J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 6(3):327-333, 2003
Protection of pancreatic b -cell by the potential antioxidant bis-o-hydroxycinnamoyl methane, analogue of natural curcuminoid in experimental diabetes.
Anusuya Srinivasan, Venugopal P. Menon1
Department of Biochemistry, Annamalai University, Annamalai Nagar, IndiaViswanathan Periaswamy
Department of Pathology, Raja Muthiah Medical College, Annamalai University, Annamalai Nagar, IndiaK.N. Rajasekaran
Department of Chemistry, University of Kerala, Trivandrum, IndiaReceived 08 April 2003, Revised 4 November 2003, Accepted 06 Novembe 2003
PDF version
Abstract
PURPOSE: To evaluate the antioxidant defense by bis-o-hydroxycinnamoylmethane, analogue of the naturally occurring curcuminoid bis-demethoxycurcumin in streptozotocin induced diabetes in male Wistar rats and its possible protection of pancreatic b-cell against gradual loss under diabetic condition. METHODS. Male wistar rats were divided into five groups. Group1 served as control rats. Group2 was control rats treated intragastrically with bis-o-hydroxycinnamoyl methane at a dose of 15mg/kg body weight for 45 days. Group3, 4 and 5 rats were injected with 40mg /kg body weight of streptozotocin to induce diabetes. Group4 rats were treated with the drug similar to group2 and group5 rats treated with the reference drug glibenclamide intragastrically for a similar period. After 45 days, the levels of plasma glucose, glycated hemoglobin, enzymic antioxidants (SOD, CAT) and non-enzymic antioxidants Vit C, Vit E was determined. Histopathological sections of the pancreas were examined. RESULTS. The levels of plasma glucose and glycated hemoglobin which were elevated in group3 diabetic rats were reduced after treatment with the drug. The antioxidant levels showed an increase in the case of treated diabetic rats as compared to group3 diabetic rats. The islets were shrunken in group3 diabetic rats in comparison to normal rats. In the treated diabetic rats there was expansion of islets. CONCLUSIONS. The experimental drug bis-o-hydroxycinnamoylmethane enhances the antioxidant defense against reactive oxygen species produced under hyperglycemic conditions and thus protects the pancreatic b -cell against loss and exhibits antidiabetic property
Introduction
Reactive oxygen species play an important role in the pathogenesis and development of complications of diabetes. Reactive oxygen species are produced mainly by the glycation reaction under diabetic conditions (1). Glycation reaction in diabetes occurs in various tissues including b -cells (2, 3). The activity of antioxidant enzymes such as superoxide dismutase, catalase and glutathione peroxidase which is low in islet cells when compared to other tissues becomes further worsened under diabetic conditions (4). Earlier reports have shown apoptosis of b -cells (5) and reduced insulin gene transcription (6) by glycation mediated reactive oxygen species. The antioxidant treatment which suppresses apoptosis of β-cells was shown to preserve b -cell function in diabetic mice (7).
The active principle isolated from the rhizomes of the Curcuma Longa Linn consists of the components namely curcumin, demethoxycurcumin and bisdemethoxycurcumin collectively known as curcuminoids. All the three constituents are powerful antioxidants and reported to be more active than α-tocopherol (8). The antioxidant activity of bisdemethoxycurcumin (BDMC), which is bis-p-hydroxycinnamoylmethane, was reported to be maximum at a lower concentration than the other two curcuminoids (9, 10).
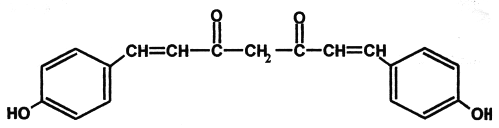
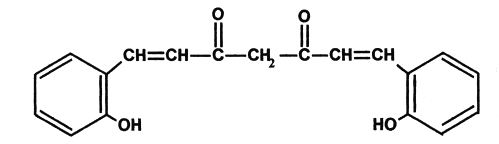
The synthetic analogs of curcuminoids have also been reported to have antioxidant, antitumor and antimutagenic activities (11). The antioxidant activity of naturally occurring curcuminoids and their synthetic analogs were attributed to their potency by inducing enzymes like superoxide dismutase (SOD), catalase (CAT) and also this potential was reported to be more than 30 fold when hydroxyl group is introduced in ortho position (12). Hence in our present study we investigated the protective role of bis-o-hydroxycinnamoylmethane (BDMCA), which is an analogue of bisdemethoxycurcumin BDMC against the oxidative stress in experimental diabetes and its possible protection of pancreatic b-cells. Chemical structures of BDMC and its analog BDMCA are shown in figure 1 and 2 respectively.
Figure 1: Structure of Bisdemethoxy curcumin (BDMC).
Figure 2: Structure of Bisdemethoxy curcumin analog (BDMCA)
MATERIALS AND METHODS
Animals
Male albino rats of wistar strain were procured from the central animal house, Rajah Muthiah Medical College, Annamalai University and maintained under standard condition with food and water ad libitum. The experimental protocol for the study has the approval of the animal ethics committee of our institution.
Chemicals and Reagents
Streptozotocin was purchased from Sigma Chemical St. Louis Mo USA. Glucose kit was purchased from Span Diagnostics India Ltd, Nitrobluetetrazolium, phenazinemethosulphate, hydrogen peroxide, thiobarbituric acid, acetyl acetone and salicylaldehyde were purchased from Himedia Laboratories, Mumbai, India. All other reagents were of analytical grade.
Drug
The experimental drug bis-o-hydroxycinnamoylmethane was prepared by refluxing a mixture of acetyl acetone, salicylaldehyde and boric acid in DMF adding few drops of catalyst namely 1:1 glacial acetic acid and diethanolamine for 5 to 6 hours. The mixture then poured to 10 % acetic acid was stirred for 2 to 3 hrs. The crude yellow mass was filtered and purified using silica gel column chromatography and the purity checked by TLC and the spectral data verified with the earlier reports (13). The drug was administered orally by intragastric intubation by dissolving a known weight of the drug in water and DMSO in the ratio 100:1(v/v) to get 15mg/kg body weight. The volume 2ml of the drug administered was kept a constant in all groups of rats.
Experimental Induction of diabetes
Diabetes was induced in 9 to 10 weeks old male Wistar rats (150 to 160g body weight) by injection of streptozotocin 40mg kg-1 body weight, dissolved in 0.1M trisodium citrate buffer pH=4.5 intraperitoneally and the control rats were injected with citrate buffer alone. Development of diabetes was first determined by the observations of three classical symptoms polyuria, polydypsia and polyphagia. It was once again verified after two weeks of STZ injections by measuring the plasma glucose levels. Rats with fasting plasma glucose levels less than 10mM/L were excluded from the study.
Experimental Design
Group 1: Control rats given 1ml of vehicle (1% DMSO) alone for 45 days.
Group 2: Rats given 15 mg/kg body weight of BDMCA for 45 days.
Group 3: STZ diabetic rats treated as in group1.
Group 4: STZ diabetic rats treated with the drug as in group2.
Group 5: STZ diabetic treated with the reference drug glibenclamide for a similar period.
The experiment was terminated at the end of 45 days and the animals were fasted overnight.
Blood Sampling
Blood was collected into heparinized tubes by reteroorbital plexus puncture and plasma was separated by centrifugation at 2000g for 10 min.
Preparation of hemolysate
After the separation of plasma, the buffy coat was removed and packed cells (RBCs) were washed thrice with cold physiological saline. RBC lysate was prepared by lysing a known volume of RBC with cold phosphate buffer pH 7.4. The hemolysate was separated by centrifuging at 3000g for 10 min at 2°C and used to measure the activities of enzymic antioxidants superoxide dismutase (SOD) and catalase (CAT).
Histological sections of the Pancreas
The animals from each group were anaesthetized with ether and intra cardiac perfusion of normal saline followed by 10% formalin was done. Pancreas was dissected out, blotted and all the groups were processed for routine paraffin embedding. Sections were cut and stained with haemotoxylin and eosin.
Biochemical Investigations
Plasma glucose
It was measured by enzymic method using kit supplied by Span Diagnostics, India Ltd (14). To 10 ml of plasma 1 ml of glucose reagent was added and incubated at 37°C for 15 min Absorbance was read at 510 nm against reagent blank and a standard was also processed similarly.
Glycated hemoglobin
It was measured by the method of Sudhakar Nayak and Pattabiraman (15). 200 ml of the lysate was mixed with 1.8 ml of 0.3 M oxalic acid and the mixture was hydrolyzed for 4hours. Cooled and added 1.0 ml of 40% TCA. After centrifugation for 20 minutes 1.5 ml of the supernatant was treated with 0.05ml of 80% phenol and 3ml of concentrated sulfuric acid. Tubes were incubated at 37°C for 30 minutes and the color developed was read at 480 nm. Standard fructose in the range of 10-40 mg was processed similarly.
Non protein thiol
It was measured by the method of Ellman (16). 0.5 ml of plasma was pipetted out and precipitated with 2.0 ml of 5% TCA. After the centrifugation 1.0ml of the supernatant was taken and 0.5ml of Ellman's reagent and 3.0 ml of phosphate buffer were added. The yellow color developed was read at 412nm. A series of standard was treated in a similar manner along with a blank containing 3.5ml of buffer. The values were expressed as mg/dl.
Vitamin C
It was measured using DNPH by the method of Roe and Kuther (17). Mixed 0.5 ml of plasma with 1.5 ml of 6 % TCA. Allowed to stand for 5 minutes and centrifuged. To the supernatant
0.3g of acid washed norit was added, shaken vigorously and filtered. 0.5 ml DNPH was added to 2 ml of the filtrate, stoppered and placed in a water bath for 3 hours at 37°C. The tubes were removed from water bath and placed in ice-cold water and added 2.5 ml of 85 % H2SO4 drop by drop. The tubes were mixed well and allowed to stand for 30 minutes at room temperature. A set of standards containing 20-100μg of ascorbic acid were taken and processed similarly, along with a blank containing 2.0 ml of 4 % TCA. The yellow color developed was read colorimetrically at 540nm.
Superoxide Dismutase
The activity of SOD was assayed by the method of Kakkar et al (18). To 1.2 ml of sodium pyrophosphate buffer, 0.1 ml of phenazine methosulphate, 0.3 ml of nitroblue tetrazolium, 0.2 ml of NADH, 1.1 ml of water and 0.1 ml of hemolysate was added and incubated exactly for 90 seconds at 30°C. The reaction was started by the addition of NADH and stopped by the addition of 1 ml of glacial acetic acid. Control was carried out simultaneously. The reaction mixture was stirred vigorously and shaken with 4 ml of n-butanol. The mixture was allowed to stand for 10 minutes, centrifuged and the chromogen extracted in butanol layer was read at 560 nm against butanol blank. One unit of the enzyme activity is defined as the enzyme reaction which gave 50% inhibition of NBT reduction in one minute under the assay conditions and expressed as specific activity in units / mg Hb.
Catalase
It was assayed by the method of Sinha (19). 0.1 ml of hemolysate and 1.5ml of phosphate buffer were added. To this, 0.4ml of hydrogen peroxide was added and the reaction was arrested after 30 and 60 second by the addition of 2.0 ml dichromate acetic acid reagent. A control was also carried out simultaneously. All the tubes were heated in a boiling water bath for exactly 10 minutes, cooled and absorbance read at 620nm. Standards in the range of 2-10 m moles were taken and processed as the test. The activity of catalase was expressed as m moles of hydrogen peroxide consumed/min/mg Hb.
Statistical Analysis
All data are presented as the mean ± SD from 6 rats in each group. One-way analysis of variance (ANOVA) followed by least significant difference point test was used to identify significantly different groups. P<0.05 indicates significant differences between group mean.
RESULTS
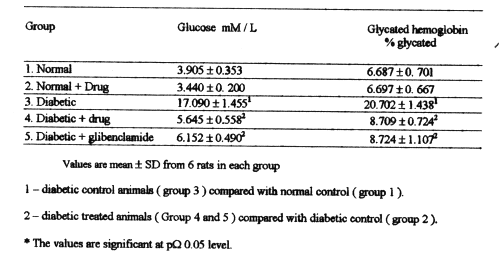
Table-1 shows the levels of plasma glucose and glycated hemoglobin in normal and experimental rats. The levels of glucose and glycated hemoglobin were elevated significantly in the group3 diabetic control rats when compared with normal rats in group1 and 2. After treatment with BDMCA the levels of glucose and glycated hemoglobin were significantly lowered.
Table 1: Levels of plasma glucose and glycated hemoglobin in expermental diabetic rats.
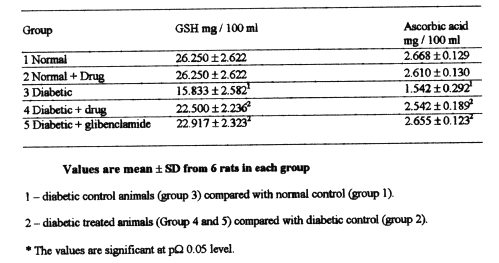
Table-2 shows the levels of plasma non protein thiols and ascorbic acid. The levels were significantly lowered in diabetic rats of group3 when compared with those in normal control rats of group1 and 2.Administration of BDMCA and glibenclamide to STZ diabetic rats in group 4 and 5 increased significantly the levels of non protein thiols and ascorbic acid as compared to that of diabetic rats in group 3.
Table 2: Levels of non-protein thiols and ascorbic acid in the plasma of experimental diabetic rats.
Table-3 depicts the activities of superoxide dismutase (SOD) and catalase in the RBC lysate of normal and experimental rats. Diabetic rats in group3 showed a significant decrease in the activity of these enzymes when compared with normal rats in group1 and 2. Administration of BDMCA and glibenclamide to diabetic rats in group 4 and 5 increased the activity as compared with that of diabetic rats in group3 for both enzymes.
Table 3: Levels of superoxide dismutase and catalase in the RBC lysate of experimental diabetic rats.
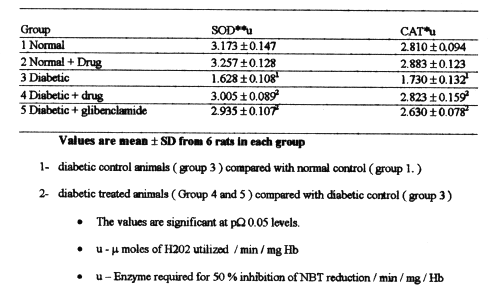
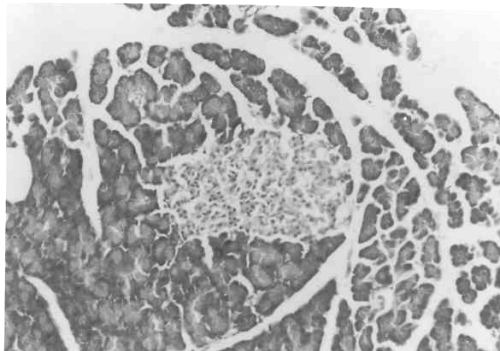
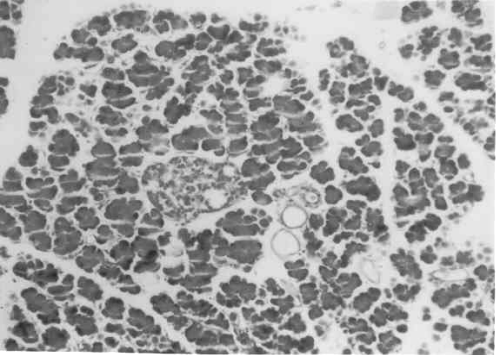
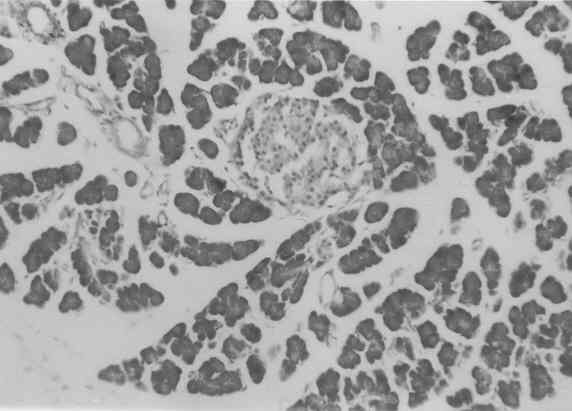
Figure 3 to 7 depicts the islet cells of the pancreas of rat in different groups. Fig 3 shows normal islet cells. The islets were shrunken in group 3 diabetic rat when compared with group1 control rat. Treatment of diabetic rat with the drug BDMCA in group 4 and glibenclamide in group 5 showed moderate expansion of islets.
Figure 3: Typical photomicrograph of the pancreas of control rat H and E x 10.
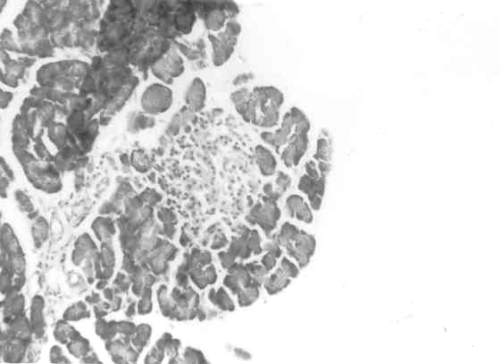
Figure 4: Typical photomicrograph of the pancreas of 15mg / kg b.wt of BDMCA treated control rat H and E x 10 shows normal islets.
Figure 5: Typical photomicrograph of the pancreas of STZ induced diabetic control rats H and E x 10 shows shrunken islets.
Figure 6: Typical photomicrograph of the pancreas of STZ induced diabetic rats treated with 15mg / kg b.wt of BDMC-A. H and E x 10 shows moderate expansion of islets.
Figure 7: Typical photomicrograph of the pancreas of STZ induced diabetic rats treated with 600 mg/kg b.wt of Glibenclamide. H and E x 10 shows moderate expansion of islets.
Discussion
The islet b cells are susceptible to damage caused by oxygen free radicals (20) since the antioxidant defense system is weak under diabetic condition (21). The levels of antioxidant defense system are altered in STZ induced diabetic rats which is in good correlation with the present observation (22).
Non protein thiols like glutathione are one of the important primary defenses that counteract the oxidative stress. We observed a lower level of plasma non protein thiols in STZ diabetic rats which is consistent with the earlier report (22, 23). The observed decrease may be due to utilization of non protein thiols by increased oxygen free radicals produced in hyperglycemic conditions associated with diabetes mellitus.
Vitamin C is a well known antioxidant and quenches singlet molecular oxygen in aqueous medium (24). We observed a decrease in level of plasma vitamin C. Decreased level of plasma ascorbic acid have been reported in experimental diabetes(25). The decrease in the level of vitamin C may be due to increased utilization against ROS produced in large amounts in diabetes or decrease in the non protein thiols like GSH as GSH is required for the recycling of vitamin C.
The two important enzymic antioxidants namely superoxide dismutase and catalase in the RBC lysate is found to have decreased activity in STZ diabetic control rats. A similar decrease in the activity of these two enzymes in hemolysate of STZ diabetic rats was reported earlier (26). The increase in the level of oxygen radicals superoxide O2*- and OH- in experimental diabetes and the protection by SOD and catalase were reported by Uchigata et al (27).
Treatment of diabetic rats with the drug BDMCA for 45 days increased the levels of non-enzymic antioxidants vitamin C and non protein thiols in plasma. The activities of SOD and CAT were also increased in RBC lysate of treated diabetic rats. The antioxidant activity of the drug BDMCA might have been due to the inhibition of glycation of the antioxidant enzymes SOD and CAT. Glucose which forms Schiff's base with proteins has been reported to have high affinity for proteins especially those containing transition metal ions (28). Increased glycated Cu-Zn-SOD has been reported in diabetes. Earlier reports have shown that curcumin inhibits advanced glycatin end products in STZ diabetes in rats (29). In the present study also we observed a decrease in the glycated hemoglobin in rats treated with the drug BDMCA. The inhibitory action may be due to the presence of the central 1, 3-diketone system as in curcumin. Enhancement of antioxidants SOD and CAT by BDMCA thus might be explained as due to prevention of glycation. Furthermore the presence of -OH group in the O-position increase its antioxidant potential through intermolecular hydrogen bonding involving the -SH group of non protein thiols and enzymes (30).
Increase in the activity of the enzymes catalase and SOD might have protected b -cells against damage by reactive oxygen species. Treatment with the antioxidant N-acetyl cysteine in diabetic mice was shown to have suppressed b -cell apoptosis and decrease blood glucose level (7). Hence we conclude that the drug used in the present study offers protection of b -cells against ROS mediated damage by enhancing antioxidants and reduces hyperglycemia in chemically induced diabetes.
ACKNOWLEDGEMENT
The author Anusuya Srinivasan is grateful to the University Grants Commission, New Delhi for the financial assistance extended in the form of fellowship.
References
Hunt, J.V., Smith, CC. and Wolff, SP., Auto-oxidative glycosylation and possible involvement of peroxides and free radicals in LDL modification by glucose. Diabetes, 39: 1420 – 1424, 1991.
Myint , T., Hoshi, S., Ookawara , T., Miyazawa , N., Suzuki, K. and Taniguchi N., Immunological detection of glycated proteins in normal and streptozotocin – induced diabetic rats using anti hexitol – lysine IgG. Biochem Biophys Acta, 1272 : 73 – 79 , 1995.
Tajiri , Y., Moller, C-and Grill, V., Long term effects of aminoguanidine on insulin release and biosynthesis: evidence that the formation of advanced glycosylation end products inhibits b-cell function. Endocrinology , 138 : 273 - 280,1997.
Tiedge, M., Lortz;S.,Drinkgern, J. and Lenzen. S., Relationship between antioxidant enzyme gene expression and anti oxidative defense status of insulin producing cells. Diabetes, 46: 1733 – 1742 , 1997
Kaneto , H.,Fuji, J., Myint T., Miyazawan., Islam , KN., Kawasaki Y., Suzuki K., Nakamura, M., Tatsumi, H., Yamasaki, Y.and Taniguchi, N., Reducing sugars triggers oxidative modification and apoptosis in pancreatic b - cells by provoking oxidative stress through the glycation reaction. Biochem.J, 320: 855 – 863 , 1996
Matsuoka , T., Kajimoto Y., Watada, H., Kaneto , H., Kishimoto , M., Umayahara, Y., Fujitani, Y., Kamada , T., Kawamori , R. and Yamasaki Y., Glycation – dependent , reactive oxygen species mediated suppression of the insulin gene promoter activity in HIT cells. J Clin Invest, 99: 144 – 150, 1997.
Kaneto, H., Kajimoto, Y., Miyagawa, J., Matruoka, T., Fujitani, Y., Umayahara, Y., Hanafusa, T., Matsuzawa , Y., Yamasaki , Y.,. and Hori , M., Benefecial effects of antioxidants in diabetes. Possible protection of pancreatic b - cells against glucose toxicity. Diabetes, 48: 2398 – 2406 , 1999.
Sreejayam. and Rao, MN., Curcuminoids as potent cohibitors of lipid peroxidation. J. Pharm Pharmacol , 46 : 1013 – 1016 , 1994.
Ruby, A.J., Kuttan, G., Babu, KD., Rajasekaran, KN. Kuttan, R., Antitumor and antioxidant activity of natural curcuminoids . Cancer Lett, 94 : 79 – 83 , 1995.
Kim, DS., Park , SY and Kim, JK., Curcuminoids from curcuma long L ( Zingiberaceae ) that protect PC-12 rat pheochromocytoma and normal human umbilical vein endothelial cells from beta A ( 1-42) insult. Neuroscience Lett, 303 : 57 – 61 , 2001.
Anto,RJ.,George,J.,Dinesh Babu, KV., Rajasekaran KN and Kuttan, R., Antimutagenic and anti carcinogenic activity of natural and synthetic curcuminoids. Mutant Res, 370: 127 – 131 , 1996.
Dinkova Kostova , AT. And Talalay, P., Relation of structure of curcumin analogs to their potencies as inducers of detoxification enzymes. Carcinogensis, 20 : 911 – 914 , 1999.
Dinesh Babu , KV and Rajasekharan KN., Simplified conditions for the synthesis of curcumin 1 and other curcuminoids. Org. Prep.Proc.Intl, 26: 674 – 677 , 1994.
Trinder., Enzymatic GOD – POD end point colorimetry. Ann. clin. Biochem, 6 : 24,1969.
Sudhakar Nayak, S and Pattibiraman TN., A new colorimetric method for the estimation of glycosylated hemoglobin. Clin. Chim. Acta,109 : 267-274, 1981.
Ellman , GL., Tissue sulfhydryl groups. Arch . Biochem. Biophys. 82 : 70 – 77 , 1959.
Roe, JM. And Kuether , CA., Detection of ascorbic acid in whole blood and urine through the 2 , 4 – DNPH derivative of dehydroascorbic acid J. Biol chem.. 147: 399 – 407 , 1943.
Kakkar , P., Das, B and Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase ( SOD). Ind. J. Biochem. Biophys, 21: 130 –1 32 , 1984.
Sinha , KA., Colorimetric assay of catalase. Anal. Biochem, 47 : 389 - .394 , 1972.
Gorey, KC., Soresso, D.. and Moak , SA., Comparison of superoxide dismutase activities in isolated rat and guinea pig islets of langerhans. Horm. Metab.Res. 25 : 649 – 650 , 1993.
Grankvist, K., Markud, S.and Toijedahl , IB, Superoxide dismutase is a prophylactic against alloxan diabetes. Nature , 294 : 158 – 160 , 1981.
Prince , PSM. And Menon, VP., Effect of Syzigium Cumini in plasma antioxidants on alloxan induced diabetic rats. J Clin Biochem. Nutr. 25 : 81 – 86 , 1998.
Ihm ,SH., Yoo, HJ., Park , SW. and Ihm. J., Effect of aminoguanidine on lipid peroxidation in streptozotocin induced diabetic rats. Metabolism , 48 : 1141 – 1145 1999 .
Chou, PT and khan , AU., L- Ascorbic acid quenching of singlet delta molecular oxygen in aqueous media. Generalized antioxidant property of Vitamin C. Biochem. Biophys. Res. Commun, 115 : 932 – 937 , 1983.
Prince , PSM and Menon, VP., Antioxidant activity of Tinospora Cordifolia roots in experimental diabetes. J. Ethnopharmacol, 65 : 277 – 281 , 1999.
Matakovics , B., Kotorman , M., Varga, Isz., Hai , DQ and Varga, Cs., Oxidative stress in experimental diabetes induced by streptozotocin. Acta . Physiol Hung. 85 : 29 – 38 , 1998.
Uchigata , Y., Yamamoto,, H., Kawamura, A and Okamoto, H., Protection by superoxide dismutase, catalase and poly ( ADP – Riobose ) synthetase inhibitors against alloxan and streptozotocin induced islet DNA. Strand breaks and against the inhibition of proinsulin synthesis. J. Biol.Chem, 251 : 6084 – 6088 , 1982.
Sajithlal, GB., Chithra, P and Gowri, C., Effect of curcumin on the advanced glycation and cross – linking of collagen in diabetic rats. Biochem. Pharamacol , 56 : 1 – 8 , 1998.
Taniguchi , N., Kaneto , H., Asahi, M., Takahasi, M., Wenyi , C., Higashiyama, S., Fuji, J., Suzuki , K . and Kayanoki, Y., Involvement of glycation and oxidative stress in diabetic macroangiopathy. Diabetes, 45 : S 81 – 83 , 1996.
Dinkova kostova, AT., Massi8ah , MA., Bozak , RE., Hicks , RJ and Talaly, P.,Potency of Michael reaction acceptors as inducers of enzymes that protect against carginogenesis depends on their reactivity with sulfhydryl groups. Proc. Natl. Aced. Sci. 98 : 3404 – 3409 , 2001.
Corresponding Author: Venugopal P.Menon, Center for Micronutrient Research, Annamalai University, Annamalai Nagar, Tamilnadu-608002, India. cmrana@sify.com
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps