J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 7(1):70-75, 2004
Studies on anti-diarrhoeal activity of calotropis gigantea r.br. in experimental animals.
Havagiray R. Chitme1
Institute of Pharmacy, Bundelkhand University, Jhansi, Uttar Pradesh, IndiaRamesh Chandra
Bundelkhand University, Jhansi, Uttar Pradesh, IndiaSadhna Kaushik
Department of Pharmacology, Maharani Laxmi Bai Medical College, Jhansi, Uttar Pradesh, IndiaReceived 03 April 2003, Revised 18 November 2003, Accepted 10 February 2004, Published 25 February 2004
PDF Version
Abstract
PURPOSE: Calotropis gigantea R. Br. (Asclepiadaceae) a wildly growing plant has been reported to possess number of medicinal properties and other purposes. The purpose of the present study was to evaluate scientifically the anti-diarrhoeal effects of C. gigantea used traditionally in Indian system of medicine using castor oil-induced diarrhoea model. METHODS : The anti-diarrhoeal effect of hydroalcoholic (50:50) extract of aerial part of Calotropis gigantea was studied against castor oil-induced-diarrhoea model in rats. The gastrointestinal transit rate was expressed as the percentage of the longest distance traversed by the charcoal divided by the total length of the small intestine. The weight and volume of intestinal content induced by castor oil were studied by enteropooling method. RESULTS : Like atropine (3mg/kg, i.p.) there were significant reductions in fecal out put and frequency of droppings when the plant extracts of 200 and 400 mg/kg doses were administered intraperitoneally compared with castor oil treated rats. All doses of the plant extracts also significantly retarded the castor-oil induced enteropooling and intestinal transit. The dose 100 (P<0.01), 200 and 400 mg/kg significantly inhibited (P<0.001) weight and volume of intestinal content. CONCLUSIONS : The remarkable anti-diarrhoeal effect of C.gigantea extract against castor oil-induced diarrhoea model attests to its utility in a wide range of diarrhoeal states.
Introduction
In developing countries, the majority of people living in rural areas almost exclusively use traditional medicines in treating all sorts of disease including diarrhoea. There are large numbers of epidemiological and experimental evidence pertaining to worldwide acute-diarrhoeal disease, which is one of the principal causes of death in the infants, particularly in malnourished and which is of critical importance in developing countries (1, 2). It thus becomes important to identify and evaluate commonly available natural drugs as alternative to currently used anti-diarrhoeal drugs, which are not completely free from adverse effects (3). Several studies have evaluated the effectiveness of some traditional medicines in treating diarrhoea, in all different continents (4-7). India has a great environmental and biological diversity compared with the rest of the world. A range of medicinal plants with anti-diarrhoeal properties has been widely used by the traditional healers; however, the effectiveness of many of these anti-diarrhoeal traditional medicines has not been scientifically evaluated.
Calotropis gigantea R. Br. (Asclepiadaceae) a wildly growing plant has been reported to possess number of medicinal properties (8) and other purposes (9). In 1980, Pal and Sinha had isolated, crystallized and studied the properties of calotropins D1 and D2 from C.gigantea (10). The plant is considered crude drug of Bangladesh (11) and medicinal plant of Indonesia (12). The new oxiopregnane-oligoglycosides named calotropis A and B have been isolated from the root of C.gigantea and their chemical structure have been elucidated by chemical and spectroscopy methods (11). The cytotoxic principles of 'Akond mul' (Root of C.gigantea) cardenoloids glycosides, calotropin frugoside and 4-O-Beta-D-glucopyranosyl frugoside were obtained as the cytotoxic principles (12).
This study reports on the anti-diarrhoeal effects of Calotropis gigantea R.Br. used traditionally in Indian system of medicine using castor oil-induced diarrhoea model.
Materials and Methods
Plant material
The aerial parts of C.gigantea were collected around Jhansi city in 2001 from wild. A voucher specimen has been deposited at the Institute of Pharmacy, Bundelkhand University, Jhansi, Uttar Pradesh, India. The aerial part were dried under shade, each dehydrated plant powdered to a fine texture and 100 g of the dried plant was repeatedly extracted with water:ethanol (50:50). The extract was concentrated under vacuum and the residue was used in the experiments. The dried plant extracts were freshly re-dissolved in normal saline and given to adult albino Swiss rats fed a standard animal diet. Unless otherwise stated, henceforth, the term `extract' means the water:ethanol (50:50) extract of aerial part of C.gigantea.
Animals
Albino Swiss rats of either sex weighing 150-180 g were used for castor oil-induced anti-diarrhoeal, anti-secretory and intestinal transit activity. All animals were fed standard animal feed and tap water ad libitum before the experiments. Each experimental group consisted of six animals housed in separate cages.
Castor oil-induced diarrhoea
Rats were divided into five groups of six animals each, diarrhoea was induced by administering 1 ml of castor oil orally to rats. Group 1 served as control (2 ml/kg, i.p. saline), group 2 received atropine (3mg/kg, i.p.) served as standard and group 3, 4, and 5 received extract (100, 200 and 400 mg/kg, i.p.) 1 h before castor oil administration. The number of both wet and dry diarrhoeal droppings were counted every hour for a period of 4 h mean of the stools passed by the treated groups were compared with that of the positive control group consisted of animals given an intraperitoneal injection of saline (2ml/kg, ip) (13).
Castor oil-induced enteropooling
Intraluminal fluid accumulation was determined by the method of Robert et al., (1976). Overnight fasted rats were divided five groups of six animals each. Group 1 received normal saline intraperitoneally (2 ml/kg, i.p.) served as a control, group 2 received atropine (3mg/kg, i.p.) and groups 3, 4 and 5 received the extract of 100, 200 and 400 mg/kg intraperitoneally respectively 1h before the oral administration of castor oil. Two hours later the rats were sacrificed, the small intestine was removed after tying the ends with thread and weighed. The intestinal contents were collected by milking into a graduated tube and their volume was measured. The intestine was reweighed and the difference between full and empty intestines was calculated (14).
Small intestinal transit
Rats were fasted for 18 h divided into six groups of six animals each, Group1 received 2 ml normal saline orally, group 2 received 2 ml of castor oil orally with saline 2 ml/kg intraperitoneally, group 3 received atropine (3 mg/kg, i.p.), group 4, 5 and 6 received 100, 200 and 400 mg/kg intraperitoneally of the plant extract respectively, 1 h before administration of castor oil. One ml of marker (10% charcoal suspension in 5% gum acacia) was administered orally 1 h after castor oil treatment. The rats were sacrificed after 1h and the distance traveled by charcoal meal from the pylorus was measured and expressed as percentage of the total length of the intestine from the pylorus to caecum (15).
Statistical analysis
The experimental results are represented as mean ± S.E. (Standard error of the mean). Student's t-test was used for the evaluation of data and P<0.05 accepted as significant.
Results
Castor oil-induced diarrhoea
30 min after administration of castor oil the diarrhoea was clinically apparent in all the animals of control group, for the next 4 h. This was markedly reduced by the intraperitoneal injection of atropine, 3 mg/kg (39.84%) (Table 1). A similar marked reduction in the number of defecations over four hours was achieved with G. gigantea when in doses of 200 or 400 mg/kg i.p. 400 mg/kg, i.p. dose of extract delayed the onset of diarrhoea and only 30% of animals showed diarrhoea at first hour (P<0.01). The 100 mg/kg, i.p. dose of the extract did not affect the severity and onset of diarrhoea.
Table 1: Effect of Calotropis gigantea extract on castor oil-induced diarrhoea in rats.
Effect of extract on castor oil-induced diarrhoea in rats. Extract was administered ip 1 h before castor oil administration. Values are expressed as mean ± SEM from the experiments. *P<0.01, **P<0.001 when compared with CO + saline-treated group.
Castor oil-induced enteropooling
Castor oil caused accumulation of water and electrolytes in intestinal loop. Castor oil-induced enteropooling is not influenced by atropine in rats (3 mg/kg, i.p.). Each dose of the extract produced a dose-dependent reduction in intestinal weight and volume. 100 mg/kg, i.p. dose of extract produced 22.16% inhibition of volume of intestinal content (P<0.01). However, 200 and 400 mg/kg, i.p. dose produced 38.82 and 55.31% inhibition of volume of intestinal content respectively with significance (P<0.001). The weight of intestinal content was also reduced significantly at all the doses. 100, 200 and 400 mg/kg, i.p. dose dependently inhibited weight of intestinal content of 25.49, 41.11 and 56.4% respectively (Table 2).
Table 2: Effect of Calotropis gigantea extract on castor oil induced enteropooling in rats.
Effect of extract on castor oil-induced enteropooling in rats. Extract was administered ip 1 h before castor oil administration. Values are expressed as mean ± SEM from the experiments. *P<0.01, **P<0.001 when compared with CO + saline-treated group.
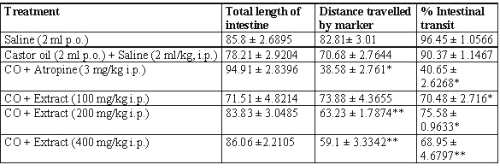
Small intestinal transit
The percent intestinal transit was increased with castor oil (90.37 ± 1.1467), but was reduced in each of the three concentrations of extract, and much more markedly by atropine (40.65 ± 2.6268). 100 and 200 mg/kg, i.p. dose of extract produced 70.48 ± 2.716 and 75.58 ± 0.9633% intestinal transit induced by castor oil respectively. Whereas, 400 mg/kg, i.p. dose produced 68.95 ± 4.6797% of castor oil induced charcoal meal transit (Table 3).
Table 3: Effect of Calotropis gigantea extract on castor oil-induced small intestinal transit in rats.
Effect of extract on castor oil-induced small intestinal transit in rats. Extract was administered ip 1 h before castor oil administration. Values are expressed as mean ± SEM from the experiments. *P<0.01, **P<0.001 when compared with CO + saline-treated group.
Discussion and Conclusion
Diarrhoea results from an imbalance between the absorptive and secretory mechanisms in the intestinal tract, accompanied by hurry, resulting in an excess loss of fluid in the faeces. In some diarrhoeas, the secretory component predominates, while other diarrhoeas are characterized by hypermotility. The use of castor oil induced diarrhoea model in our study is logical because the autocoids and prostaglandins are involved these have been implicated in the causation of diarrhoeas in man (16, 17). The liberation of ricinoleic acid from castor oil results in irritation and inflammation of the intestinal mucosa, leading to release of prostaglandins, which stimulate motility and secretion (18). The results of the present study show that the extract of C.gigantea produced a statistically significant reduction in the severity and frequency of diarrhoea produced by castor oil. It is also noted that the extract significantly inhibited castor oil induced intestinal fluid accumulation and the volume of intestinal content, dose dependently more than atropine. The extract significantly reduced the castor oil induced intestinal transit. In this study, atropine produced a significant reduction in the number of stools and increased intestinal transit time possibly due to its anti-cholinergic effect (19). However, it did not inhibit castor oil induced enteropooling and gain in weight of intestinal content suggesting thereby that mediators other than acetylcholine are involved in castor oil induced enteropooling. An increase in intestinal transit time with atropine could also result due to reduction in gastric emptying (20).
Castor oil is also reported to induce diarrhoea by increasing the volume of intestinal content by prevention of the reabsorption of water. The liberation of ricinoleic acid results in irritation and inflammation of the intestinal mucosa, leading to release of prostaglandins, which results in stimulation of secretion (18). Thereby prevents the reabsorption of NaCl and H2O (21). Probably extract increased the reabsorption of NaCl and water by decreasing intestinal motility as observed by the decrease in intestinal transit by charcoal meal. The anti-diarrhoeal activity of the extract may also be due to the presence of denature proteins forming protein tannates, protein tannates make the intestinal mucosa more resistant and reduce secretion (22). The secretory diarrhoea is associated with an activation of Cl- channels, causing Cl- efflux from the cell, the efflux of Cl- results in massive secretion of water into the intestinal lumen and profuse watery diarrhoea (23). The extract may inhibit the secretion of water into the lumen by reverting this mechanism.
Anti-dysentric and antidiarrhoeal properties of medicinal plants were found to be due to tannins, alkaloids, saponins, flavonoids, sterols and/or triterpenes and reducing sugars (24). The sesquiterpene lactones, a large group of compounds with anti-inflammatory properties have the ability to relax smooth muscles and thereby relieve gastrointestinal distress (25). The phytochemical analysis of the extract revealed the presence of sugars (26), flavonoids (27), flavonol glycosides (28), oxypregnane-oligoglycosides (29), terpenes, terpene derivatives, pentacyclic triterpenoids and triterpenoids and they have been isolated from C.gigantea (30). These constituents may mediate the anitdiarrhoeal property of the C.gigantea extract. Although the antidiarrhoeal properties of the reported active terpenoids are well established, aspects of their mechanism of action remain poorly understood. Sesquiterpenes, diterpenes, terpenes, flavonoids and terpenoid derivatives are known for inhibiting release of autocoids and prostaglandins, thereby inhibit the motility and secretion induced by castor oil (31-34).
Castor oil is a suitable model of diarrhoea in rats, since it allows the observation of measurable changes in the number of stools, enteropooling and intestinal transit. The extract resulted in a marked reduction in the number of diarrhoea stools and the reduction in the weight and volume of the intestinal contents, as well as a modest reduction in intestinal transit. This signifies the usefulness of this model and the clinical effect of the extract.
The remarkable anti-diarrhoeal effect of C.gigantea extract against castor oil diarrhoea, model attest to wide range of utility in secretory and functional diarrhoeas (35). This study did not go further, to demonstration as to whether the extract altered the activity of Na+K+ATPase or activation of chloride channels. Whatever, may be the mechanism of action, C.gigantea extract may be useful in a wide range of diarrhoeal states, due to both disorders of transit e.g. functional diarrhoeas, radiation diarrhoea or due to abnormal secretory mechanisms like in cholera or E.coli enterotoxin induced diarrhoea. Further studies are needed to completely understand the mechanism of anti-diarrhoeal action of C.gigantea extract.
References
Syder, J.D. and Merson, M.H., The magnitude of the global problems of acute diarrhoeal disease a review of active surveillance data. Bulletin of the world health organization, 60: 605-613, 1982.
Lutterodt, G.D., Inhibition of gastrointestinal release of acetylcholine by quercetin as possible mode of action of Psidium guajava leaf extracts in the treatment of acute diarrhoeal disease. Journal of Ethnopharmacology, 25:235-249, 1989.
Hardman, J.G., Limbird, L.E., (Eds), Goodman and Gilman’s The pharmacological Basis of therapeutics 10th Edition, MacGraw Hill, New York, pp 914-931, 1992.
Offiah, V.N. and Chikwender, U.A., Antidiarrhoeal effects of ocimum gratissimum leaf extract in experimental animals. Journal of Ethnopharmacology, 68:327-330, 1999.
Mukherjee, P.K., Saha, K., Murugesan, T., Mandal, S.C., Pal, M. and Saha, B.P., Screening of antidiarrhoeal profile of some plant extracts of a specific region of west Bengal, India. Journal of Ethnopharmacology, 60:85-89, 1998.
Rani, S., Ahamed, N., Rajaram,S., Saluja, R., Thenmozhi, S. and Murugesan, T., Antidiarrhoeal evaluation of clerodendrum phlomidis Linn, leaf extract in rats. Journal of Ethnopharmacology, 68:315-319, 1999.
Zavata, M.A., Perez, S., Perez, C., Vargus, R. and Perez, R.M., Antidiarrhoeal activity of Waltheria Americana, commelinacoelestis and Atternenthera repens. Journal of Ethnopharmacology, 61:41-47, 1998.
Kartikar, K.R. and Basu, N., Indian medicinal plants, Lolit Mohan Basu, Allahabad, p1606, 1935.
Duke, J.A., Hand book of medicinal herbs, Calotropis gigantea, CRC Press, Orlando, pp 90-92, 1985.
Pal, G. and Sinha, N.K., Isolation, crystallization and properties of calotropins D1 and D2 from calotropis gigantean. Archives of Biochemistry and Biophys. 202:321-329, 1980.
Kitagawa Isao, Ru-Song Zhang, Jony Dae Park, Nam In Back, Yasuyuki, Takeda, Mayasuki, Yoshikawa and Hirotaka Shibuya, Indonesian medicinal plants. I. Chemical structures of calotroposides A and B, Two new oxypregnane-oligoglycosides from the root of calotropis gigantea (Asclepiadaceae). Chem.Pharm.Bull.,40:2007-2013, 1992.
Kiuchi, F., Fukao, Y., Maruyama, T., Tanaka, M., Saraki, T., Mikage, M., Hague, M.E. and Tsuda, Y., Cytotoxic principles of a Bangladeshi crude drug, akondmul (roots of calotropis gigantea). Chem./Pharm.Bull., 46:528-530, 1998.
Awouters, F., Niemegeers, C.J.E., Lenaerts, F.M., Janseen, P.A.J., Delay of castor oil diarhoea in rats; a new way to evaluate inhibitors of prostaglandin biosynthesis. Journal of Pharmacy Pharmacology, 30:41-45, 1978.
Robert A., Nezamis, J.E., Lancaster, C., Hanchar, A.J., Klepper, M.S., Enteropooling assay; a test for diarrhea produced by prostaglandins, Prostaglandins, 11:809-828, 1976.
Mascolo, N., Izzo, A.A., Avtore, G., Barboto, F., Capasso, F., Nitric oxide and castor oil induced diarrhea, Journal of Pharmacology and Experimental therapeutics, 268: 291-295, 1994.
Horton, E.W., Main, I.H.M., Thampson, C.J. and Wright, P.M., Effects of orally administered, PGE on gastric secretion and gastrointestinal motility in man, Gut, 9: 655-658, 1968.
Greenbargena, N.J., Arwanitakis, C. and Hurwitz, A.,: Azarnoff, D.L. (eds), In drug development of gastrointestinal disorders, Churchill Livingstone, NewYork, pp 155-156, 1978.
Pierce, N.F., Carpenter, C.C.J., Elliot, H.Z. and Greenough, W.B., Effects of prostaglandins, theophylline and Cholera exotoxin upon transmucosal water and electrolyte movement in canine jejunum, Gastroenterology, 60:22-32, 1971.
Brown J.A. and Taylor, P., Muscarinic receptor agonists and antagonist. In: Hardman, J.G., Limbird, L.E., (Eds), Goodman and Gilman’s The pharmacological Basis of therapeutics 10th Edition, MacGraw Hill, New York, pp 115-158, 2000.
Izzo, A.A., Mascolo, N., Capasso, R., Germano, M.P..,DePasquel, R., Capasso, F., Inhibitory effectof caannabinoid agonists on gastric emptying in the rat, Archives of Pharmacology, 360:221-223, 1999.
Galvez, J., Zavzuelo, A., Crespo, M.E., Lorente, M.D., Ocete, M.A.and Jimenez, J., Anti-diarrhoeic activity of Euphorbia hirta extract and isolation of an active flavanoid constituent, Planta Medica, 59:333-336, 1993.
Tripathi, K.D., Essentials of Medical Pharmacology, Jaypee Brothers Medical Publishers (P), New Delhi, pp 775, 1994.
Ammon, H.V. and Soergel, K.H., Diarrhea In Berk J.E. (eds): Bockus Gstroenterology, Philadelphia, Saunders, pp, 125-141, 1985.
Longanga_Otshudi, A. Vercruysse, A. and Foriers, A., Contribution to the ethnobotanical, phytochemical and pharmacological studies of traditionally used medicinal plants in the treatment of dysentery and diarrhea in Lomela area, Democratic Republic of Congo (DRC), J Ethnopharmacol, 71, 3, 411-23, 2000.
Heinrich, M., Robles, M., West, J.E., Ortiz_de_Montellano, B.R. and Rodriguez, E., Ethnopharmacology of Mexican asteraceae (Compositae), Annu Rev Pharmacol Toxicol, 38, 539-65, 1998.
De, S., 1996, On the morphology and biochemical aspects of starch grains in latex sera of five laticiferous plants, Geobios Jodhpur, 23, 4, 267-268.
Rahman, M. A. and Wilcock, C. C., 1991, A report on flavonoid investigation in some Bangladesh Asclepiads, Bangladesh J. Bot., 20, 2, 175-178.
Sen, S. and Sahu, N. P., 1992, Flavonol glycosides from Calotropis gigantea, Phytochem., 31, 8, 2919-2921.
Shibuya, H. and Zhang, R. S., 1992, Indonesian medicinal plants: V. Chemical structures of calotroposides C, D, E, F, and G: Five additional new oxypregnane-oligoglycosides from the root of Calotropis gigantea (Asclepiadaceae), Chem. Pharm. Bull. Tokyo, 40, 10, 2647-2653.
Gupta, J. and Ali Mohd, 2000, Rare chemical constituents from Calotropis gigantea roots, Indian J. Pharm. Sci., 62, 29-32.
Vimala, R., Nagarajan, S., Alam, M., Susan, T., and Joy, S., Anti-inflammatory and antipyretic activity of Michelia champaca Linn., (white variety), Ixora brachiata Roxb. and Rhynchosia cana (Willd.) D.C. flower extract, Indian J Exp Biol, 35, 12, 1310-4, 1997.
Veiga, V.F., Zunino, L., Calixto, J.B., Patitucci, M.L. and Pinto, A.C., Phytochemical and antioedematogenic studies of commercial copaiba oils available in Brazil, Phytother Res, 15, 6, 476-80, 2001.
Milanova, R., Han, K. and Moore, M., Oxidation and glucose conjugation of synthetic abietane diterpenes by Cunninghamella sp. II. Novel routes to the family of diterpenes from Tripterygium wilfordii, J Nat Prod, 58, 1, 68-73, 1995.
Nikiema, J.B., Vanhaelen_Fastre, R., Vanhaelen, M., Fontaine, J., De_Graef, C. and Heenen, M., Effects of anti-inflammatory triterpenes isolated from Leptadenia hastata latex on keratinocyte proliferation, Phytother Res, 15, 2, 131-4, 2001.
The wealth of India, Raw materials Publication and Information Directorate, CSIR, New Delhi, Vol.3, pp 78-84, 1992.
Corresponding Author: Havagiray R. Chitme, Institute of Pharmacy, Bundelkhand University, Jhansi, Uttar Pradesh, India. hrchitme@rediffmail.com
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps