrix former swells in the presence of liquid solvent due to polymer relaxation and is characterized by the formation of a gel-like network surrounding the system. The mechanical property of the surface-hydrated gelatinous barrier plays an important role in overall drug release rate (4). Although it is desirable for a controlled release device to deliver drug in zero-order kinetics, it is extremely difficult to attain such pattern as the kinetics of release is affected by the physico-chemical composition of surrounding medium and processing variables. The values of n had no definite relationship with polymer content for any of the drugs. This observation was in agreement with the findings of Nokhodchi (31)
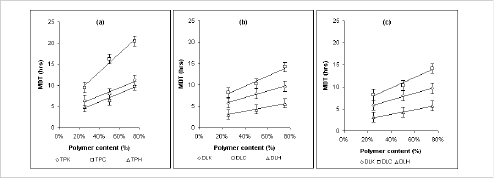
MDT value is used to characterize the drug release rate from the dosage form and the retarding efficacy of the polymer. A higher value of MDT indicates a higher drug retarding ability of the polymer and vice-versa. The MDT value was also found to be a function of polymer loading, polymer nature and physico-chemical property of the drug molecule. Figure 5(a-c) shows that a direct relationship can be found with MDT value and polymer loading irrespective of drug and polymer type which is linear in nature (r2>0.98).
Figure 5: Effect of polymer level on Mean Dissolution Time (MDT) of (a) Theophylline (b) Diclofenac sodium and (c) Diltiazem HCl in dissolution study at pH 6.8 (n=3).
TPK: Theophylline-Kollidon SR system, TPC: Theophylline-Carnauba wax system, TPH: Theophylline-HPMC system, DSK: Diclofenac sodium-Kollidon SR system, DSC: Diclofenac sodium-Carnauba wax system, DSH: Diclofenac sodium-HPMC system; DLK: Diltiazem HCl-Kollidon SR system, DLC: Diltiazem HCl-Carnauba wax system and DLH: Diltiazem HCl-HPMC system
This finding was also in agreement with Reza et al. (42). Supportive to drug release data, it was found that, Carnauba wax matrix system showed a higher MDT value for all three classes of drug whereas HPMC based systems showed the least value of MDT. This can be attributed to the higher drug retaining ability of Carnauba wax due to their hydrophobic and water repelling nature. Kollidon SR showed the MDT value ranging between the above two systems.
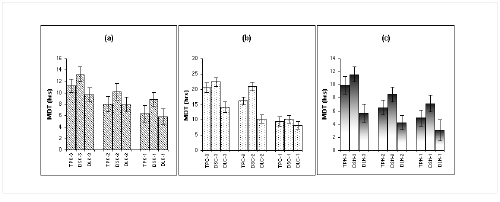
From all the polymers, the MDT of soluble drugs (Diltiazem HCl and Theophyllline) were significantly lower than those of less soluble drugs (e.g. Diclofenac sodium) as illustrated in Figure 6 (a-c).
Figure 6: Mean Dissolution Time (MDT in hours) value of different drugs from (a) Kollidon SR (b) Carnauba wax and (c) HPMC-15 cps matrix system in dissolution study at pH 6.8 (n=3).
TPK: Theophylline-Kollidon SR system, TPC: Theophylline-Carnauba wax system, TPH: Theophylline-HPMC system, DSK: Diclofenac sodium-Kollidon SR system, DSC: Diclofenac sodium-Carnauba wax system, DSH: Diclofenac sodium-HPMC system; DLK: Diltiazem HCl-Kollidon SR system, DLC: Diltiazem HCl-Carnauba wax system and DLH: Diltiazem HCl-HPMC systemThe difference was significant at P < 0.0001 (unpaired t-test). This indicates that the release of soluble drugs is faster than the release of insoluble drug from all the matrix systems under investigation. This discrepancy in release rate between soluble and less soluble drugs can be attributed to the difference in their release mechanisms. This finding was in accordance with Talukder et al (4).
CONCLUSIONS
At present, all the polymers being studied are used extensively in pharmaceuticals to control the release of drug. The approach of the present study was to make a comparative evaluation among these polymers and to assess the effect of physico-chemical nature of the active ingredient on drug release profile. The study reveals that, the release of water soluble drugs was higher than the drugs with lower solubility and the mechanism of release was changed with the nature and content of polymer in the matrix. The type of polymers used imparts a conspicuous effect on release mechanism. The data generated in this study also shows that, the drug release from plastic and hydrophobic matrix was less than hydrophilic polymer. Again, the release pattern of drug from hydrophilic matrices was closer to zero-order kinetics than that from other classes of matrices. However, a number of critical parameters such as granulation process, tabletting conditions, hardness and porosity of the tablet and compression pressure will markly affect drug release pattern from different matrices. These factors, although beyond the scope of this study, should be taken into consideration during formulation design. The wide range of polymers available for controlling the release of drug from dosage form endows the formulator with higher degree of flexibility and the present study clearly manifests the necessity of combining different classes of polymers to get an acceptable pharmacokinetic profile in the fluctuating in vivo environment.
ACKNOWLEDGEMENTS
The author would like to thank Square Pharmaceuticals Bangladesh Ltd., for anhydrous theophylline and diclofenac sodium and also to Drug International Ltd. for their generous donation of diltiazem HCl. The authors express their gratitude to BASF Bangladesh Ltd. for the gift sample of kollidon SR and ludipress.
References
Merkus, F.W.H.M., Controlled and rate-controlled drug delivery ; principal characteristics, possibilities and limitations, in Struyker-Boudier, H.A.J. (eds), Rate- Controlled Drug Administration and Action, CRC Press, Boca Raton, FL, USA, pp 15-47, 1986.
Cardinal, J.R., Matrix systems, in Langer R. S. and Wise D.L. (eds), Medical Applications of Controlled Release, Volume 1, Classes of Systems, CRC Press, Boca Raton, FL, USA, pp 41-67, 1984.
Lordi, N.G., Sustained Release Dosage Forms, in Lachman L: Lieberman H A and Kanig J L (eds), The Theory and Practice of Industrial Pharmacy. 3rd ed., Varghese Publishing House, Bombay, pp 430-456, 1990.
Talukder, M.M., Michoel, A., Rombaut, P. and Kinget, R., Comparative Study on Xanthun Gum and Hydroxypropylmethylcellulose as Matrices for Controlled-Release Drug Delivery I. Int J Pharm, 129: 231-241, 1996.
Bain, J.C., Tan, S.B., Ganderton, D. and Solomon, M.S., Comparison of the in vitro release characteristics of a wax matrix and a hydrogel sustained release diclofenac sodium tablet. Drug Dev Ind Pharm, 17: 215-232.
Lee, B.J., Ryu, S.G. and Cui, J.H., Formulation and release characteristics of hydroxypropyl methylcellulose matrix tablet containing melatonin. Drug Dev Ind Pharm, 25 (4): 493-501, 1999.
The Dow Chemical Company, Formulation for controlled release with METHOCEL cellulose ethers, USA, 1987.
Cabelka, T.D. and Sheskey, P.J., Evaluation of granulation, excipient characteristics and drug solubility in the preparation of hydrophilic controlled-release matrix tablets. Dow Chemicals Publications, <http: // www.dow.com>
Reza, M.S., Quadir, M.A. and Haider, S.S., Development of Theophylline Sustained Release Dosage Form Based on Kollidon SR. Pak J Pharm Sci , 2002. (Accepted)
Shao, Z.J., Farooqi, M.I., Diaz, S., Krishna, A.K. and Muhammad, NA., Effect of formulation variables and post-compression curing on drug release from a new sustained-release matrix material: polyvinylacetate-povidone. Pharm Dev Technol, 6(2): 247-254, 2001.
BASF, Technical Information, ME 397e, June 1999.
Dakkuri, A., Schroeder, H.G. and Deluca, P.P., Sustained release from inert matrixes II: effect of surfactants on Tripelennamine Hydrochloride release J Pharm Sci, 67: 354-357, 1978a
Al-Shora, H., Said, S. and Hammad, A.L., Sustained release from inert matrixes II. Effect of polyethylene glycols on theophylline release. Int J Pharm, 7: 77-82, 1980.
Goodhart, F.W., McCoy, R.H. and Ninger, G.C., Release of a water-soluble drug from wax matrix time-release tablets. J Pharm Sci, 63: 1748-1751, 1974.
Verma, P.R.P. and Banu, V., Sustained release of theophylline from eudragit RLPO and RSPO tablet. Drug Dev Ind Pharm, 22 (12): 1243-1247, 1996.
Chattaraj, S.C. and Das, S.K., Effect of formulation variables on dissolution profile of diclofenac sodium from ethyl- and hydroxypropylmethyl cellulose tablets. Drug Dev Ind Pharm, 22(7): 555-559, 1996.
Shah, D., Shah, Y. and Pradhan, R., Development and evaluation of controlled-release diltiazem HCl microparticles using cross-linked poly (vinyl alcohol). Drug Dev Ind Pharm, 23 (6): 567-574, 1997.
Ritger, P.L. and Peppas, N.A., A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Control Rel, 5: 37-42, 1987.
Shato, H., Miyagawa, Y., Okabe, T., Miyajima, M. and Sunada, H., Dissolution mechanism of diclofenac sodium from wax matrix granules. J Pharm Sci, 86 (8): 929-934, 1997.
Hakim, M.; Jalil, R., Evaluation of a New Rate Retarding Polymer Kollidon SR as Matrix Tablets. M.Pharm thesis, DU, Bangladesh , 2001.
Mockel, J.E. and Lippold, B.C., Zero-order release from hydrocolloid matrices. Pharm Res, 90 (7): 1066-1070, 1993.
Salsa, T., Veiga, F. and Pina, M.E., Oral controlled-release dosage forms.I. Cellulose ehter polymers in hydrophillic matrices. Drug Dev Ind Pharm, 23 (9): 929-938, 1997.
Monographs on drug substances: Diclofenac sodium, in Lund W (eds), The Pharmaceutical Codex. 12th ed., The Pharmaceutical Press, London, pp 835-838, 1994.
Sheu, M.T., Chou, H.L., Kao, C.C., Liu, C.H. and Sokoloski, T.D., Dissolution of diclofenac sodium from matrix tablets. Int J Pharm, 85: 57-63, 1992.
Billa, N., Yuen, K.H. and Peh, K.K., Diclofenac release from eudragit containing matrices and effect of thermal treatment. Drug Dev Ind Pharm, 24 (1): 45-50, 1998.
Alderman, D.A., A review of cellulose ethers in hydrophillic matrices for oral controlled-release dosage forms. Int J Pharm Tech Prod Mfr, 5(3): 1-9, 1984.
Li Wan Po, A., Wong, L.P. and Gilligan, C.A., Characterisation of commercially available theophylline sustained or controlled-release systems: in-vitro drug release profiles. Int J Pharm, 66: 111-130, 1990.
Boza, A., Caraballo, I., Alvarez-Fuentes, J. and Rabasco, A.M., Evaluation of eudragit® RS-PO and Ethocel ® 100 matrices for the controlled release of lobenzarit disodium. Drug Dev Ind Pharm, 25 (2): 229-233, 1999.
Nokhodchi, A., Farid, Dj., Najafi, M. and Adrangui, M., Studies on controlled release formulation of diclofenac sodium. Drug Dev Ind Pharm, 23 (11): 1019-1023, 1997.
Quadir, M.A., Reza, M.S. and Haider, S.S., Effect of polyethylene glycols on release of diclofenac sodium from directly compressed carnauba wax matrix tablets. J Bang Acad Sci, 26 (1): 1-8, 2002.
Sungthongjeen, S., Pitaksuteepong, T., Somsiri, A. and Sriamornsak, P., Studies on pectins as potential hydrogel matrices for controlled-release drug delivery. Drug Dev Ind Pharm, 25 (12): 1271-1276, 1999.
Wong, L.P., Gilligan, C.A. and Li Won Po, A., Preparation and characterisation of sustained-release ibuprofen-cetostearyl alcohol spheres. Int J Pharm, 83: 96-114, 1992.
Dakkuri, A., Butler, L.D. and Deluca, P.P., Sustained release from inert wax matrices III: Effect of povidone on tripellenamine hydrochloride release. J Pharm Sci, 67: 357-360, 1978a.
Dakkuri, A., Schroeder, H.G. and Deluca, P.P., Sustained release from inert wax matrices II: Effect of surfactants on tripellenamine hydrochloride release. J Pharm Sci, 67: 354-357, 1978b.
Schwartz, J.B., Simonelli, A.P. and Higuchi, W.I., Drug release from wax matrices I: Analysis of data with first order kinetics and with the diffusion controlled model. J Pharm Sci, 57: 274-277, 1968a.
Schwartz, J.B., Simonelli, A.P. and Higuchi, W.I., Drug release from wax matrices II: Application of a mixture theory to the sulfanilamide-wax system. J Pharm Sci, 57: 278-282, 1968b.
Goodhart, F.W., McCoy, R.H. and Ninger, F.C., Release of a water-soluble drug from a wax matrix timed-release tablet. J Pharm Sci, 63: 1748-1751, 1974.
Parab, P.V., Oh, C.K. and Ritschel, W.A., Sustained release from Precirol® (glycerol palmito-stearate) matrix: Effects of mannitol and hydroxypropyl methylcellulose on the release of theophylline. Drug Dev Ind Pharm, 12: 1309-1327, 1986.
Peterlin, A., Diffusion with discontinuous swelling. VI. Type II diffusion in spherical particles. Polym Eng Sci, 20: 238-251, 1980.
Reza, M.S., Quadir, M.A. and Haider, S.S., Development of theophylline sustained release dosage form based on kollidon SR. Pak J Pharm Sci, 15(1): 63-70, 2002.
Corresponding Author: Md. Selim Reza, Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, University of Dhaka, Dhaka-1000, Bangladesh selimreza@hotmail.com
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps