J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 7(1):88-91, 2004
Fluorometric variable -temperature kinetics investigations of the transesterification reaction of procaine with aliphatic alcohols.
Ali Reza Harifi-Mood, Saeed Haghighi, Mohammad Reza Gholami1
Department of Chemistry, Sharif University of Technology, Tehran, Iran; Pasteur Institute, Tehran, IranReceived 08 February 2004, Revised 13 April 2004, Accepted 14 April, 2004, Published 29 April, 2004
PDF Version
Abstract
PURPOSE: Variable-Temperature Kinetics has been used to obtain the rate constants of the reaction at various temperatures during one kinetic run. METHODS. Pseudo-first-order rate constants for the transesterification of procaine with aliphatic alcohols ethanol, n-propanol and tert-butanol were obtained by the fluorescence spectroscopy using the variable-temperature kinetics (VTK) method. RESULTS. The activation parameters of the reactions were calculated (24-28 kcal.mol-1). The half-life of the procaine decreases in the ethanol solution compared with the other alcoholic solutions in the presence of sodium ethoxide. CONCLUSIONS. The investigation time of the reactions is reduced to one-tenth of the one used for usual kinetic methods.
Introduction
Some drugs and common preservatives, such as esteric drugs and parabens are degraded by the reaction of transesterification. Degradation of parabens by transesterification with several aliphatic alcohols and polyols reveal that the reaction leads to a sharp decrease in the half-life of drugs (1-2). The transesterification can be carried out in alcoholic solution or in presence of the alcoholic solution containing the corresponding alkoxide ions.
The study of reaction mechanism is a very important step in understanding the sub microscopic world and it is often useful for practical application (3). A great deal of work is often necessary in order to clarify the detail behaviour of the studied systems, which is time consuming.
The transesterification of procaine with aliphatic alcohols such as ethanol, n-propanol and tert-butanol was studied by variable-parameter kinetics (VPaK) method, using the fluorometric measurements (4). In VPaK method, a chemical reaction is carried out by changing the value of a physical parameter (i.e. temperature, concentration, pressure, pH and ion strength) which influences the rate constants of the reaction. The kinetic profile obtained contains all the information necessary in produce the dependence of the observed rate constant, kobs, on the parameters (5-7). Consider a general reaction:
(1)
The general rate law under pseudo-first order condition can be given by equation 2:
(2)
where Pari (t) is the varying parameter i with respect to the time and kobs [Par i (t)] is the specific rate coefficient depending on the parameter. The varying parameter in variable-temperature kinetics (VTK) is the temperature (8). This method, based on a generalization of non-isothermal analysis (9), was used to illustrate the influence of the temperature on the reaction kinetics only with one variable-temperature kinetic (VTK) run.
Materials and Methods
Kinetics of transesterification of the procaine with the alkoxide ions in alcoholic solution were followed fluorometrically by VTK runs. A fluorescence spectrophotometer model HITACHI MPF-4 coupled with PC computer and a Microsoft FLUORESCENCE version 1.97 were used for fluorometric measurement.
Solution of procaine and sodium alkoxide in alcohol was thermostated separately in a water-bath (+0.05°C). The solutions were then rapidly mixed to give ester and alkoxide ion concentration of 4x10 -3 M and 1.00 M, respectively. These concentrations, however, provide pseudo-first-order condition for reaction (3). Reaction kinetics was followed directly by fluorescence spectroscopy at a linearly increasing temperature T = T 0 + αt, where T is initial temperature, a is slope, T and t are variables of temperature and time, respectively. Temperature was controlled by a computer controlled water circulation thermostatic bath model RTE-8 Instruments Inc. NESLAB with accuracy at +0.05 °C.
The excitation wavelengths are centered at 322nm, 327nm and 322nm for the reaction of procaine with ethanol, n-propanol and tert-butanol respectively. The width of slits was chosen 2nm to avoid the overlapping of signals of the reactant and product. The fluorescence signal was filtered by the emission monochromator centered at 350nm for all of the reactions. In this wavelength, fluorescence intensity of product (III) was measured (scheme 1).
After the fluorometric experiment, a blank scanning was carried out on the final solution so that the dependence fluorescence intensity of the product on the temperature was recorded (8). The temperature dependence of the molar extinction coefficient and the thermal expansion of the solution, which can have effect on the absorbance, are negligible due to the short temperature range (ΔT<30 °C) in VTK experiments.
Data processing
Kinetic data was processed by a differential method simply dividing the first derivative of the kinetic profile (eq. 2) and using a Pascal program by the Savitzky-Golay method for the evaluation of the derivative (10).
Results and Discussion
The transesterification is a reversible reaction and its equilibrium depends on the relative concentrations and nucleophilic abilities of the alkoxide ions (11). In these studies, the reverse reaction can be negligible due to extremely low concentration of living group compared with the alkoxide ion.
The general rate law for the reaction is:
(3)
where kobs is the rate constant under pseudo-first-order condition and is temperature-dependent.
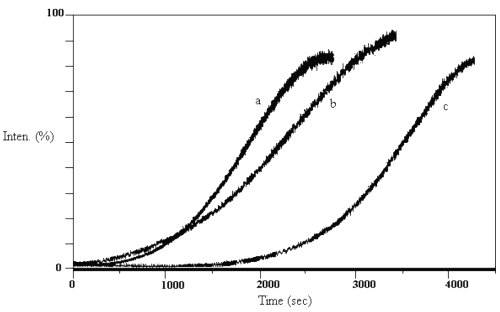
By increasing the temperature, the gradient of fluorescence signal of the products increases and a typical shape of a sigmoid is obtained due to the acceleration of the reaction at the beginning of the reaction. Figure 1 shows the spectrofluorometric variable-temperature kinetics profile obtained for the transesterification of the procaine with ethanol, n-propanol and tert-butanol at a linearly increasing temperature T = T0 + αt in which T0 = 313.5 + 0.1 K and α=0.010 + 0.001 K.s-1 .
Figure 1: Change in fluorescence intensity of the products of the reaction at 322nm, 327nm and 322nm for the ethanolic solution (a), n-propanolic solution (b) and tert-butanolic solution (c) at the variable temperature, respectively.
In this particular experiment, we can replace the concentration of the ester in eq. 3 by the fluorescence intensity of the reaction mixture. The fluorescence intensity is dependent on the concentration and temperature, and can be expressed by equation 4:
(4)
where S is a proportionality constant depending on the quantum efficiency, F an instrumental proportionality constant, I0 the excitation intensity, e the extinction coefficient, b the cell width, and C is the molar concentration of the fluorescent species (12). The temperature favours radiationless decay, therefore S is strongly dependent on the temperature. Both S and C, as a result, vary during the VTK run. The data correction was performed using the values of intensities acquired during a temperature-dependent experiment of the final concentration of the product. During the blank scanning, C is constant while S varies with temperature to some extent as for the VTK experiment. Point by point ratio of the fluorescence intensity values leads to the normalized values I N = S To FI 0 ebC, which is concentration-dependent.
By replacing these values in equation 3, equation 5 is obtained:
(5)
where IN,t and IN,¥ are the normalized fluorescence intensities at t time and at the end of the reaction, respectively. To calculate the kobs [T(t)] values, the derivative of the corrected fluorescence intensity is substracted by the normalized value (I N,t -I N,¥ ), point-by-point. The derivatives were obtained by using the simulation of the spectrofluorometric variable-temperature kinetics profile obtained for the reaction. This procedure is a differential method and is different from previous work (8).
It is obvious that a considerable increase in free Gibbs activation energy is obtained when the steric hindrance of the alkoxide ion is increased (table 1).
In other words, half-life of the procaine in the presence of sodium ethoxide decreases compared with the sodium tert-butoxide at a given temperature. The basicity of the nucleophile is a dominant factor governing the relative reactivities of ester and alkoxide ions when the electrophilic center of the substrate is carbon of the carbonyl group (i.e., a hard Lewis acid). Almost all of the alkoxide ions are classified as hard Lewis bases, hence tert-butoxide has a greater basicity compared with n-propoxide and ethoxide. In the alcoholic solution, the mechanism of the reaction can be proposed as follows (scheme 1):
Scheme 1
We therefore expect the rate of the reaction in the presence of tert-butoxide ion to be more than other reactions. On the other hand, when the steric hindrance of the alkoxide ion increases, the activation complex of the reaction will be unstable and the rate of the reaction decreases. The tert-butoxide ion has the most steric hindrance among these alkoxide ions, thus the rate of transesterification with tert-butanol is expected to be lower than the other reactions. However, both steric hindrance and basicity of nucleophile influence the rate of the reaction, but in this case, the former has a larger effect and therefore the rate of reaction decreases.
Table 1: Activation parameters for transesterification of the procaine with three alcohols. # has been calculated at 328.15 K.
References
Hensel A., Leisenheimer S., Muller A., Busker E. and Wolf-Heuss E., Transestesification reactions of parabens (Alkyle 4-hydroxybenzoates) with polyols in aqueous solution. J. Pharm. Sci., 85:115-118, 1995.
Runesson B. and Gustavii K., Stability of parabens in the presence of polyols. Acta Pharm. Suec., 23:151-162, 1986.
Moore J. W. and Pearson R. G., Kinetics and mechanism, Wiley, New York, 1981.
Alibrandi G., Variable-concentration kinetics. J. Chem. Soc., Chem. Commun., 2709-2710, 1994.
Alibrandi G., Coppolino S., Micali N. and Villari A., Variable pH kinetics: An easy determination of pH-rate profile. J. Pharm. Sci., 90(3):270-274, 2001.
Ficarra R., Villari A., Micali N., Tommasini S., Calabro M. L., Di Bella M. R., Melardi S., Agresta M. F., Cappolino S. and Stancanelli R., Stability study of piroxicam and cinnoxicam in solid pharmaceuticals. J. Pharm. Bio. Anal., 20:283-288, 1999.
Alibrandi G., Coppolino S., D’Aliberti S., Ficarra R., Micali N. and Villari A., Temperature-rate profiles by polarimetric variable-temperature kinetic experiments to study racemization reactions. J. Pharm. Bio. Anal., 29:1025-1029, 2002.
Alibrandi G., Micali N., Trusso S. and Villari A., Hydrolysis of aspirin studied by spectrophotometric and fluorometric variable-temperature kinetics. J. Pharm. Sci., 85:1105-1108, 1996.
Koch E., Non-isothermal reaction analysis. Academic Press, London, 1977.
Savitzky A. and Golay M. J. E., Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem., 36:1627-1639, 1964.
11. Sanderland V. B. and Watts D. W., Alkaline ethanolysis of methyl 4-hydroxybenzoate and hydrolysis of methyl and ethyl 4-hydroxy. Int. J. Pharm., 27:1-15, 1985.
Lakowicz J. R., Principles of fluorescence specroscopy. Plenum Press, New York and London, 1983.
Corresponding Author: Mohammad Reza Gholami, Department of Chemistry, Sharif University of Technology, P.O. Box 11365-9516, Tehran, Iran. gholami@sharif.eduPublished by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps