J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 7(2):260-264, 2004
Synthesis of 4-aryl substituted semicarbazones of some terpenes as novel anticonvulsants.
Navneet Aggarwal
Department of Pharmaceutical Chemistry, Hindu College of Pharmacy, Sonepat, Haryana, IndiaPradeep Mishra1
Department of Pharmaceutical Sciences, Dr. Hari Singh Gour University, Sagar, M.P., IndiaReceived 22 September 2003, Revised 14 June 2004, Accepted 28 June 2004, Published 11 August 2004
PDF Version
Abstract
PURPOSE: A series of 4-aryl substituted semicarbazones of citral and R- (-) carvone were designed and synthesized to meet the structural requirements essential for anticonvulsant activity. METHODS: TLC evaluated purity of synthesized compounds and their structure confirmed by infrared spectroscopy, proton magnetic resonance spectroscopy and by nitrogen estimation. All the compounds were evaluated for anticonvulsant activity by maximal electroshock (MES) and subcutaneous metrazol (ScMet) induced seizure methods and minimal motor impairment was determined by rotorod test. RESULTS: All the synthesized compounds exhibited significant protection after intraperitoneal (i.p.) administration in MES. Seventy two percent of the compounds exhibited protection in ScMet test. Some of them also showed good activity after oral administration. The results showed that anticonvulsants with cyclic and acyclic terpenoid moiety retain activity in MES as well as ScMet test. The p-fluoro aryl substituted semicarbazones emerged as the most active analogue in both cyclic and acyclic terpenes. CONCLUSION: Semicarbazones with terpenoid as the lipophilic moiety resulted in compounds with broad spectrum of anticonvulsant activity and therefore, they may be utilized for the future development of novel anticonvulsants with broad spectrum of anticonvulsant activity. The results also validated pharmacophore model with four binding sites essential for anticonvulsant activity.
Introduction
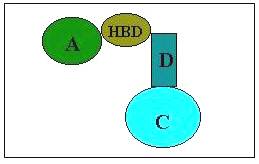
Epilepsy is a neurological disorder of varied etiology. It is characterized by paroxymal, excessive and hypersynchronous discharges of large number of neurons [1]. Approximately 50 million people worldwide suffer from epilepsy, making this condition the second leading neurological disorder [2]. It is estimated that 25% of the epileptic population have seizures that are not responsive to presently available medical therapies. Despite the optimal use of available antiepileptic drugs, many patients fail to experience seizure control and others do so only at the expense of significant toxic effects that range in severity from minimal brain impairment to death from aplastic anaemia or hepatic failure [3]. It is estimated that available medication controls the seizures in only 50% of patients or decrease incidence in only 75% of patients. These facts make the field of anticonvulsant drug discovery a high priority. Semicarbazones present a wide range of bioactivities, and their pharmacological applications have been extensively investigated [4]. Recently 4-aryl substituted semicarbazones have acquired an important place as anticonvulsants and can be considered a new class of anticonvulsants with oral activity [5, 6]. Recently Pandeya et al. [7] have suggested a new pharmacophore model for semicarbazones displaying anticonvulsant activity (Fig 1). They proposed that the terminal amino function of the semicarbazones was not essential for activity and could be substituted with a lipophilic aryl ring. Proposed pharmacophore model contain four binding sites for interaction with a macromolecular complex in vivo . These binding sites include
- An aryl hydrophobic binding site (A) with halo substituent preferably at para position
- A hydrogen bonding domain (HBD)
- An electron donor group (D)
- Another hydrophobic -hydrophilic site controlling the pharmacokinetic properties of the anticonvulsant (C)
Figure 1: Suggested pharmacophore model for semicarbazones displaying anticonvulsant activity.
These new aspects might be useful for designing prototypic molecules with potential anticonvulsant activity. In the recent study, the halo substituents selected are F, Cl and Br, because these substituents have been found to be active [8, 9]. The effect of other substituents at para position has also been studied. The major change in this study has been to explore the size and functional requirement of the hydrophobic-hydrophilic site controlling the pharmacokinetic properties. One cyclic terpene [R-(-) carvone] and one acyclic terpene with unsaturated hydrocarbon chain (citral) have been selected because they are expected to increase lipophilicity of the molecule and this may result in improved anticonvulsant activity.
Experimental
Chemistry: All the chemicals used were of BDH and Merck except wherever mentioned. The melting points were determined in open capillary tubes on a Superfit India melting point apparatus and are uncorrected. The purity of the compounds was confirmed by TLC using silica gel G as stationary phase and Benzene: ethanol (9:1) as the solvent system. NMR spectra were recorded on a Hitachi R-600 high-resolution NMR spectrometer and IR spectra were recorded on Perkin Elmer spectrum 2000 FT-IR spectrometer (Department of Chemistry, University of Delhi). UV λ max of the synthesized compounds were taken on Cintra 10 UV visible spectrometer (Department of Pharmaceutical Sciences, Dr. Harisingh Gour University) and was found to be in accordance with the proposed structures. Nitrogen estimations were undertaken (USIC, University of Delhi) and were found to be within 0.4% of the calculated values.
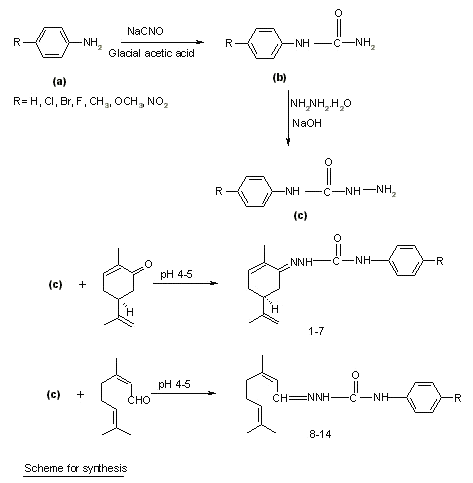
Figure 2: Scheme for synthesis.
Synthesis of substituted semicarbazones : Different para substituted aryl semicarbazides were prepared by method previously described by Pandeya et al. [10]. Equimolar quantities of terpene (0.005 mol) and the appropriate substituted phenyl semicarbazide (0.005 mol) were dissolved in 20 ml of ethanol (95%) and pH of the reaction mixture was adjusted between 4-5 by adding glacial acetic acid. The mixture was refluxed for 45 min to 1.5 h and then cooled in an ice bath. In some cases, the solution was poured on crushed ice to induce crystallization. The resultant precipitates were filtered, dried and recrystallized from aqueous ethanol (95%). Physical data of synthesized compounds are given in Table 1.
Table 1: Physical data of synthesized compounds.

*Compound melted with decomposition.
The spectral data of some of the synthesized compounds are as follows: Compound 1: UV (λ max , nm, methanol) 235. IR (KBr, υcm -1 ) 3421 (secondary NH), 3314 (amide NH), 1597 (C=N), 1658 (NH-CO-NH), 2921 (ArCH stretch). 1 HNMR (DMSOd 6 , δ) 1.82 (s, 3H, CH 3 ), 1.9 (s, 3H, CH 3 ), 2.28 (m, 4H, 2CH 2 ), 2.68 (p, 1H, CH2-CH-CH2), 4.87 (s, 2H, =CH2), 5.1 (t, 1H, CH=C), 6.32 (s, 1H, -CONH), 7.25 (br s, 5H, Ar), 9.02 (s, 1H, =NNH). Compound 4: UV (λ max , nm, methanol) 232. IR (KBr, υcm -1 ), 3426 (secondary NH), 3317 (amide NH), 3046 (CH stretch), 2924 (ArCH stretch), 1655 (NH-CO-NH), 1596 (C=N), 1094 (C-F stretch). 1 HNMR (DMSOd 6 , δ) 1.86 (s, 3H, CH 3 ), 2.1 (s, 3H, CH 3 ), 2.2 (m, 4H, 2CH 2 ), 2.7 (p, 1H, CH 2 -CH-CH 2 ), 4.9 (t, 1H, CH=C), 5.3 (s, 2H, =CH 2 ), 5.9 (s, 1H, -CONH), 7.55-7.8 (m, 4H, p -fluorophenyl), 9.2 (s, 1H, =NNH). Compound 10: UV (λ max , nm, methanol) 244. IR (KBr, υcm -1 ) 3425 (secondary NH), 3310 (amide NH), 1610 (C=N), 1655 (NH-CO-NH), 2929 (Ar CH stretch). 1 HNMR (DMSOdd 6 , δ) 1.7 (s, 3H, CH 3 ), 1.9 (s, 6H, 2CH 3 ), 2.24 (m, 4H, 2CH 2 ), 4.8 (t, 1H, =CH), 5.46 (d, 1H, =CH-CH=N), 5.7 (s, 1H, -CONH) 7.2-7.5 (m, 4H, p-bromophenyl), 7.8 (d, 1H, CH=N), 8.75 (s, 1H, =NNH). Compound 14: UV (λ max , nm, methanol) 238, 371. IR (KBr, υcm -1 ) 3428 (Secondary NH), 3308 (amide NH), 1619 (C=N), 1658 (NH-CO-NH), 1533 (asymmetric ArNO 2 stretch), 1355 (symmetric NO 2 stretch). 1 HNMR (DMSOd 6 , δ) 1.75 (s, 3H, CH 3 ), 1.9 (d, 6H, 2CH 3 ), 2.32 (m, 4H, 2CH 2 ), 5.25 (t, 1H, =CH), 5.6 (d, 1H, =CH-CH=N), 5.9 (s, 1H, -CONH), 7.6-7.8 (m, 4H, p -nitrophenyl), 7.95 (d, 1H, CH=N), 9.1 (s, 1H, =NNH).
Pharmacology
All the compounds were screened by maximal electroshock test (MES) and subcutaneous metrazol (ScMET) test for anticonvulsant activity. The neurotoxicity (NT) was measured by the rotorod test.
The results are summarized in Table 2.
Anticonvulsant screening : Anticonvulsant evaluation of semicarbazones was undertaken by following the anticonvulsant drug development program protocol [11, 12]. Male albino mice (18-25 g) and male albino rats (100-125 g) were used as experimental animals. The semicarbazones were suspended in 0.5% methyl cellulose/water mixture. All the compounds were administered intraperitoneally in doses of 30, 100 and 300 mg/kg to one to four animals. Some selected compounds were examined for oral activity in rats.
The results of oral activity are summarized in table 3.
Table 2: Anticonvulsant evaluation of compounds in the MES, ScMet and NT screens.
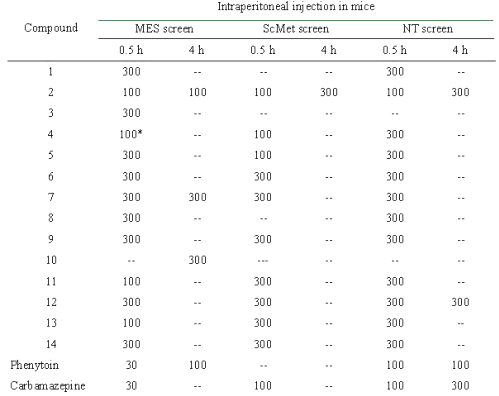
The figures in the table indicate the dose in mg/kg at which bioactivity was observed in a majority of the animals. The (--) sign indicates absence of activity at the maximum dose administered. * Compound 4 showed activity at 30 mg/kg after 0.25 h.
Table 3: Anticonvulsant evaluation of compounds after oral administration in rats.
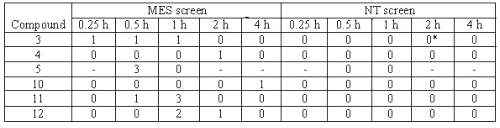
The compounds were administered in a dose of 30 mg/kg. The figure indicates the number of rats out of four, which were protected. *Hyperesthesia was observed after 2 h. (--) Indicates observation not taken.
Neurotoxicity screen: Minimal motor impairment was measured in mice by the rotorod test. The mice were trained to stay on an accelerating rotorod of diameter 3.2 cm that rotates at 10 revolutions per min. Previously trained mice were given test compounds intraperitoneally in doses of 30, 100, and 300 mg/kg. Neurotoxicity was indicated by the inability of the animal to maintain equilibrium on the rod for at least one min in each of the three trials.
Results
The candidate anticonvulsants have been often evaluated in the maximal electroshock and subcutaneous metrazol tests. Compounds affording protection in the maximal electroshock test may prove to be useful in treating generalized tonic-clonic and complex partial seizures, while activity in the subcutaneous metrazol is claimed to denote agents of value in treating absence seizures [11]. Neurotoxicity in mice may be measured by the rotorod test. All the compounds were screened at 30, 100 and 300 mg/kg for these tests. The results of intraperitoneal screens are summarized in table 2. The data reveals that all the compounds afforded protection in the maximal electroshock test. All of the compounds except 1, 3, 8 and 10 were active in subcutaneous metrazol test, a test used to identify compounds that elevate seizure threshold. In general, carvone semicarbazones showed activity at a lower dose in subcutaneous metrazol test in comparison to corresponding citral semicarbazones. The p -fluoro phenyl substituted semicarbazones of both cyclic and acyclic terpene were most active, showing broad spectrum of activity at 100 mg/kg and at the same time showing low neurotoxicity. R-(-) carvone 4-( p -fluoro phenyl) semicarbazone (compound 4) showed activity at 30 mg/kg after 0.25 h. In general, anticonvulsant activity was noted at the end of 30 min rather than 4 h. Therefore, it can be said that the onset of action for the compounds is rapid. Only compound 10 showed a delayed activity at the end of 4 h in a dose of 300 mg/kg. In the neurotoxicity screen, all the compounds (except 2, 3 and 10) showed neurotoxicity at 300 mg/kg. Compounds 3 and 10 were not found to be neurotoxic at maximum administered dose and compound 10 showed neurotoxicity at 100 mg/kg.
Compounds 3, 4, 5, 10, 11 and 12 were evaluated for activity and neurotoxicity after oral administration in rats. For these compounds were administered in a dose of 30 mg/kg and effects observed at 0.25, 0.5, 1, 2, and 4 h intervals. All the compounds evaluated showed some protection. Compound 3 showed marginal protection at three time points. Compound 10 showed delayed activity at 4 h. None of the compounds showed neurotoxicity in this test. Only compound 3 showed hyperesthesia after 2 h.
Discussion
The most common structural elements of the older generation clinically active anticonvulsants can be defined as a nitrogen heteroatomic system bearing one or two phenyl rings and at least one carbonyl group. Many investigations indicated that the presence of at least one aryl group, one or two electron donor atoms and/or an NH group in a special spatial arrangement seems to be necessary for anticonvulsant activity [13]. In the semicarbazone series, Pandeya et al. [7] have proposed a new pharmacophore model with four binding sites essential for anticonvulsant activity. In the present series of compounds, 4-aryl substituted semicarbazones of some terpenes were designed and synthesized to meet the structural requirements essential for anticonvulsant activity. The results obtained show that all the compounds afforded protection in the maximal electroshock test and all (except 1, 3, 8 and 10) were active in subcutaneous metrazol test. Thus, the results validated the pharmacophore model with four binding sites essential for anticonvulsant activity. The structural requirement for activity in the maximal electroshock screen has been stated to be the presence of a large hydrophobic group in close proximity to at least two electron donor atoms. For activity in the subcutaneous metrazol screen, a hydrophobic group smaller than required for activity in maximal electroshock screen should be present [14]. One of the main features of the results obtained from the present study is the broad spectrum of activity shown by majority of the compounds. This shows that terpenes fulfill the structural requirement of hydrophobic moiety for both the activities. Thus, they may be utilized in the future development of novel anticonvulsants with broad spectrum of activity. In conclusion, our results validated the pharmacophore model with four binding sites essential for anticonvulsant activity. Semicarbazones with terpenoid as the lipophillic moiety may be utilized for the future development of novel anticonvulsants with broad spectrum of anticonvulsant activity.
Acknowledgements
The authors would like to thank J P Stables and other members of anticonvulsant drug development programme, USA, for their extraordinary assistance in anticonvulsant evaluation. We would also like to thank Dr. Gurmeet Singh, University of Delhi, for spectral & elemental analysis and Dr. S.N. Pandeya, Banaras Hindu University, for helpful discussions.
References
Craig, C.R., Anticonvulsant Drugs, in, Craig, C.R., and Stitzel, R.E., (eds), Modern Pharmacology With Clinical Application, 5th edition, Little Brown and Company, New York, 391-405, 1997.
Porter, R.J. and Rogawski, M.A., New antiepileptic drugs: from serendipity to rational discovery, Epilepsia, 33 (suppl. 1): S1-S6, 1992.
McNamara J.O., Drugs effective in the therapy of the epilepsies, in Hardman, J.G.; Limbird, L.E. and Gilman A.G. (eds), Goodman and Gilman’s, The Pharmacological Basis of Therapeutics, 10th edition, The McGraw –Hill, New York, pp 521-547, 2001.
Beraldo, H. and Gambino, D., Wide pharmacological versatility of semicarbazones, thiosemicarbazones and their metal complexes, Mini Rev. Med. Chem., 4:159-165, 2004.
Pandeya, S.N.; Ponnilavarsan, I.; Pandey, A.; Lakhan, R. and Stables, J.P., Evaluation of p-nitrophenyl substituted semicarbazones for anticonvulsant properties, Pharmazie, 54: 923-925, 1999.
Pandeya, S.N.; Yogeeswari, P. and Stables, J.P., Synthesis and anticonvulsant activity of 4-bromophenyl substituted aryl semicarbazones, Eur. J. Med. Chem., 35: 879-886, 2000.
Pandeya, S.N.; Raja, A.S. and Stables, J.P., Synthesis of isatin semicarbazones as novel anticonvulsants-role of hydrogen bonding. J. Pharm. Pharmaceut. Sci., 5(3): 266-271, 2002.
Dimmock, J.R. and Baker, G.B., Anticonvulsant activities of 4-bromobenzaldehyde semicarbazone. Epilepsia, 35 (3): 648-655, 1994.
Pandeya, S.N.; Mishra, V.; Ponnilavarsan, I. and Stables, J.P., Anticonvulsant activity of p-chloro phenyl substituted aryl semicarbazones –the role of primary terminal amino group. Pol. J. pharmacol., 52: 283-290, 2000.
Pandeya, S.N.; Aggarwal, N. and Jain, J.S., Evaluation of semicarbazones for anticonvulsant and sedative hypnotic properties. Pharmazie, 54: 300-302, 1999.
Krall, R.L.; Penry, J.K.; White, B.G.; Kupferberg, H.J. and Swinyard, E.A., Anticonvulsant drug development: Anticonvulsant drug screening. Epilepsia, 19: 409-428, 1978.
Porter, R.J.; Hessie, B.J.; Cereghino, J.J.; Gladding, G.D.; Kupferberg, H.J.; Scoville, B. and White, B.G., Advances in the clinical development of antiepileptic drugs. Fed. Proc., 44(10): 2645-2649, 1985.
Malawska, B., Application of pharmacophore models for the design and synthesis of new anticonvulsant drugs, Mini Rev. Med. Chem., 3: 159-165, 2003.
Jones, G.L. and Woodbury, D.M., Anticonvulsant structure activity relationships: historical development and probable causes of failure. Drug Dev. Res., 2:333-355, 1982.
Corresponding Author: Pradeep Mishra Department of Pharmaceutical Sciences, Dr. Harisingh Gour University Sagar (M.P.) 470003 INDIA. mishrap51@yahoo.com
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps