J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 7(1):8-12, 2004
Effect of a lipoidic excipient on the absorption profile of compound UK 81252 in dogs after oral administration.
Rong-Kun Chang
Shire Laboratories, Inc., Rockville, Maryland, USAAmir H Shojaei1
Shire Laboratories, Inc., and Shire Pharmaceutical Development, Rockville, Maryland, USAReceived 01 December 2003, Revised 05 January 2004, Accepted 05 January 2004, Published 23January 2004
PDF version
Abstract
PURPOSE: Effect of caprylocaproyl macrogolglycerides (Labrasol), as a lipoidic excipient/vehicle in an oral capsule formulation, on pharmacokinetic disposition of a BCS Class 3 compound, UK-81252, was investigated in vivo in a canine model. METHODS: The control and lipoidic formulations were administrated to six Beagle dogs in a crossover, single dose design with a 2-week washout period in between treatments. The plasma concentration-time profile for the lipoidic formulation was compared to that of the control formulation (lactose-based oral capsule). RESULTS: Although the lipoidic formulation resulted in a markedly increased oral bioavailability (based on mean pharmacokinetic parameters, AUC0-48hr and Cmax ), a double-peaking phenomenon was observed with this formulation. The most likely cause of this double-peak effect was the gastric emptying retardation attribute of the lipoidic vehicle/excipient. The initial peak (Tmax1 ) was due to the absorption enhancing properties of the lipoidic formulation and the second peak (Tmax2 ) was most likely the result of a shutdown in gastric emptying for a period of up to 2 hours (this value varied between dogs) after which the remaining Compound UK 81252 emptied from the stomach to generate the second peak. CONCLUSIONS: Caprylocaproyl macrogolglycerides enhanced the absorption of Compound UK 81252. After oral administration, the liquid-filled formulation consistently produced a double-peak phenomenon in the plasma profile. Labrasol was determined to be the most likely culprit for this double peaking phenomenon.
Introduction
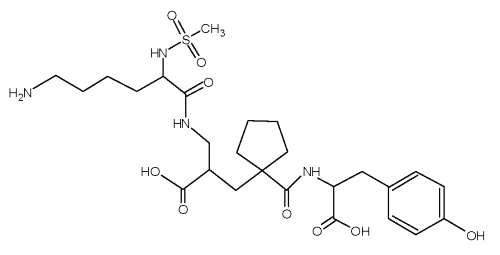
Compound UK 81252, (S, S, S)-N-{1-[2-carboxy-3-(N-mesyllysylamino) propyl]-1-cyclopentylcarbonyl} tyrosine, is a dual inhibitor of angiotensin-converting enzyme and neutral peptidase with potential application as an antihypertensive agent as well as a treatment of congestive heart failure [1]. Compound UK 81252 (Figure 1) is a hydrophilic compound containing one weakly acidic phenolic group, two more strongly acidic carboxylic acid groups, and one strong basic primary amine group.
Figure 1: Chemical structure of Compound UK 81252.
Solubility of Compound UK 81252 can be described as freely soluble in water and diluted acid/alkali solution, specifically 40 mg/mL at pH 1 and 5 mg/mL at pH 5. Permeability of Compound UK 81252 as determined by Caco-2 permeability assay is low with a permeability coefficient of 1.5 x 10 -7 cm/s. According to the Biopharmaceutics Classification System (BCS), Compound UK 81252 is classified as a Class 3 high solubility-low permeability compound [2]. Earlier studies demonstrated that the compound has low oral bioavailability, which is potentially caused by the low permeability of the compound. To increase the oral bioavailability of UK 81252, lipid based formulations were investigated using caprylocaproyl macrogolglycerides (Labrasol, Gattefosse, NJ) as the lipoidic vehicle. Here we report the effect of Labrasol on the pharmacokinetic disposition of compound UK 81252 in vivo in a canine study.
Materials and Methods
Preparation of Compound UK 81252 Capsules
The control formulation was prepared by using lactose (Spectrum, Gardena, CA) as the filler. Preweighed amounts of Compound UK 81252 (100 mg) and lactose (1900 mg) were triturated and mixed using a mortar and pestle. Appropriate amount (200 mg) of this powder blend was encapsulated in size 00 Swedish orange hard gelatin capsules (Capsugel, Greenwood, SC) by hand filling. The filled capsules were then sealed with a hydroalcoholic solution of gelatin. For lipoidic formulation, caprylocaproyl macrogolglycerides (Labrasol, Gattefosse, and Westwood, NJ) was used as the vehicle. Compound UK 81252 (120 mg) was homogeneously dispersed in polyglycolyzed glycerides (8280 mg) using a mortar and pestle. Appropriate amount (700 mg) of this dispersion was encapsulated in size 00 Swedish orange hard gelatin capsules by hand filling.
Drug Administration, Blood sampling, and Analysis of Plasma Samples
The control and lipoidic formulations were administrated to six Beagle dogs in a crossover, single dose design with a 2-week washout period. The study was conducted in accordance with the international guiding principles for biomedical research involving laboratory animals and in compliance with the International Conference on Harmonization (ICH) guidelines for Good Clinical Practice. Each dog, after fasting for at least 18 hours with free access to water, was dosed at approximately 1 mg/kg on the first day. For each dosing, blood samples (3 mL) were collected from the jugular vein by venipuncture (repeated punctures) immediately before treatment and after Compound UK 81252 administration at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 24, and 48 hours. The blood samples were collected into lithium heparin vacutainer tubes and were stored at -80°C until analysis.
Plasma samples, plasma standards, and a plasma blank were allowed to thaw at room temperature. All samples were mixed by vortexing. The samples were then centrifuged at 3000 rpm for 3 minutes. The drug was extracted from the plasma using a quaternary amine solid phase extraction column (J.T. Baker, Philipsburg, NJ). The column was first conditioned with water and ethanol. Then, 1 mL of the plasma sample was transferred to the solid phase extraction column and the column was allowed to dry. The column was washed by adding sequentially 2 mL of water, ethanol, hexane, ethanol, and then water. The column was transferred to a new test tube and eluded with 1 mL of 1M ammonium acetate (pH 4.0). The samples were mixed well and analyzed by liquid chromatography-mass spectrometry (LC-MS). The LC-MS system consisted of a Waters 2690 Alliance Separation Module and a Micromass Quattro LC. A Phenomenex Luna column, C-18(2) (150 x 2.0 mm, 5 μm Phenomenex, Torrance, CA) was used with a gradient elution from 5% acetonitrile to 42% acetonitrile in 5 mM ammonium acetate for 8 minutes, then 42% acetonitrile for 2 additional minutes. Subsequently, the column was re-equilibrated at the initial conditions for 5 minutes. The flow rate was controlled at 0.25 ml/min; the injection volume was 40 mL. The method had a linearity factor of 0.98 and was validated for specificity with samples of known concentrations.
The samples were analyzed on-line by a Quattro LC and quantitated using MassLynx software after the samples eluted from the LC system. A standard curve comprising of six calibration standards (10 ng/mL, 20 ng/mL, 50 ng/mL, 100 ng/mL, 500 ng/mL, and 1000 ng/mL) was constructed with a weighted quadratic regression. The amount of Compound UK 81252 present in each plasma sample was then calculated from the standard curve using the chromatographic peak area.
Results and Discussion
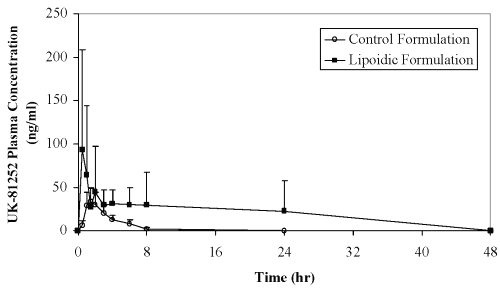
The mean plasma concentration-time profiles of Compound UK-81252 after oral administration of lactose-based control capsules and caprylocaproyl macrogolglycerides-based liquid-filled capsules (containing 10 mg Compound UK 81252) to Beagle dogs are shown in Figure 2.
Figure 2: Plasma concentration (Mean + SD, n=6) of UK-81252 after oral administration in dogs.
At the 10 mg dose, the plasma concentrations for the control formulation were very low and at numerous time points, the plasma levels were below the assay sensitivity (10 ng/mL). The results obtained form the current study further confirms low bioavailability of Compound UK 81252 from the earlier studies. Both control and liquid-filled formulations gave 100% drug release at the 15-minute dissolution time point in both deionized water and 0.1 N HCl as the dissolution medium. Permeability of Compound UK 81252 as determined in Caco-2 permeability assay is low with permeability coefficient of 0.15 x 10 -6 cm/s. Permeability of Compound UK 81252 in Caco-2 cell lines in presence of 5% polyglycolyzed glycerides in Hank's Buffer solution markedly increased to 17.5 x 10 -6 cm/s, which coincides with the increased passage of mannitol (permeability coefficient of mannitol from 0.71 x 10 -6 cm/s without polyglycolyzed glycerides to 22.4 x 10 -6 cm/s with polyglycolyzed glycerides). The enhanced permeation of mannitol and Compound UK 81252 indicates that caprylocaproyl macrogolglycerides may cause the tight junction opening and paracellular diffusion may be the main absorption route for Compound UK 81252. In dogs, the lipoidic vehicle, caprylocaproyl macrogolglycerides, clearly enhanced the absorption of Compound UK 81252 effectively, compared to lactose-based control formulation. The absorption enhancement observed in the present study further confirms the potential application of caprylocaproyl macrogoglycerides as a bioavailability enhancer for oral liquid and capsule formulations
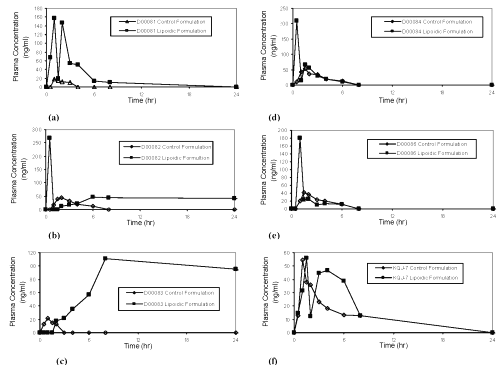
Visual examination of the mean plasma concentrations-time profile indicates the presence of double peaks; further inspection of the individual plasma concentrations-time profiles reveals that five out of six subjects showed a clear double-peak phenomenon (Figure 3a-f). Several mechanisms can trigger this atypical double-peak plasma profile: 1) enterohepatic cycling, 2) the presence of two absorption regions along the gastrointestinal tract, 3) gastric emptying regulated absorption [3-8]. Enterohepatic cycling may be ruled out as a cause of the double peaks in plasma concentrations-time profile, because the phenomenon is not observed after oral administration of lactose-based control formulation and after intravenous administration of Compound UK 81252 in buffer solution at 2 mg/mL concentration (from a previous study). The absence of the double peaks after oral dosing of lactose-based formulation also may be used to negate the presence of two absorption windows along the gastrointestinal tract.
Figure 3: UK-81252 Plasma concentration-time profiles for individual dogs.
Caprylocaproyl macrogolglycerides is a lipid-based surface-active agent. It is known that natural triglycerides inhibit gastric motility, linseed oil and olive oil being the most effective [9]. It is also known that surfactants can inhibit the gastric emptying (i.e., slow down gastric emptying or shut-down gastric emptying for a period of time), which is mediated by the formation of viscous mass in gastric and intestinal lumen or by a substance (or substances) formed after contact of the intestinal mucosa with the surface-active agents [10, 11]. The pharmaceutical formulation may thus influence drug absorption through this indirect physiological effect.
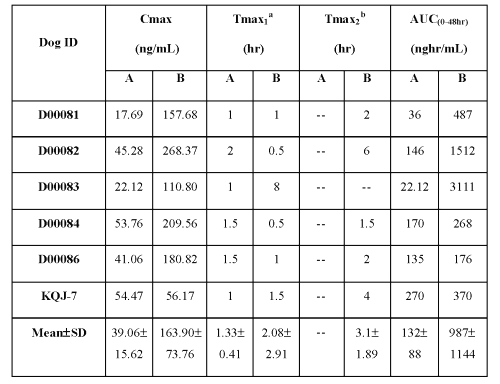
These scenarios presented in the literature are consistent with the double peaks observed in the current case, where caprylocaproyl macrogolglycerides enhance the absorption of Compound UK 81252 to generate the first peak and provide a shutdown of gastric emptying for a variable period (up to two hours) to limit the supply of Compound UK 81252. Subsequently remaining Compound UK 81252 emptied form the stomach to produce the second peak in the plasma profile. One of the six dogs did not show the double-peak phenomenon, but the plasma profile for this dog showed a significant lag time (1.5 hours), before the appearance of Compound UK 81252 in the blood. This long lag time further demonstrates that caprylocaproyl macrogolglycerides shuts down the gastric emptying. The basic pharmacokinetic parameters for individual subjects along with the mean data are listed in Table 1.
Table 1: Basic pharmacokinetic parameters from individual animals along with mean data after oral administration of lactose-based control formulation and caprylocaproyl macrogolglycerides-based liquid-filled formulation.
aTmax1=first Tmax; bTmax2= second Tmax
A = lactose-based control formulation; B = caprylocaproyl macrogolglycerides-based liquid-filled formulation
The remarkable contrast between with and without caprylocaproyl macrogolglycerides in the formula in terms of basic pharmacokinetic parameters suggests that caprylocaproyl macrogolglycerides has a strong permeability enhancing and gastric emptying inhibitory effects.
Conclusion
The results from this dog study further confirm the low bioavailability of Compound UK 81252 and the compound's permeability-limited oral absorption. Caprylocaproyl macrogolglycerides enhanced the absorption of Compound UK 81252. The mean area under the plasma curve from time 0 to 48 hours (AUC0-48hr) for the caprylocaproyl macrogolglycerides-liquid filled formula was more than 7 fold greater than that for the control formulation. After oral administration, the liquid-filled formulation consistently produced a double-peak phenomenon in the plasma profile. Caprylocaproyl macrogolglycerides was found to be the most likely culprit for this double peaking.
Acknowledgements
The authors would like to acknowledge the contribution of Ms. Kathy Yu and Dr. Ali Shokri.
References
Norton, G.R., et al., Sustained antihypertensive actions of a dual angiotensin-converting enzyme neutral endopeptidase inhibitor, sampatrilat, in black hypertensive subjects. American Journal of Hypertension, 1999. 12: p. 563-571.
Amidon, G., et al., A Theoretical Basis for a Biopharmaceutics Drug Classification: The Correlation of In Vitro Drug Product Dissolution and In Vivo Bioavailability. Pharm Res, 1995. 12: p. 413-420.
Wang, Y., et al., A Double-Peak Phenomenon in the Pharmacokinetics of Alprazolam after Oral Administration. Drug Metabolism and Disposition, 1999. 27(8): p. 855-859.
Bressolle, F., et al., Double Weibull input function describes the complex absorption of sustained-release oral sodium valproate. J Pharm Sci, 1994. 83(10): p. 1461-1464.
Charman, W.N., et al., Absorption of danazol after administration to different sites of the gastrointestinal tract and the relationship to single and double peak phenomena in the plasma profiles. J Clin Pharmacol, 1993. 33: p. 1207-1213.
Lennernas, H. and C.-G. Regardh, Evidence for an interaction between the beta-blocker pafenolol and bile salts in the intestinal lumen of the rat leading dose-dependent oral absorption and double peaks in the plasma concentration-time profile. Pharm Res, 1993. 10(6): p. 879-883.
Cook, C., et al., Pharmacokinetics of a novel antiarrhythmic drug, Actisomide. Pharm Res, 1993. 10(3): p. 427-433.
Mummaneni, V. and J.B. Dressman, Intestinal uptake of cimetidine and ranitidine in rats. Pharm Res, 1994. 11(11): p. 1599-1604.
Cooke, A., Control of gastric emtying and motility. Gastroenterology, 1975. 68(4): p. 804-816.
Lish, P., Some pharmacologic effects of dioctyl sodium sulfosuccinate on the gastrointestinal tract of the rat. Gastroenterology, 1961. 41: p. 580-584.
Necheles, H. and J. Sporn, Effect of detergents on gastric motility. American Journal of Gastroenterology, 1966. 46: p. 481-483.Corresponding Author: Amir H. Shojaei, Shire Pharmaceutical Development, 1801 Research Boulevard, Rockville, Maryland, USA, 20850. ashojaei@us.shire.com
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps