J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 6(3):315-320, 2003
Permeation prediction of M100240 using the parallel artificial membrane permeability assay.
Kin-Kai Hwang1, Nancy E Martin and Lan Jiang
Department of Drug Metabolism and Pharmacokinetics, Aventis Pharmaceuticals Inc., Bridgewater, New Jersey, USAChengyue Zhu
Department of Chemistry, Aventis Pharmaceuticals Inc., Bridgewater, New Jersey, USAReceived 20 June 2003, Revised 1 October 2003, Accepted 9 October 2003
PDF version
Abstract
PURPOSE: Kansy et al [5] first introduced the Parallel artificial membrane permeation assay (PAMPA) in 1998. In this system, the permeability through a membrane formed by a mixture of lecithin and an inert organic solvent on a filter support is assessed. PAMPA shows definite trends in the ability of molecules to permeate membranes by transcellular passive diffusion. Its simplicity, low cost, high throughput, and wide pH range make it very attractive in modern drug discovery. Based on this concept, Whohnsland et al[1], Sugano et al[12] and Zhu et al [9] modified the assay and used it to screen compound permeability. We used PAMPA for the permeation prediction of M100240, which was unable to be determined by cell-based assays due to compound instability. METHODS: In this study, 92 commercially available agents provided the structural diversity used to generate a mathematical prediction model for human fraction absorbed, M100240 -- an acetate thioester of MDL 100,173. Permeation of M100240 and MDL 100,173 was evaluated using the parallel artificial membrane permeability assay (PAMPA). The donor and recipient solutions consisted of 0.5N HCl (pH 1.5) or phosphate-buffered saline (pH 5.5 or 7.4) with 2% dimethyl sulfoxide. The donor solution also contained 200 mM M100240 or MDL 100,173. RESULTS: M100240 had a medium permeation at pH 5.5 (2.99%), corresponding to a high predicted Fa in humans (92%). Permeation of MDL 100,173 was low at this pH (0.72%), corresponding to a medium-to-low predicted Fa (46). At pH 7.4, the permeation of M100240 was low (~ 1%) and no permeation was apparent for MDL 100,173. CONCLUSIONS: We predicted M100240 is likely to be well absorbed via passive diffusion across the human gastrointestinal tract following oral administration.
Introduction
Many investigative compounds with good therapeutic potential fail to progress beyond the early developmental stages,[1] primarily because of poor biopharmaceutical properties such as insufficient oral bioavailability.[2,3] The drug development process has, therefore, relied increasingly upon in vitro methods to screen a large number of potentially therapeutic oral compounds in terms of rates of permeation and, in turn, anticipated fraction absorbed (Fa) in humans. This approach serves to identify the absorption potential of new chemical entities while they are in the early discovery phase, thereby facilitating an effective lead candidate selection and its optimization in the development phase.
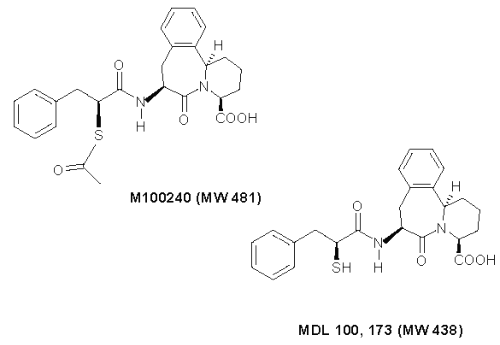
Several in vitro methods are available to assess the oral absorption potential of new molecular entities, such as immobilized artificial membrane chromatography and cell culture models of intestinal permeability such as Caco-2 monolayers.[4] However, some agents are not stable in these systems, prompting the need for alternative methods of permeability testing. In this case, M100240 was unstable and rapidly hydrolyzed to MDL100, 173 in Caco-2 system. As a result, one alternative is the parallel artificial membrane permeability assay (PAMPA).[5] We report here the use of PAMPA to assess the permeation of M100240 -- an acetate thioester of MDL 100,173 -- a dual angiotensin-converting enzyme (ACE)/neutral endopeptidase (NEP) inhibitor[6] currently in development for cardiovascular conditions (Figure 1).
Figure 1: Structure of M100240 and MDL 100,173. M100240 is acetate thioester of MDL 100,173.
METHODS
The permeation of the 92 structurally diversified commercially available compounds across a lipid bilayer at pH 5.5 and pH 7.4 were determined by PAMPA as preciously described [9]. In brief, PAMPA was performed using 96-well hydrophilic filtration plates (Millipore, Bedford, MA). Filter material was prepared by wetting each well of the filtration plate with 5 mL of the artificial membrane solution (0.8% egg lecithin in n-dodecane). The donor and recipient solutions consisted of phosphate-buffered saline (pH 5.5 or 7.4) with 2% dimethyl sulfoxide. The donor solution also contained 200 μM compound. The PAMPA receiving plate was a Millipore GV hydrophilic filter plate (Millipore, Bedford, MA) containing 272 mL of blank phosphate-buffered saline, and the donor plate was a Dynex 96-well plate (Dynex, Ashford, Middlesex, UK) filled with 272 mL of donor solution. The stacked donor-receiving plates were incubated for 2 hours at room temperature with gentle circular shaking, following which drug concentrations in the receiving solutions were assayed by high-performance liquid chromatography (HPLC) coupled with ultraviolet absorbance detection (235 and 254 nm). The HPLC system comprised a YMC AQ 4.6 × 50 mm column (YMC Inc., Wilmington, DE, USA) using a mobile phase gradient of 0.1% formic acid in water/95% acetonitrile (run time of 8 minutes). Injection volume was 40 mL. Fifty randomly selected compounds were used to generate a mathematical prediction model for human fraction absorbed and 42 compounds were used to validate this model. The artificial membrane permeation of M100240 and MDL 100,173 was evaluated in solutions consisted of 0.5N HCl (pH 1.5) or phosphate-buffered saline (pH 5.5 or 7.4) with 2% dimethyl sulfoxide. The donor solution also contained 200 mM M100240 or MDL 100,173 (relevant controls comprised naproxen, warfarin, and diltiazem at pH 1.5, and diltiazem, atenolol, and metoprolol at pH 5.5 and 7.4). The stability of M100240 and MDL 100,173 in these buffer systems was confirmed in preliminary studies, in which both compounds were stable at pH 5.5 and 7.4 throughout a 3-hour incubation period at 25°C (Aventis data on file).
Percentage transport across the lipid bilayer (%T) was calculated as follows:
%T = 100 × Ar/Ad
where Ar and Ad correspond to the HPLC peak areas of the receiving solution and initial donor solution, respectively. Permeation was ranked as low, medium or high based on %T values of <2%, 2-5% and >5%, respectively. The calculated value for %T was subsequently used to project the percent Fa in humans, based on an established correlation model:
Fa = 100 x [1-EXP x (-R x %T)]R = 0.85
Where Fa is human fraction absorbed, % of T is PAMPA percentage transport and R is the regression coefficient.
RESULTS
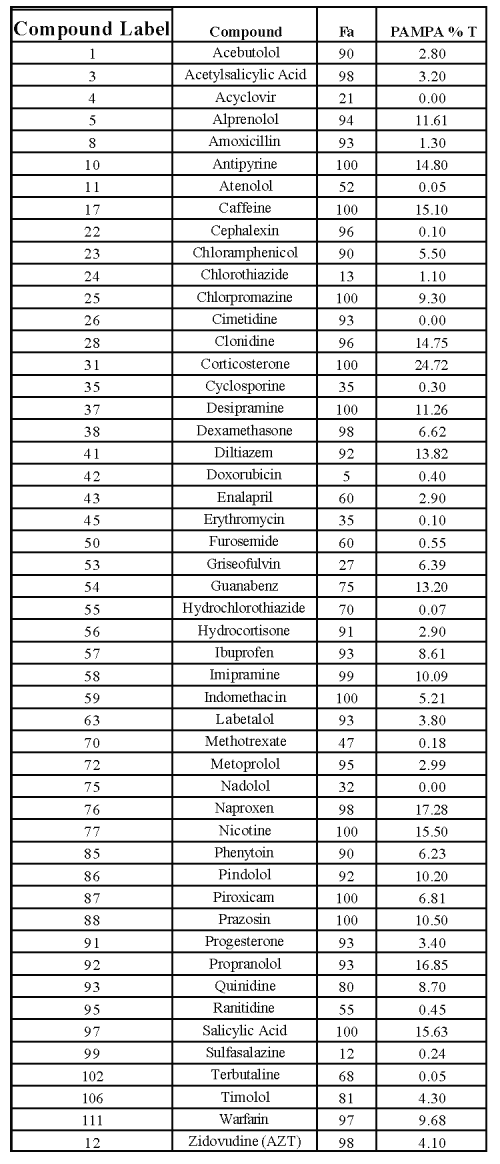
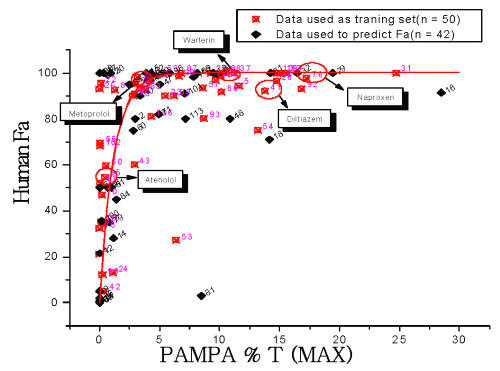
For ninety-two commercial compounds, we randomly generated a number for each compound between 0 and 1 (uniform distribution). We selected fifty compounds listed in Table I with smallest random numbers as training set to create a prediction model for human fraction absorbed (red open circles in Figure 2). This was then validated with the remaining forty-two compounds listed in Table II.
Since the pH values of human small intestine ranged from 5.0 to 8.0. The higher PAMPA percentage transports of pH 5.5 and 7.4 from these compounds were used to plot against literature human fraction absorption (Fa). A hyperbolic curve was generated by using the equation: Fa = 100 x [1-EXP x (-R x %T)], R (correlation coefficient) = 0.85.
Table 1: Commercial compounds used for prediction model.
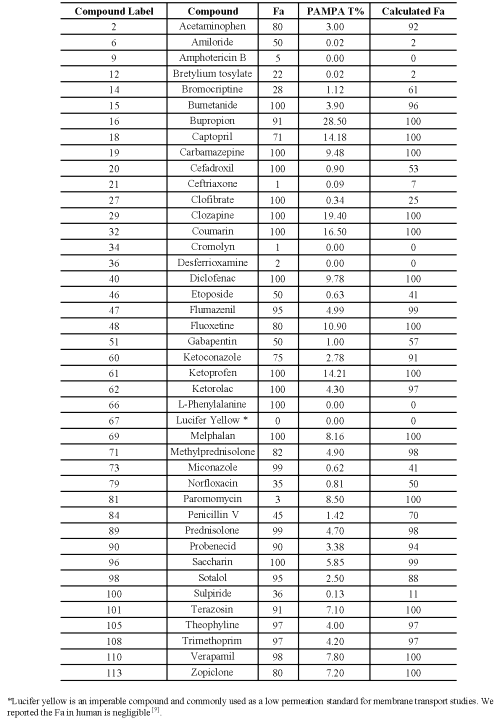
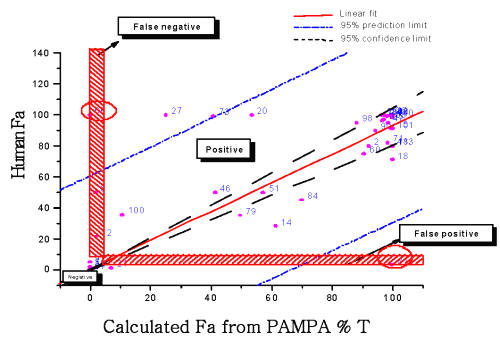
Compounds highlighted in the Figure 2 were used as controls in this study. The human Fa of another 42 commercial compounds listed in Table II (black diamonds in Figure 2) was projected by their PAMPA percentage transport and compared with literature human Fa shown in Figure 3. The straight line is the linear fit. Dash line is 95% confidence limit. The Dash-dot line is the 95% prediction limit. Compounds with Fa less than 10 and calculated Fa less than 5 are true negatives.
Table 2: Compounds used to validate prediction model.
Compounds with Fa greater than 10 and calculated Fa greater than 5 are true positives. The horizontal hatched box is the false positives when Fa is less than 10 and calculated Fa is greater than 5. The vertical hatched box is a false negative where Fa is greater than 10 but calculated Fa is less than 5.
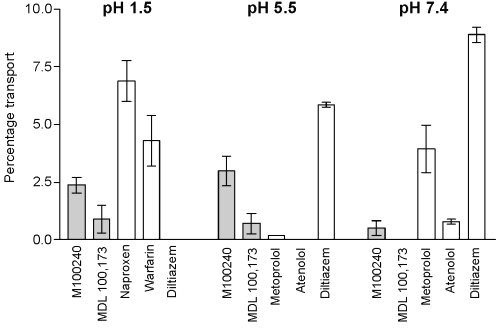
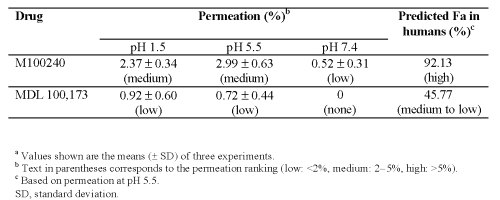
The findings of the permeation assay for M100240 and MDL 100,173 are shown in Table III. Overall, M100240 had a medium permeation at pH 5.5, corresponding to a high predicted Fa in humans.
In contrast, membrane permeation of this compound was low at pH 1.5 and 7.4 (Table III and Figure 4).
MDL 100,173 showed low permeation at both pH 1.5 and 5.5 (Figure 4), the latter corresponding to a medium-to-low predicted Fa in humans.
Permeation of MDL 100,173 was not evident at pH 7.4 (Table III and Figure 4).
Figure 2: 50 randomly selected compounds (red open circles) were used to create prediction model for human fraction absorbed. The higher PAMPA percentage transports of pH 5.5 and 7.4 from these compounds were plotted against literature human fraction absorption (Fa). A hyperbolic curve was generated, which fits the equation shown in the method. Compounds highlighted in boxes were controls in M100240 and MDL 100, 173 studies. Another 42 commercial compounds (black diamonds) were used to validate this model shown in figure 3.
Figure 3: The calculated human fraction absorbed of 42 commercial compounds from their PAMPA transport value was plotted against their literature data. The red line is linear fit. Dash dot line is 95% prediction limit. The dash line is 95% confidence limit. The horizontal hatched box is the false positives and vertical box is false negative. The area above two hatched boxes is positive and below is negative.
DISCUSSION
The advent of increasingly efficient methods to synthesize and pharmacologically screen new chemical entities is increasing the demand for an equally rapid method of determining the pharmacokinetic attributes of potential lead compounds.
Figure 4: PAMPA percentage transport of M100240 and MDL 100, 173 as well as standard compounds, Naproxen, warfarin, Diltiazem, metaprolol and Atenolol at pH 1.5, 5.5, and 7.4.
Indeed, selection of oral drug development candidates with adequate absorption characteristics is pivotal to increasing the probability of success in the developmental phase.
The prediction model of human fraction absorption (Fa) showed in Figure 2 used 50 commercial drugs as a training set (red open circles). Figure 2 shows a hyperbolic curve created by the equation discussed in the method. Another 42 commercial compounds (black diamonds) were used to confirm the accuracy of the equation. The human Fa of these 42 commercial compounds was projected by their PAMPA percentage transport and compared with literature human Fa shown in Figure 3. There is a good correlation (R=0.68) between calculated human Fa and literature human Fa with few outliers. Among them. L-Phenylalanie (66) is transported by active transporter. Paromomycin (81) is a substrate for P-glycoprotein. PAMPA only estimates the ability of molecules to permeate membranes by transcellular passive diffusion. Thus, PAMPA is not suitable for these two compounds. The correlation coefficient is 0.83 without these two compounds. Compound 6 (Amiloid), 12 (Bretylium tosylate, 21 (Ceftriaxone) all have very low human fraction absorbed. Their PAMPA percentage transports are less than 0.1 %. Therefore, PAMPA may not be sensitive enough to predict the absolute human Fa. It is accurate enough to rank compound permeability, which is solely transported by transcellular passive diffusion. For some compounds transported across membrane partly by transporter and/or paracellular routes, PAMPA may underestimate its permeation value.
The introduction of PAMPA allowed for increased throughput of permeability measurements, and was used in the present study to evaluate the intrinsic permeation of MDL 100,173 and its acetate thioester M100240.[6] We used three pH levels in order to study the absorption potential of these compounds at different pH levels in the gastrointestinal tract: pH 1.5 mimicked the acidic conditions of the stomach, whereas pH 5.5 and 7.4 mimicked the variable pH of the small intestine (the main site of absorption of orally administered drugs).
Table 3: Percentage transport of M100240 and MDL 100,173 in the parallel artificial membrane permeability assaya and predicted fraction absorption (Fa) in humans.
M100240 is a weak acid, so ionization at high pH would prevent it crossing the lipid bilayer. Indeed, permeation of M100240 was lowest at pH 7.4 (0.52%). Overall, permeation of M100240 increased with decreasing pH, with the greatest level of percentage transport apparent at pH 5.5, which is in agreement with its pKa value of 4.3. Such findings suggest that M100240 is likely to be well absorbed across the small intestine following oral administration in humans. Indeed, the small intestine has been shown to be the primary site of absorption for M100240 in rats and humans.[7,8] Nancy et. al published results demonstrated that M100240 was absorbed primarily in proximal and distal of human small intestine. The estimated relative bioavailability of M100240 in human proximal and distal small intestine were 94% and 97%, respectively [8] . Our prediction model suggested 92.13% human Fa for M100240 based on the in vitro membrane permeability findings. The validity of the prediction model was confirmed by the fact that human Fa values predicted from the PAMPA percentage transport of 42 randomly selected commercial compounds showed high rates of correlation with human Fa values from the literature[9].
Compared with M100240, however, MDL 100,173 showed lower rates of permeation at all levels of pH. Indeed, PAMPA permeation of MDL 100,173 was not evident at pH 7.4, increasing to 0.72% and 0.92% at pH 5.5 and 1.5, respectively. Using the prediction model, we calculated a human Fa for MDL 100,173 of approximately 45.77% at pH 5.5.
The findings of our PAMPA study were validated by the results for a panel of control compounds. Naproxen and warfarin, for example, have relatively low pKa values (4.2 and 5.0, respectively) and exist in non-ionized form at low pH. Both compounds therefore achieved high rates of permeation at pH 1.5. In contrast, diltiazem, a weak base (pKa 8.9), showed increasing rates of permeation as pH increased (from 0% at pH 1.5 to 8.88% at pH 7.4). We also included the β-adrenoreceptor antagonists atenolol and metoprolol, which are weakly basic and have similar pKa values (9.6 and 9.7, respectively), to highlight the effect of differences in lipophilicity on membrane penetration. Reported log D values (n-octanol-phosphate buffer; pH 7.4) for these agents are -1.29 (atenolol) and -0.16 (metoprolol), respectively.[9] Thus, at pH 7.4, metoprolol showed increased permeation versus atenolol (3.94% vs 0.8%), presumably as a result of the increased lipophilicity of this agent. Overall, these findings would appear to confirm the validity of the PAMPA system used in the present study.
Tandem Caco-2 evaluation in conjunction with in vitro liver enzyme metabolic stability rates has been employed to predict the oral bioavailability of new molecular entities [10]. This technique can be modified with the use of PAMPA in lieu of Caco-2 for bioavailability predictions in cases where stability issues pose experimental challenges.
CONCLUSION
In conclusion, the results of this study using a validated artificial membrane permeability assay suggest that M100240 is likely to be well absorbed via transcellular passive diffusion across the human gastrointestinal tract following oral administration. These in vitro findings, consistent with data obtained in humans, demonstrate the potential predictive value of PAMPA for fraction-absorbed estimates.
ACKNOWLEDGMENT
This study was supported by Aventis Pharmaceuticals Inc., Bridgewater, NJ, USA. The authors are indebted to Mr. Hong Shen (Department of Chemistry) and Ms. Sharon Porubsky (Department of Pharmacokinetics and Drug Metabolism), Aventis Pharmaceuticals Inc., Bridgewater, NJ, USA) for their support and assistance during the conduct of this study.
References
Lipper RA. E Pluribus Product: how can we optimize selection of drug development candidates from many compounds at an early stage? Mod Drug Discov 1999;2:55–60.
Prentis RA, Lis Y, Walker SR. Pharmaceutical innovation by the seven UK-owned pharmaceutical companies (1964–1985). Br J Clin Pharmacol 1988;25:387–396.
Kennedy T. Managing the drug discovery/development interface. Drug Discov Today 1997;2:436–444.
Hidalgo IJ. Assessing the absorption of new pharmaceuticals. Curr Top Med Chem 2001;1:385–401.
Kansy M, Senner F, Gubernator K. Physicochemical high throughput screening: parallel artificial membrane permeation assay in the description of passive absorption processes. J Med Chem 1998;41:1007–1010.
French JF, Flynn GA, Giroux EL, Mehdi S, Anderson B, Beach DC, et al. Characterization of a dual inhibitor of angiotensin I-converting enzyme and neutral endopeptidase. J Pharmacol Exp Ther 1994;268:180–186.
Hwang KK, Jiang L, Ren, Y, Martin LL, Martin NE. Site-specific absorption of M100240 and MDL 100,173 in rats using Sweetana–Grass diffusion chamber technology. J Pharmacological & Toxicological Methods 2002:48:97-101.
Martin NE, Read, KA, Martin LL, Tardiff S, Wilding I, Wray H, Barrett JS. Pharmacoscintigraphic assessment of the regional drug absorption of a dual ACE/NEP inhibitor, M100240, in healthy volunteers. J Clin Pharmacol 2003;43:529-538.
Zhu C, Jiang L, Chen T-M, Hwang K-K. A comparative study of artificial membrane permeability assay for high throughput profiling of drug absorption potential. Eur J Med Chem 2002;37:399–407.
Mandagere AK, Thompson TN, Hwang K-K. Graphical model for estimating oral bioavailability of drugs in humans and other species from their Caco-2 permeability and in vitro liver enzyme metabolic stability rates. J Med Chem 2002;45:304–311.
Wohnsland F.; Faller B. High-throughput permeability pH profile and high-throughput alkane/water log P with artificial membranes. J. Med. Chem. 2001; 44(6); 923-930
Kiyohiko Sugano; Hirokazu Hamada; Minoru Machida; Hidetoshi Ushio. High Throughput Prediction of Oral Absorption: Improvement of the Composition of the Lipid Solution Used in Parallel Artificial Membrane Permeation Assay. J. Biomolecular Screening 2002; 6(3); 189-196
Corresponding Author: Kin-Kai Hwang, Department of Drug Metabolism and Pharmacokinetics, Aventis Pharmaceuticals Inc., Bridgewater, New Jersey, USA. kinkai.hwang@aventis.com
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps