J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 7(1):1-7, 2004
Involvement of nitric oxide in fading of 5-hydroxytryptamine-induced vasocontraction in rat isolated vena portae smooth muscle.
J.Y. Datté1, M. A. Offoumou
Laboratoire de Nutrition & Pharmacologie, Département BA-PA, UFR-Biosciences, Cocody Université, Abidjan, Côte d'IvoireReceived 12 September 2003, Revised 19 December 2003, Accepted 19 December 2003, Published 23 January 2004
PDF version
Abstract
PURPOSE: The aim of the present study was to investigate a nitric oxide (NO) involvement in the mediation of a 5-HT-induced vasoconstrictions response in the rat portal vein in vitro. MATERIAL AND METHODS: Isolated rat portal vein preparations obtained after dissection were placed in organ baths for isometric force measurement via a strain gauge. RESULTS: 5-Hydroxytryptamine (5-HT) in concentrations ranging from 3x10-8 M to 3x10-4 M contracted portal vein preparations (EC50 = 7x10-7 M) in a concentration dependent manner. The vasoconstrictions induced by 5-HT was significantly increased in endothelium-denuded vessels. Pre-treatment with Nw -nitro-L-arginine methyl Ester (L-NAME 100 mM) enhanced the contractive response to 5-HT either in intact- or denuded endothelium vessels. Whereas, ketanserin (0.1 mM) abolished 5-HT-induced vasoconstrictions (EC50 = 4.6x10-8 M). Furthermore, a non-selective 5-HT1B/D- receptors agonist, sumatriptan, (1x10-10 M - 1x10-5 M) induced a reduction of spontaneous rhythmic contractions either in endothelium-intact or in endothelium -denuded vessels. However, 5-HT-induced vasoconstriction was unaffected by propranolol (10 mM). CONCLUSIONS: These findings are consistent with the hypothesis that the vasoconstrictor activity of 5-HT in vascular smooth muscle was mediated by activation of 5-HT1B/D and 5-HT2B receptors subtypes involving the endothelium (NO) dependent mechanism.
Introduction
The portal vein smooth muscle in several species has been shown to have unusual properties, including exhibition of spontaneous myogenic tone and vasoconstriction to acetylcholine [1- 3]. Structural and pharmacological features of portal veins offer convenient preparation of venous smooth muscle, which possibly models the pharmacological reactivity of blood vessels [4, 5-7].
Furchgott et al. postulated the existence of endothelium-derived vascular relaxant factor (EDRF) when they observed that acetylcholine paradoxically relaxed preconstricted aortic strip preparations by an endothelium-dependent mechanism [8, 9]. This phenomenon has since been demonstrated in different blood vessels and mammalian species [10, 11]. It is well established that endothelium-derived relaxing factor has been identified as nitric oxide (NO). The nitric oxide synthases (NOS) constitute a family of closely related isoenzymes [12-14]. Several studies have shown that NO may counteract the constrictor effects of several pressor systems [15-18]. In vitro studies have indicated that nitric oxide plays important role in the modulation of the vascular tone of pharmacological tools [19-23]. In the present study, we investigate the involvement of nitric oxide in the response of rats portal vein to exogenously applied 5-HT, and we further use a selective 5-HT2-receptor antagonist and subtype selective 5-HT1-receptor agonist.
Materials and Methods
Animals
Male 18-22 weeks old Wistar Kyoto rats (WKY, 280-350 g) bred at the Department of Nutrition and Pharmacology in Abidjan (Côte d'Ivoire) were used for the experiments. All of the animals were housed at constant humidity (60 %) and temperature (25°C) and kept under a 12-hour light/dark cycle. Animals were cared for and treated according to the principles for the care and use of laboratory animals approved by the ethical committee of Cocody University, Abidjan.
Preparation of isolated Mesenteric Portal Vein strips
The mesenteric portal veins were isolated from the animals and 5-6 mm of the vessel was removed from the connective tissue, cleaned and carefully placed in a Tyrode solution ( NaCl: 148.9, KCl: 2.7, CaCl2 : 1.8, NaPO4 H2 : 0.2, NaC0 3 H: 11.9, MgCl2 : 1.2, glucose: 5.5 mM). The preparations (5-6 mm) were transferred in normal Tyrode solution.
Functional endothelium in the vessels was destroyed by gently rubbing the intimal surface with a wooden rod. When a functionally intact endothelium was required special care was taken to avoid any damage to the intimae surfaces, in this case we obtained endothelium-denuded vessels.
Measurement of isometric tension
Vascular isolated preparations were mounted in 10 ml jacketed tissue baths by suspending them between two L-shaped stainless steel hooks. The lower hooks were attached to stationary support such that they could be positioned at the bottom of the bath chambers. The upper hooks were attached to force-displacement transducers Swema (Stockholm, Sweden). The bath chamber contained the appropriate Tyrode solution (37 ± 0.5 C) constantly bubbled with 95 % O2 -5 % CO2 , giving a pH of 7.4. Isometric force was measured and recorded using a Hellige polygraph (Hugo Sachs Instruments, Freiburg, Germany). A pre-load of 1g was applied. After mounting, strips were equilibrated for 30 min. Changes in isometric force were measured and recorded by means of a force transducer.
Experimental Protocol
The effects of 5-HT were evaluated on isometric spontaneous myogenic contractions of portal veins. To each preparation, norepinephrine (NE, 10 mM) was added and the maximum contractile response to NE had been obtained in order to assess the presence or absence of functional endothelium. A rapid and marked reduction of the induced tone was taken as evidence that a significant amount of functional endothelium was present. The absence of a relaxant response was taken as indicative for the disappearance of functional endothelium.
After the 30 min period of equilibration, the contractile response to depolarizing potassium solution was assessed as a test for viability. The depolarizing KCl solution (80 mM) had the same composition as the Tyrode solution used, except for the NaCl that had been completely replaced by an equimolar amount of KCl. The preparations were washed with Tyrode solution four times and re-equilibrated for another 30 min. 5-HT was directly administered for 4 min in the organ bath and the magnitude of the spontaneous and rhythmic contractions was evaluated. After the control value and after a pre-incubation with L-NAME for 30 min; 5-HT was added into the Tyrode solution concentration-dependently.
After equilibration, cumulative concentration-response curves for 5HT contraction were done. NE (10 mM) contraction in the presence of endothelium did not begin fading away for at least 30 min after stimulation.
Drugs used
Norepinephrine was purchased from Hoechst AG, Darmstadt, Germany. Serotonin (5 hydroxytryptamine), ketanserin, sumatriptan, Nw -nitro-L-arginine-methy ester (L-NAME) and sodium nitroprusside, all were purchased from Sigma Chemical Co, St Louis, Mo, USA.
Statistical Analysis
Concentrations response curves were analysed using GraphPad software (GrapPad Software Inc., San Diego, CA, USA) to determine pEC50 values [negative logarithm of the concentration eliciting 50% of the maximal contractile response (Emax )]. When a plateau in the concentration response curve was not reached, the response observed with the highest concentration used (100 mM) was considered as Emax .
All values in the text and illustrations are presented as mean ± SEM, with n representing the number of different preparations. Differences between pEC50 and Emax values of the compounds were evaluated with Tukey's test, once an analysis of variance (ANOVA) for data had revealed that the samples represented different populations. Values of p <0.05 were considered to indicate significant differences.
Results
Effects of 5-HT on the spontaneous contractions of portal vein preparations
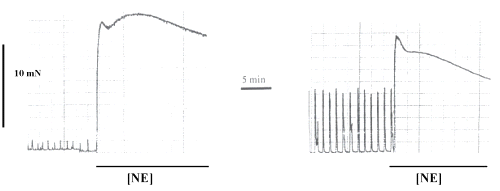
Figure 1 illustrated the effect of NE (10 mM) in preparations before and after L-NAME incubation.
Figure 1: Original recording of contractile force of the spontaneous rhythmic activity of a strip isolated from WKY-rats portal vein in Tyrode solution and the effect of norepinephrine (NE) at a concentration of 10mM, before (first panel) and after L-NAME incubation (second panel). NE-effect is indicated by a rectangle. The horizontal bar shows the time of recording and the vertical bar represents the magnitude of contractions.
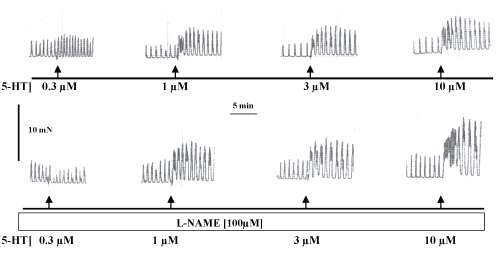
The contractile response evoked by 5-HT in endothelium intact isolated mesenteric portal vein preparations in absence and presence of L-NAME are illustrated in Figure 2.
Figure 2: Original recording of contractile force of the spontaneous rhythmic activity of a strip isolated from WKY-rats portal vein in Tyrode solution and the effect of 5-hydroxytryptamine (5-HT) in concentrations ranging from 0.3mM to 10mM; control (upper panel) and L-NAME pre-incubation (lower panel). The horizontal bar shows the time of recording and the vertical bar represents the magnitude of contractions.
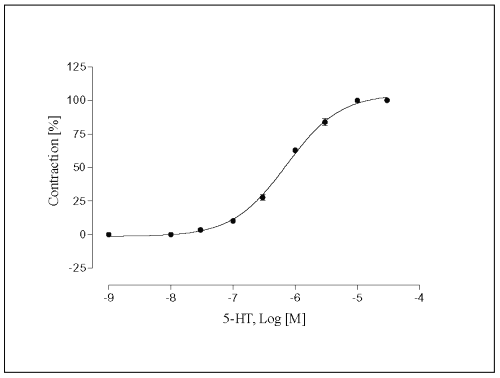
The concentration-effect graph shows 5-HT, in concentrations ranging from 3x10 -8 M to 3x10-4 M, induced an increase of the contractile force of vessels, causing a graded enhancement of the rate and force of contraction in a concentration-related manner (Fig. 3). The half-maximum contractile response was achieved at (-log EC50 ) 6.1 ± 0.02.
Figure 3: Concentration-response curve for 5-HT in isolated portal vein preparations of WKY-rats. Concentration of 5-HT are expressed as the log10 of the molar concentration. Each Point represents the mean values obtained using 6-7 rings. Each obtained from different animal. Brackets indicate SEM (n=6-7).
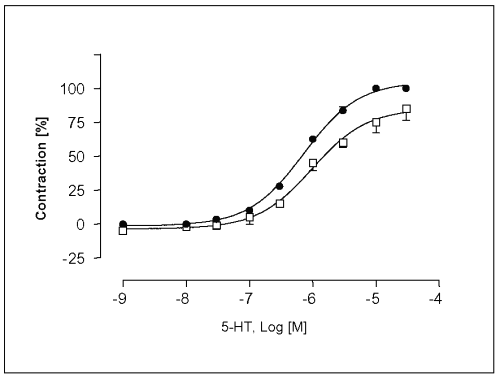
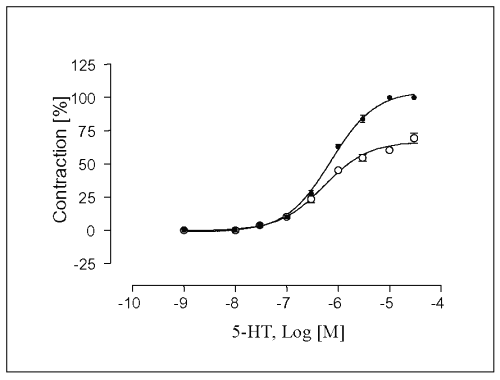
The maximal contraction response (Emax ) of the concentration-response curve for 5-HT was enhanced in the presence of L-NAME at 10-4 M by 14.6 ± 2.5%, and the EC50-value in L-NAME-treated endothelium intact vessels was slightly affected (EC50 = 7.2 x 10-7 M. The sensitivity of vessels to 5-HT was increased in L-NAME-treated endothelium intact vessels (Figure 4).
Figure 4: Concentration-response curves for 5-HT in isolated portal vein preparations of WKY-rats in the absence ( ) and presence (·) of L-NAME at a concentration of 100mM. Concentration of 5-HT are expressed as the log10 of the molar concentration. Each Point represents the mean values obtained using 6 rings. Each obtained from different animal. Brackets indicate SEM (n=6).
Effects of 5-HT on the spontaneous contractions of denuded vessels.
Further study for the importance of endothelium for 5-HT-induced vasoconstrictions was obtained from experiments with L-NAME pre-treated vessels without functional endothelium. All experiments were carried out with endothelium-denuded vessels.
In mechanically denuded vessels without L-NAME incubation, the contractile force induced by 5-HT was enhanced and the maximal contraction response to 5-HT was achieved and corresponded to 131.5 ± 2.2 % compared to control endothelium intact vessels (Fig. 5).
Figure 5: Contractile responses induced by 5-HT in segments of rat portal veins. Vascular preparations were isolated with care taken to preserve functional endothelium (o) or rubbed on the intimal surface to destroy functional endothelium (·). Rings were mounted for recording of isometric tension and, after equilibration. References responses were obtained and each preparation was tested for the presence or absence of functional endothelium as described in Materials and methods.
After 30 min of L-NAME incubation in the organ bath, addition of L-NAME increased 5-HT-induced contractions in denuded vessels. The maximal contraction response (Emax ) of concentration-response curve for 5-HT was significantly different of values observed in vessels impregnated of L-NAME at a concentration of 100 mM (Emax= 134 ± 3.6 %, EC50 = 1.3 x 10-7 M, Figure 6). Moreover, significant enhancement of the frequencies rate was observed.
Figure 6: Contractile responses induced by 5-HT in segments of rat portal veins vein in the absence (·) and presence (à) of L-NAME at a concentration of 100mM in rubbed on the intimal surface to destroy functional endothelium. Rings were mounted for recording of isometric tension and, after equilibration. References responses were obtained and each preparation was tested for the presence or absence of functional endothelium as described in Materials and methods. Concentrations of 5-HT are expressed as the log10 of the molar concentration. Each bar represents the mean values obtained using 7 rings. Each obtained from different animal. Brackets indicate SEM.
Influence of ketanserin and sumatriptan on the contractions evoked by 5-HT.
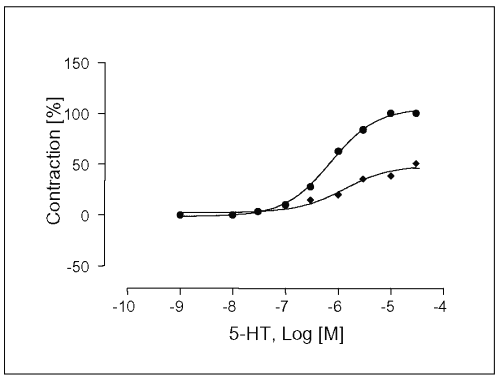
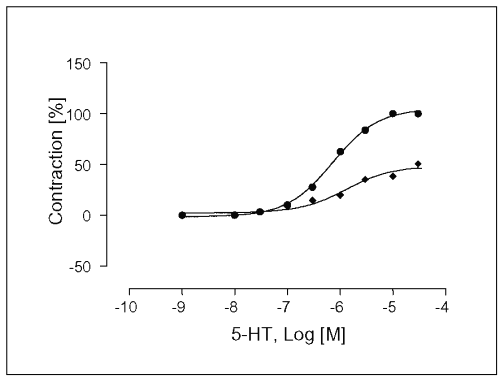
The experiments were carried out in the presence of ketanserin, a 5-HT2A and 5-HT1c -receptor antagonist. After 30 min of ketanserin incubation at a concentration of 10 mM, Emax-value of the concentration response curve for 5-HT was significantly prevented to 12.8 ± 3.1% (see Table 1), and the EC50 -value was affected (EC50 = 4.6x10 -8 M, Figure 7).
Table 1: Contractionsa (%) to 5-HT.

Figure 7: Concentration-response curves for 5-HT in isolated portal vein preparations of WKY-rats in the absence (·) and presence (à) of ketanserin at a concentration of 100mM. Concentration of 5HT are expressed as the log10 of the molar concentration. Each Point represents the mean values obtained using 5 rings. Each obtained from different animal. Ordinate: Contraction as percentage of maximal response; Abscissa: Logarithms of molar concentrations of 5-HT. Brackets indicate SEM (n=5).
Figure 8: Original recording of contractile force of the spontaneous rhythmic activity of a strip isolated from WKY-rats portal vein in Tyrode solution and the effect of sumatriptan (SUMA) at a concentration of 10M before (first panel) and after L-NAME incubation (second panel). SUMA- effect is indicated by a rectangle. The horizontal bar shows the time of recording and the vertical bar represents the magnitude of contractions.
The pre-treatment with ketanserin showed a slight increase of the rate of the frequencies of rhythmic spontaneous contractions. Whereas, sumatriptan, 5-HT1B and 5-HT1D receptors agonist in concentrations ranging from 10-4 mM to 100 mM, greatly increased the rate of frequencies of contractions 2.5 fold in comparison with endothelium-intact vessels (267 ± 8%, Fig. 3B); but decreased contractions of vessels to 78 ± 1.5% of control value. However, 5-HT-induced contractions in portal vein vessels was unaffected by propranolol pre-treatment at a unique concentration of 10 mM (data not shown).
Discussion
The study showed that 5-HT enhanced the contractile force of preparations isolated from rat's portal vein smooth muscle. Moreover, the results obtained with NE results suggest that decrease in vascular tone in rat Vena portae after the plateau of 5-HT contraction has been reached is in part due to NO derived from vascular endothelial and non-endothelial cells.
NO inhibitors affected 5-HT sensitivity in portal vein smooth muscle by increasing the contractile activity. Similar observations were obtained in the presence of L-NAME in endothelium intact vessels or in endothelium denuded vessels. The vasoconstriction obtained in the experiments indicated the effect on blood vessels involved NO-dependent mechanism as demonstrated by Summer [24], Martin et al. [25], and Wallis and Martin [26].
It is known that the presence or absence of functional endothelium can influence contractile responses in blood vessels [27, 28, 29, 30]. These data suggest that a dual endogenous vasocontractory mechanism present in mesenteric portal vein and those products of the EDRF-NO pathway may interact to regulate 5-HT stimulation via 5-HT receptors, as obtained by Yamano et al. [31], Hinton et al. [32] and Nieto et al. [33].
The endothelium-dependent contraction induced by 5-HT was antagonized by ketanserin, but not affected by propranolol. The strips contracted in the presence of β-adrenoceptors antagonists [25]. The receptor mediating the contractile response has been shown to be a 5-HT1-like receptor (positively coupled to adenyl cyclase) located on the vascular smooth muscle [32, 35-37].
The endothelium dependent response to 5-HT was not mimicked by sumatriptan. The effect of sumatriptan was not related to the endothelial quality; though in a large post hoc study, the authors have previously demonstrated that portal vein contraction in response to sumatriptan slightly decrease with increasing functional integrity of the endothelium [27, 36-40]. Sumatriptan induced only an increase in the rate of the rhythmic spontaneous contractions in the presence of L-NAME.
Conclusion
In conclusion, our results confirm the vasoconstricting effects of 5-HT in isolated portal vein smooth muscle. The nitric oxide inhibitors induced a slight and variable enhancement of contractile activity. 5-HT contracts rat isolated Vena portae preparations by both an endothelium-dependent and an endothelium-independent mechanism. 5-HT-induced contraction in rats is mediated via 5-HT1 and 5-HT2 receptors on the muscle, but not by release of noradrenaline via beta-adrenoceptors. 5-HT activates 5-HT2A and 5-HT1D and/or 5-HT1B receptors to cause contraction.
Acknowledgements
We would like to thank the Deutsche Akademisches Austausch Dienst (DAAD) for the financial support) and Prof. JP TILLEMENT, at the department of Pharmacology of Paris XII University for the critical analyze of the manuscript.
References
Bourreau, J. P., Feletou, M. and Tricoche, R. In vitro effects of S.3341 on the spontaneous contractile activity of the rat portal vein: interference with a1-adrenergic stimulation: Arches International Pharmacodyn. Ther, 296: 29-44., 1988.
Sutter M.C. The pharmacology of isolated portal veins. Br. J. Pharmacol., 24: 742, 1965.
Zimmerman B.G. Effect of acute sympathectomy on responses to angiotensin and norepinephrine. Circ. Res., 11: 780, 1962.
Datte J.Y., Gohlke P., Pees C. and Ziegler A. Short treatments of normotensive and hypertensive rats by angiotensin II and nitric oxide inhibitor induce an increase of noradrenaline sensitivity in isolated Vena portae preparations. Pharmacol. Res., 41:641-8, 2000.
Feletou M., Alya G., Walden M. and Tricoche R.Proposition de deux modèles pharmacologiques : veine porte, artère coronaire gauche de mouton. J. Physiol. Paris, 79: 23A, 1984.
Johansson B., Jonsson O., Axelson J. and Wallstrom B. Electrical and mechanical characteristics of vascular smooth muscle response to norepinephrine and isoproterenol. Circ. Res., 21: 619, 1967.
Leprêtre, N. and Mironneau, J. α2-adrenoceptors activate dihydropyridine-sensitive calcium channels via Gi-proteins and protein kinase C in rat portal vein myocytes. Pflugers Arch-Eur. J. Physiol, 429: 253-261, 1994.
Furchgott R.F. and Vanhoutte P.M. Endothelium – derived relaxing and contracting factors. FASEB J., 3:2007-2018, 1989.
Furchgott R.F. and Zawadzki J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature , 288: 373, 1980.
Ignarro L.G., Buga M.M., Wood K.S., Byrns R.E. and Chandhuri G. Endothelium-derived hyperpolarizing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sciences,84: 9265-9269, 1987.
Mombouli J.V., Bissiriou I., Agbeton V. and Vanhoutte P.M. Endothelium-derived hyperpolarizing factor: A key mediator of the vasodilator action of bradykinin. Immunopharmacol., 33: 46-50, 1996.
Griffith, T.M., Edwards D.H., Lewis, M.J., Newby, A.C. and Henderson, A..H. The nature of endothelium-derived vascular relaxant factor. Nature, 308 :645-7, 1984.
Moncada S., Palmer R.M.J. and Higgs E.A. Nitric oxide: Physiology, phathophysiology and Pharmacology. Pharmacol. Res., 43:109-141, 1991.
Palmer R.M.J., Ashton D.S. and Moncada, S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature, 333: 664-666, 1988.
Datte J.Y., Gohlke P., Pees C. and Ziegler A. At receptor inhibition affects the noradrenaline sensitivity in isolated portal vein of normotensive rat. Clin. Exp. Hypertens., 23:177-87, 2001.
Heim R., Pertz H. and Elz, S. Derivatives of 3 (-2-aminoethyl)-2-4-quinazolinedione: Partial agonism on vascular 5-HT2A receptors. Naunyn Schmiedeberg´s Arch. of Pharmacol. Supplement., 357:106 R30, 1998.
Manning R.D.Jr., Hu L., Mizelle H.L., Montani J-P. and Norton M.W. Cardiovascular responses to long-term blockade of nitric oxide synthesis. Hypertension, 22: 40–48, 1993.
Rees D.D., Palmer R.M.J., Schulz R., Hodson H.F. and Moncada S. Characterization of three inhibitors of nitric oxide synthase in vitro and in vivo. Br. J. Pharmacol., 101: 746-752, 1990.
Ohnuki A and Ogawa Y. Differential modulation by the endothelium of contractile responses to 5-hydroxytryptamine, noradrenaline, and histamine in the rabbit isolated basilar artery. Gen. Pharmacol, 28: 681-7, 1997.
Tuladhar B.R., Costall B. and Naylor R.J. Pharmacological characterization of the 5-hydroxytryptamine receptor mediating relaxation in the rat isolated ileum. Br. J. Pharmacol., 119:303-10, 1996.
Valentin J.P., Bonnafous R. and John G.W. Contractile responses evoked by dihydroergotamine, naratriptan and sumatriptan in the canine isolated coronary artery. Fundam. Clin. Pharmacol, 12:152-7, 1998.
Valentin J.P., Bonnafous R. and John G.W. Influence of the endothelium and nitric oxide on the contractile responses evoked by 5-HT1D receptor agonists in the rabbit isolated saphenous vein. Br. J. Pharmacol., 119: 35-42, 1996.
Ward J.K., Fox A.J., Barnes P.J., and Belvisi M.G. Inhibition of excitatory non-adrenergic non-cholinergic bronchoconstriction in guinea-pig airways in vitro by activation of an atypical 5-HT receptor. Br. J. Pharmacol., 111:1095-102, 1994.
Sumner, M.J.Characterization of the 5-HT receptor mediating endothelium-dependent relaxation in porcine vena cava. Br. J. Pharmacol., 102:938-42, 1991.
Martin G.R., Bolofo M.L. and Giles H. Inhibition of endothelium-dependent vasorelaxation by arginine analogues: a pharmacological analysis of agonist and tissue dependence. Br. J. Pharmacol, 105: 643-52, 1992.
Wallis S.J. and Martin W. Conditions permitting suppression of stretch-induced and vasoconstrictor tone by basal nitric oxide activity in porcine cerebral artery. Br. J. Pharmacol., 130:567-74, 2000.
MaassenVanBrink, A., van den Broek, R.W.M., de Vries, R., Upton, N., Parsons, A.A. and Saxena, P.R. The potential anti-migraine compound SB-220453 does not contract human isolated blood vessels or myocardium; a comparison with sumatriptan. Cephalalgia, 20: 538-545, 2001.
Nyborg N.C. and Mikkelsen E.O. 5-Hydroxytryptamine does not release endothelium-derived relaxing factor in rat isolated coronary arteries. Eur. J. Pharmacol., 186:295-300, 1990.
Stuehr D.J. and Griffith O.W. Mammalian nitric oxide synthases. Adv. Enzymol., 65: 287-346, 1992.
Sward K., Josefsson M., Lydrup M.L. and Hellstrand P. Effects of metabolic inhinbition on cytoplasmic calcium and contraction in smooth muscle of rat portal vein. Acta Physiol. Scand., 148: 265-272, 1993.
Yamano M., Ito, H. and Miyata, K. Species differences in the 5-hydroxytryptamine-induced contraction in the isolated distal ileum. Jpn. J. Pharmacol., 74:267-74, 1997.
Hinton J.M., Hill P., Jeremy J. and Garland C. Signalling pathways activated by 5-HT (1B)/5-HT (1D) receptors in native smooth muscle and primary cultures of rabbit renal artery smooth muscle cells. Journal of Vascular Research, 37:457-68, 2000.
Nieto J.E., Snyder J.R., Kollias-Baker C. and Stanley S. () In vitro effects of 5-hydroxytryptamine and cisapride on the circular smooth muscle of the jejunum of horses. Am. J. Vet. Res., 61:1561-5, 2000.
Ortiz J.L., Labat C., Norel X., Gorenne I., Verley J. and Brink C. Histamine receptors on human isolated pulmonary arterial muscle preparations: effects of endothelial cell removal and nitric oxide inhibitors. J. Pharmacol. Exp. Ther., 260:762-7, 1992.
Costa B.R. and Naylor R.J. Pharmacological characterization of the 5-Hydroxytryptamine receptor mediating relaxation in the rat isolated ileum. Br. J. Pharmacol., 119:303-10, 1996.
Ellwood A.J. and Curtis M.J. Mechanism of actions of sumatriptan on coronary flow before and after endothelial dysfunction in guinea-pig isolated heart. Br. J. Pharmacol., 120:1039-48, 1997
Whiting M.V. and Cambridge D. Canine renovascular responses to sumatriptan and 5-carboxamidotryptamine: modulation through endothelial 5-HT1-like receptors by endogenous nitric oxide. Br. J. Pharmacol., 114:969-74, 1995.
Coulie B., Tack J., Sifrim, D., Andrioli A. and Janssens J. Role of nitric oxide in fasting gastric fundus tone and in 5-HT1 receptor-mediated relaxation of gastric fundus. Am. J. Physiol., 276: G373–G377, 1999.
Mathias R.S., Carvalho J.C., Cavalcante M.T. and de Moraes, S. Effects of simulated myocardial ischemic conditions on the responses of isolated human umbilical artery strips to 5-hydroxytryptamine and prostaglandin F2 alpha. Gen. Pharmacol., 29:483-7, 1997.
Seager J.M., Clark A.H. and Garland C.J. Endothelium-dependent contractile responses to 5-hydroxytryptamine in the rabbit basilar artery. Br. J. Pharmacol., 105:424-8, 1992.
Corresponding Author: J.Y.Datte, Laboratoire de Nutrition & Pharmacologie, Department of BA-PA, UFR-Biosciences, Cocody University, Abidjan, 20 BP 947 Abidjan 20, Cote d’Ivoire. yaodj@ci.refer.org
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps