J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 7(2):274-283, 2004
Comparative effects of curcumin and an analog of curcumin on alcohol and PUFA induced oxidative stress.
Rajagopalan Rukkumani, Kode Aruna, Penumathsa Suresh Varma, Kallikat Narayanan Rajasekaran, Venugopal Padmanabhan Menon1
Department of Biochemistry, Faculty of Science, Annamalai University, Annamalainagar, Tamil Nadu, India; Department of Chemistry, Faculty of Science, Kerala University, Kariavattom, Kerala, IndiaReceived 23 March 2004, Revised 29 April 2004, Accepted 27 May 2004, Published 20 August 2004
PDF Version
Abstract
PURPOSE: Alcoholic liver disease is a major medical complication of alcohol abuse and a common liver disease in western countries. Increasing evidence demonstrates that oxidative stress plays an important etiologic role in the development of alcoholic liver disease. Alcohol alone or in combination with high fat is known to cause oxidative injury. The present study therefore aims at evaluating the protective role of curcumin, an active principle of turmeric and a synthetic analog of curcumin (CA) on alcohol and thermally oxidised sunflower oil (DPUFA) induced oxidative stress. METHODS: Male albino Wistar rats were used for the experimental study. The liver marker enzymes: g-glutamyl transferase (GGT), alkaline phosphatase (ALP), the lipid peroxidative indices: thiobarbituric acid reactive substances (TBARS) and hydroperoxides (HP) and antioxidants such as vitamin C, vitamin E, reduced glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) were used as biomarkers for testing the antioxidant potential of the drugs. RESULTS: The liver marker enzymes and lipid peroxidative indices were increased significantly in alcohol, DPUFA and alcohol + DPUFA groups. Administration of curcumin and CA abrograted this effect. The antioxidant status which was decreased in alcohol, DPUFA and alcohol + DPUFA groups was effectively modulated by both curcumin and CA treatment. However, the reduction in oxidative stress was more pronounced in CA treatment groups compared to curcumin. CONCLUSION: In conclusion, these observations show that CA exerts its protective effect by decreasing the lipid peroxidation and improving antioxidant status, thus proving itself as an effective antioxidant.
Introduction
Oxidative stress plays an important role in the development of alcohol induced tissue injury (1). Oxidative stress is generally considered as an imbalance between pro oxidant/antioxidant (2). Intake of alcohol results in excessive generation of free radicals (3), which alter the bio membranes and cause severe damage. Alcohol alone or in combination with high fat is known to cause oxidative injury.
Fat is an important dietary component, which affects both growth and health. It is widely accepted that a high level of fat in the diet is detrimental to health. Replacing the traditional cooking fats, considered atherogenic with refined vegetable oils promoted as `heart friendly' because of their PUFA content, has resulted in increased prevalence of heart disease in India (4). Current data on dietary fats indicate that it is not just the presence of PUFA but the type of PUFA that is important. A high PUFA n-6 content and a high n-6/n-3 ratio in dietary fats are considered to be dangerous (4). The newer heart friendly oils like sunflower oil possess this undesirable PUFA content and thus excess intake of these vegetable oils is actually detrimental to health. Moreover heating of oil is known to alter its nutritional properties especially when it is rich in PUFA. During deep fat frying many volatile and non-volatile products are produced, some of which are toxic depending on the level of intake (5).
Alcoholics usually after a heavy binge of alcohol, take fried food items normally made up of PUFA. Our previous studies have shown that the intake of sunflower oil along with alcohol aggravates the toxicity, especially when it is heated (6, 7).
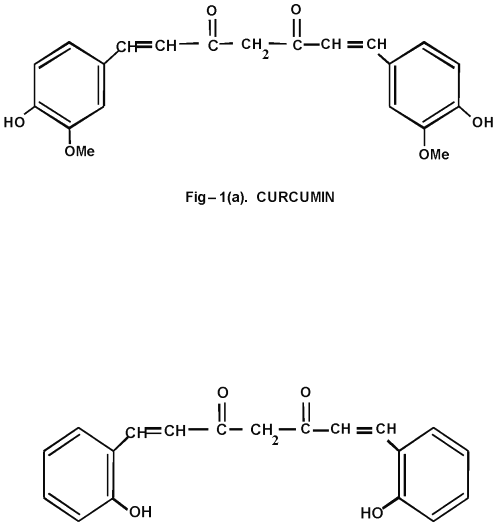
Curcumin (C) and bisdemethoxy curcumin are natural phenolic curcuminoids present in turmeric (8), a spice used in Indian food. Curcumin (Figure 1a), the active principle of turmeric has been extensively investigated for its antioxidant potential. In experimental animals, curcumin has been shown to prevent lipid peroxidation (9).
Figure 1: (a) Curcumin and (b) Curcumin Analog
A report has shown that an ortho hydroxyl group substituted analog of curcumin is very effective compared to all other existing curcuminoids in treating skin tumours (10). Previous studies from our lab have shown that this curcuminoid is effective against colon cancer (11) and diabetes (12). Since little or no work has been done to evaluate the other therapeutic strategy of this curcuminoid, in the present study, we synthesized an o-hydroxy substituted analog of curcumin (Figure 1b) and compared its effects with curcumin over alcohol and PUFA induced oxidative stress.
Materials and Methods
Animals
Male Albino rats, Wistar strain of body weight ranging 140-160 g bred in Central Animal House, Rajah Muthiah Medical College, Tamil Nadu, India, fed on standard pellet diet (Agro Corporation Private Limited, Bangalore, India) were used for the study and water was given ad libitum . The standard pellet diet comprised 21% protein, 5% lipids, 4% crude fibre, 8% ash, 1% calcium, 0.6% phosphorus, 3.4% glucose, 2% vitamin and 55% nitrogen free extract (carbohydrates). It provides metabolisable energy of 3600 K Cal.
The animals were housed in plastic cages under controlled conditions of 12 h light/12 h dark cycle, 50% humidity and at 30° ± 2°C. The animals used in the present study were maintained in accordance with the guidelines of the National Institute of Nutrition, Indian Council of Medical Research, Hyderabad, India and approved by the Animal Ethical Committee, Annamalai University.
Materials used
Ethanol: Absolute ethanol (AR) was obtained from Hayman limited, England.
Thermally oxidised PUFA (D PUFA): Sunflower oil (Gold Winner) was subjected to heating at 180°C for 30 minutes, twice (Fatty acid composition given in Table 1) (6).
Table 1: Fatty Acid Composition of Sunflower Oil (Percentage of Fatty Acid/g Oil)
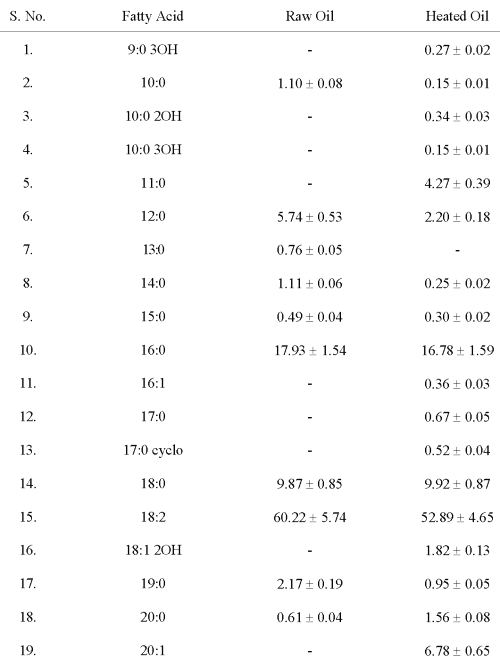
Values are mean ± S.D. of six values.
Curcumin: Curcumin was obtained from Central drug house private limited, Mumbai, India.
Curcumin analog: Curcumin analog was synthesized as per the method described by Dinesh Babu and Rajasekaran (13). Briefly, the procedure is as follow, Acetyl acetone was mixed with boric acid and dimethyl formamide (DMF), heated for 15 minutes. To that hot mixture, salicylaldeyde was added and heating was continued further for 5min. Few drops of catalyst (2:1 mixture of glacial acetic acid and diethanolamine) was added, refluxed for 5 to 6 hours and kept overnight. Few ml of DMF was added, warmed and the flowy paste was poured to 10% acetic acid slowly with stirring. The drug separates as a yellow solid mass. The product thus obtained was purified by column chromatography packed with silica gel using chloroform as solvent. Purity was checked by thin layer chromatography and the structure was further confirmed by FTIR and H1 NMR.
All other chemicals and reagents used in the present study were of analytical grade and were obtained from Sigma Chemical Company, Saint Louis, USA and Hi media laboratories, Mumbai, India.
Experimental design
The animals were divided into 12 groups of 6 rats each.
Group 1 (Control): Control rats were given glucose solution isocalorific to ethanol and high fat diet.
Group 2 (Alcohol): Rats given 20% ethanol (7.9 g/kg body weight) (14) orally, using an intragastric tube.
Group 3 ( D PUFA): Rats given high fat diet (15% thermally oxidised sunflower oil) mixed with the diet.
Group 4 (Alcohol + D PUFA): Rats given 20% ethanol + 15% thermally oxidised sunflower oil.
Group 5 (Alcohol + C): Rats given curcumin (80 mg/kg body weight) dissolved in 20% ethanol.
Group 6 (Alcohol + CA): Rats given curcumin analog (80 mg/kg body weight) dissolved in 20% ethanol.
Group 7 ( D PUFA + C): Rats given 15% thermally oxidised sunflower oil + curcumin (80 mg/kg body weight) dissolved in distilled water.
Group 8 ( D PUFA + CA): Rats given 15% thermally oxidised sunflower oil + curcumin analog (80 mg/kg body weight) dissolved in distilled water.
Group 9 (Alcohol + D PUFA + C): Rats given curcumin (80 mg/kg body weight) dissolved in 20% ethanol + 15% thermally oxidised sunflower oil.
Group 10 (Alcohol + D PUFA + CA): Rats given curcumin analog (80 mg/kg body weight) dissolved in 20% ethanol + 15% thermally oxidised sunflower oil.
Group 11 (Curcumin): Rats given curcumin (80 mg/kg body weight) dissolved in distilled water orally using an intragastric tube.
Group 12 (CA): Rats given Curcumin analog (80 mg/kg body weight) dissolved in distilled water orally using an intragastric tube.
Rats were maintained in isocalorific diet using glucose solution. (Total calories per day: 508 K Cal/kg body weight). At the end of the experimental period of 45 days, the rats were killed by cervical decapitation and the blood and tissues (liver, heart and kidney) were collected for various biochemical estimations.
Preparation of Plasma: Blood was collected in a heparinised tube and plasma was separated by centrifugation at 1000 g for 15 min for the estimation of GGT and ALP.
Preparation of Tissue Homogenate: Known amount of tissue was weighed and homogenised in appropriate buffer for the estimation of lipid peroxidative indices and enzymic and non-enzymic antioxidants.
Biochemical investigation
Estimation of liver marker enzymes
Hepatic damage was assessed by estimating the activities of ALP by King and Armstrong method (15) and GGT by Orlowski and Meister (16) method. Based on the method of King and Armstrong, alkaline phosphatase activity was assayed using disodium phenyl phosphate as substrate. After preincubation of buffer (0.1 M Bicarbonate buffer pH 10) with substrate for 10 minutes, 0.2 ml of serum was added and incubated for 15 min at 37°C. The liberated phenols from substrate, reacts with Folin - Phenol reagent (1 ml). The suspensions were centrifuged and supernatant was collected. 2 ml of 10% sodium bicarbonate was added to supernatant and the colour developed was read at 680 nm after 10 min.
GGT was analysed by adding 2ml buffer (Tris HCl 120 mm , MgCl2 12mM glycyl glycine 90 mM , pH 7.8) to 0.2ml substrate (L- g-glutamyl p-nitro anilide 48 mm in 150mM HCl ), warmed to 37°C. 0.1 ml serum was added, mixed and incubated at 37°C. The reaction was then stopped by adding 2ml of glacial acetic acid and the absorbance was read at 405nm.
Estimation of lipid peroxidative indices
Lipid peroxidation as evidenced by the formation of TBARS and HP were measured by the method of Niehaus and Samuelsson (17) and Jiang et al. (18) respectively. In brief, 0.1 ml of tissue homogenate (Tris-Hcl buffer, pH 7.5) was treated with 2 ml of (1:1:1 ratio) TBA-TCA-HCl reagent (thiobarbituric acid 0.37%, 0.25N HCl and 15% TCA) and placed in water bath for 15 min, cooled and centrifuged at room temperature for 10 min at 1,000 rpm. The absorbance of clear supernatant was measured against reference blank at 535 nm.
For hydroperoxides 0.1 ml of tissue homogenate was treated with 0.9 ml of Fox reagent (88 mg butylated hydroxytoluene (BHT), 7.6 mg xylenol orange and 9.8 mg ammonium ion sulphate were added to 90 ml of methanol and 10 ml 250 mM sulphuric acid) and incubated at 37°C for 30 min. The colour developed was read at 560 nm colorimetrically.
Determination of non-enzymic antioxidant status
Estimation of Reduced glutathione
Reduced glutathione (GSH) was determined by the method of Ellman (19). To the homogenate added 10% TCA, centrifuged. 1.0 ml of supernatant was treated with 0.5 ml of Ellmans reagent (19.8 mg of 5, 5'-dithiobisnitro benzoic acid (DTNB) in 100 ml of 0.1% sodium nitrate) and 3.0 ml of phosphate buffer (0.2M, pH 8.0). The absorbance was read at 412 nm.
Estimation of vitamin E
Vitamin E was estimated by Baker and Frank method (20). Lipid extract was prepared by the method of Folch et al . To 0.5ml of lipid extract, 1.5ml ethanol, 2.0ml of petroleum ether were added and centrifuged. The supernatant was evaporated to dryness at 80°C, to that added 0.2ml of 2-2' dipyridyl solution (0.2%) and ferric chloride (0.5%), kept in dark for 5 minutes and then 4ml of butanol was added. The colour developed was read at 520nm.
Estimation of ascorbic acid
Vitamin C was estimated by Roe and Kuether method (21). To 0.5 ml of tissue homogenate, 1.5ml 6% TCA was added, centrifuged. To the supernatant added, acid washed norit and filtered. To the filterate, added 0.5ml of DNPH and incubated at 37°C for 3 hours and then added 85% H2SO4 and incubated for30 min. The colour developed was read at 540nm.
Determination of superoxide dismutase, catalase and glutathione peroxidase
Superoxide dismutase (SOD) was assayed utilizing the technique of Kakkar et al . (22). A single unit of enzyme was expressed as 50% inhibition of NBT (Nitroblue tetrazolium) reduction/min/mg protein.
Catalase (CAT) was assayed colorimetrically at 620 nm and expressed as mmoles of H2O2 consumed/min/mg protein as described by Sinha (23). The reaction mixture (1.5ml) contained 1.0 ml of 0.01M pH 7.0 phosphate buffer, 0.1 ml of tissue homogenate and 0.4 ml of 2M H2O2 . The reaction was stopped by the addition of 2.0 ml of dichromate-acetic acid reagent (5% potassium dichromate and glacial acetic acid were mixed in 1:3 ratio).
Glutathione peroxidase (GPx) activity was measured by the method described by Ellman (19). Briefly, reaction mixture contained 0.2 ml of 0.4M phosphate buffer pH 7.0, 0.1 ml of 10 mM sodium azide, 0.2 ml of tissue homogenate (homogenised in 0.4M, phosphate buffer pH 7.0), 0.2 ml glutathione, 0.1 ml of 0.2 mM hydrogen peroxide. The contents were incubated at 37°C for 10 min. The reaction was arrested by 0.4 ml of 10% TCA, and centrifuged. Supernatant was assayed for glutathione content by using Ellmans reagent.
The total protein was estimated by total protein and albumin Kit (No-72111) from Qualigens fine chemicals, Worli, Mumbai.
Statistical Analysis
Statistical analysis was done by analysis of variance (ANOVA) followed by Duncan's Multiple Range Test (DMRT). Values were considered statistically significant when P ≤ 0.05.
Results
This study analyses the protective role of curcumin and CA on oxidative stress induced by alcohol and Δ PUFA. Our results showed that there was a significant reduction in oxidative stress in both curcumin and CA treatment.
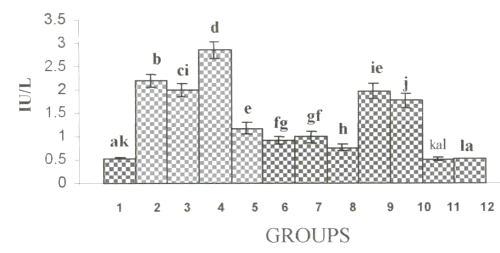
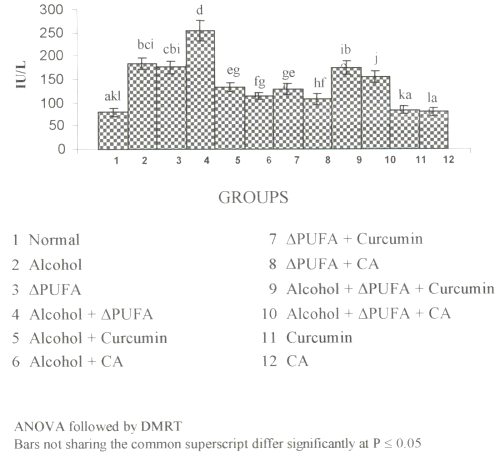
Figure 2a and 2b show the changes in the activities of ALP and GGT in plasma.
Figure 2: (a) Activities of γ-glutamyltranseferase in Plasma. (values are mean ± S.D from 6 rats in each group). Figure 2: (b) Activities of Alkaline Phosphatase in Plasma. (values are mean ± S.D from 6 rats in each group).
Their activities were increased significantly in alcohol, ∆ PUFA and alcohol + ∆ PUFA groups, which were decreased on treatment with curcumin and CA. However, CA treatment decreased their activity more significantly compared to curcumin.
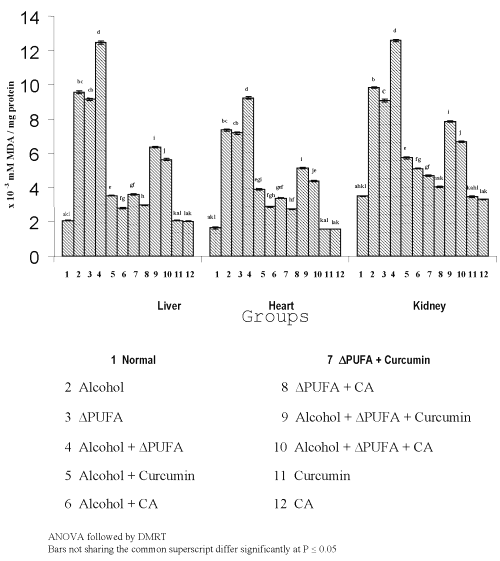
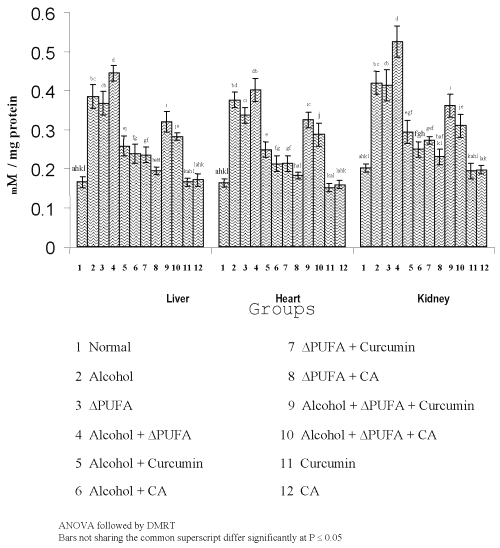
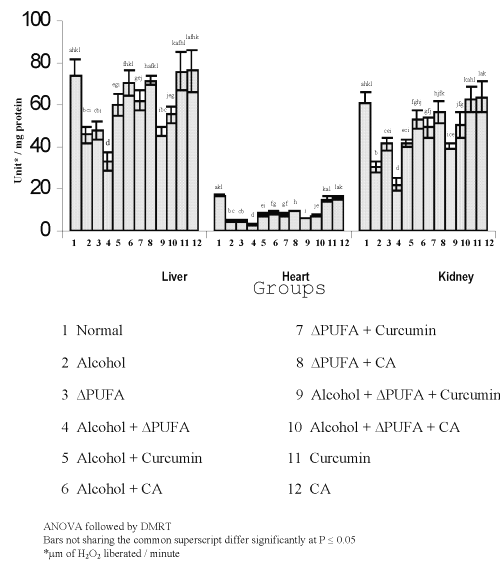
Figure 3 and 4 show the levels of lipid peroxidative indices in different tissues.
Figure 3: Levels of TBARS in Tissues. (values are mean ± S.D from 6 rats in each group)
Figure 4: Levels of Hydroperoxides in Tissues. (values are mean ± S.D from 6 rats in each group)
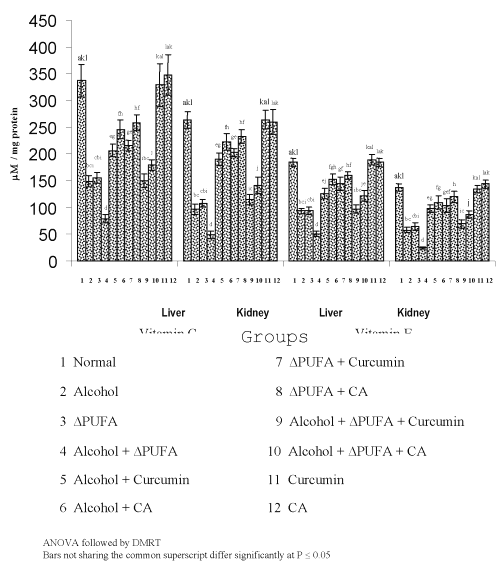
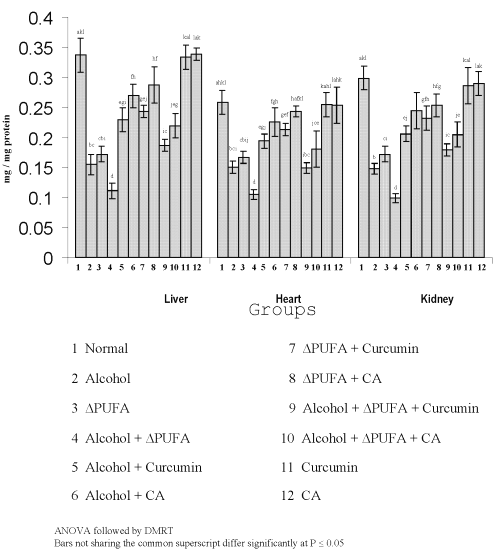
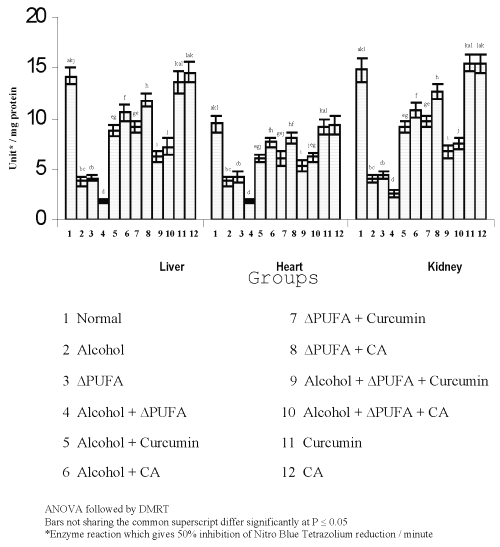
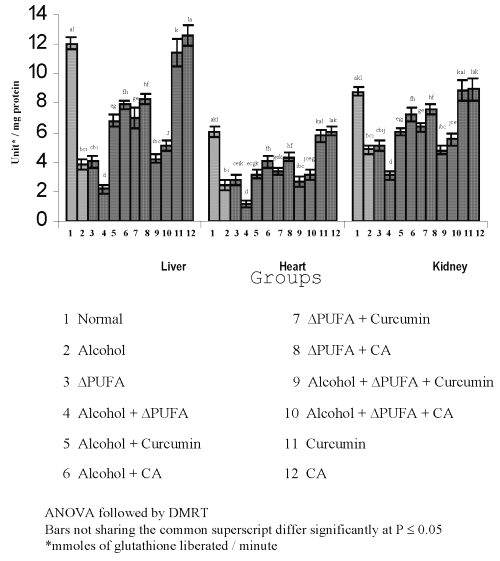
The levels of TBARS (Figure 3) and HP (Figure 4) were increased significantly in alcohol, ∆ PUFA and alcohol + ∆ PUFA groups, which were decreased significantly on treatment with curcumin and curcumin analog. The decrease was more significant in CA treated groups compared to curcumin. The levels of non-enzymic antioxidants: vitamin C, vitamin E (Figure 5) and GSH (Figure 6) and enzymic antioxidants: SOD (Figure 7), CAT (Figure 8) and GPX (Figure 9) were significantly depleted in alcohol, ∆ PUFA and alcohol + ∆ PUFA groups which were increased in both curcumin and CA treatment.
Figure 5: Levels of Vitamin C and Vitamin E in Tissues. (values are mean ± S.D from 6 rats in each group)
Figure 6: Levels of Reduced Glutathione in Tissues. (values are mean ± S.D from 6 rats in each group)
Figure 7: Activities of superoxide dismutase in Tissues. (values are mean ± S.D from 6 rats in each group)
Figure 8: Activities of Catalase in Tissues. (values are mean ± S.D from 6 rats in each group)
Figure 9: Activities of Glutathione Peroxidase in Tissues. (values are mean ± S.D from 6 rats in each group)
The CA treatment was found to be more effective compared to curcumin. Over all the reduction in oxidative stress was ~ 50% in curcumin, treated groups and ~60% in CA treated groups.
Discussion
The increase in plasma liver markers is a direct reflection of oxidative injury of liver. Various pathways play a role in ethanol induced tissue injury, including changes in cellular oxidized NAD+, NADH (24), production of acetaldehyde protein adducts (25), induction of CYP2E1 (26), formation of 1-hydroxyethyl free radicals (27), ethanol mediated mitochondrial damage (28), endotoxin derived activation of Kupffer cells and subsequent production of tumour necrosis factor a (29). These changes perturb the biomembranes and cause severe damage and leakage of liver markers into the circulation. Moreover, increased intake of PUFA increases the degree of unsaturation of the biomembrane and makes them more susceptible to lipid peroxidation (30). Wide utilization of fats which are highly susceptible to oxidation during cooking and frying may alter physiological effects of their PUFA content and generate lipid peroxides that cause membrane damage and increase lipid infiltration and hence make the membrane leaky to liver markers (31). Thus, the increased activities of GGT and ALP in our study are suggestive of severe hepatic injury during alcohol and PUFA ingestion.
The enhanced lipid peroxidation is one of the toxic manifestations of acute ethanol ingestion. evidences have indicated that free radicals or Reactive Oxygen Species (ROS) such as hydroxy ethyl radical, superoxide radical (O2°-), hydroxy radical (OH•), peroxy radical and hydrogen peroxide are implicated in ethanol induced lipid peroxidation (32). Ethanol is extensively metabolised to cytotoxic acetaldehyde by alcohol dehydrogenase enzyme in the liver and acetaldehyde is oxidised to acetate by aldehyde dehydrogenase or xanthine oxidase giving rise to ROS (33). Moreover, ethanol metabolism is associated with increase in CYP2E1 activity (34). CYP2E1 catalyses the conversion of ethanol to acetaldehyde and at the same time reduces dioxygen to a variety of ROS, including O2°- (35). These enhanced O2°- and other ROS increases the degree of LPO during alcohol ingestion. The excess LPO in alcohol-ingested group as measured by the formation of TBARS and HP in our study corroborate these findings.
The changes in the composition of erythrocyte with increased erythrocyte deformability ex vivo have been reported with increased intake of PUFA (36). It has been demonstrated that fatty acid composition of membranes can be affected by dietary variation of saturated and unsaturated fat. The increase in dietary unsaturated fat increases the degree of unsaturation of the membranes (37) and unsaturated bonds are more susceptible to lipid peroxidation. Moreover, heating of oil rich in PUFA produces various toxic metabolites (38), which may increase the lipid peroxidative changes. Thus, the observed increase in lipid peroxidative indices in our study during ∆ PUFA ingestion is in correlation with other findings.
Antioxidant defense system protects the aerobic organism from the deleterious effects of reactive oxygen metabolites. Vitamin E, a major lipophilic antioxidant and vitamin C, play a vital role in the defense against oxidative stress (39). In our study, the levels of vitamin E and C were decreased significantly during alcohol and PUFA ingestion. This is in agreement with the previous reports that chronic alcoholics are deficient in vitamin C and E (40). The increased oxidative stress due to alcohol and PUFA ingestion might have resulted in complete utilization of vitamin C and E thus depleting their levels.
Glutathione, an important cellular reductant is involved in protection against free radicals, peroxides and other toxic components (41). In addition to serving as a substrate for glutathione related enzymes, GSH acts as a free radical scavenger, a generator of a α-tocopherol and plays an important role in the maintenance of protein sulfhydryl groups (42). Previous studies have shown that acute ethanol ingestion depletes GSH levels (43). In the present study, the levels of GSH were decreased significantly in alcohol and Δ PUFA ingestion indicating the oxidative stress.
GPx has a well-established role in protecting cells against oxidative injury. GPx is non-specific for H2O2 and lack of this substrate specificity extends a range of substrates from H2O2 to organic hydroperoxides (44). Therefore, the excess H2O2 and lipid peroxides generated during alcohol and PUFA ingestion are efficiently scavenged by GPx activity. The depression of this enzyme activity reflects perturbations in normal oxidative mechanisms during alcohol and PUFA ingestion. Catalase, which acts as preventative antioxidant plays an important role in protection against the deleterious effects of lipid peroxidation (45). The inhibition of CAT activity is suggestive of enhanced synthesis of O2°- during the ingestion of alcohol and PUFA since O2°- is a powerful inhibitor of catalase (46).
SOD catalyses the dismutation of O2°- radical anions to H2O2 and O2 (47). Numerous studies have shown the importance of SOD in protecting cells against oxidative stress (48). Our study has shown a decrease in SOD activity in tissues during alcohol and PUFA ingestion. This decrease could be due to a feed back inhibition or oxidative inactivation of enzyme protein due to excess ROS generation (49). The generation of the α-hydroxy ethyl radical may also lead to inactivation of the enzyme.
Administration of curcumin and curcumin analog (CA), decreased the LPO, improved the antioxidant status and thereby prevented the damage to the liver and leakage of enzymes GGT and ALP. This is mainly because of the antioxidant sparing action of curcumin and CA.
The antioxidant mechanism of curcumin may include one or more of the following interactions. Scavenging or neutralizing of free radicals (50), interacting with oxidative cascade and preventing its outcome (51), oxygen quenching and making it less available for oxidative reaction (50), inhibition of oxidative enzymes like cytochrome P450 (50) and chelating and disarming oxidative properties of metal ions such as iron (52). Thus in this work curcumin effectively prevented tissue damage by decreasing the oxidative stress and restoring the antioxidant status.
However, the treatment with CA was found to be more effective compared to curcumin. Among many classes of compounds, phenolics have been recognized as a powerful counter measure against LPO (53). Normally phenolic compounds act by scavenging free radicals and quenching the lipid peroxidative side chain. Phenolic compounds can act as free radical scavengers by virtue of their hydrogen donating ability, forming aryloxyl radicals (54). It has been proposed that hydroxy and hydroperoxy radicals initiate H+ abstraction from a free phenolic substrate to form phenoxy radical that can rearrange to quinonemethide radical intermediate (55) which is excreted via bile.
Moreover the introduction of a hydroxyl group in the `O' and `P' position is known to increase antioxidant activity in peroxidizing lipid system. Several investigators have shown that `O' substitution with an e- donor group increases the stability of the aryloxyl radical and thus antioxidant activity (10, 54). The increased efficacy of this novel curcuminoid may be attributed to the presence of hydroxyl group at ortho position. The o -hydroxyl group, because of its resonance property, easily donates e− to free radicals and effectively neutralizes them. This property makes the CA, a novel compound for treating oxidative stress.
Conclusion
Thus, CA effectively quenches free radicals and LPO, decreases release of liver markers and positively modulates antioxidant status. Thus by the property of eliciting a significant effect on LPO, this synthetic curcuminoid may become a promising candidate for the treatment of oxidative stress. Further, more studies that are mechanistic are essential to elucidate the exact mechanism of its modulatory effects.
References
Kurose, I., Higuchi, H., Kato, S., Miura, S., Watanabe, N., Kamegaya, Y., Tomita, K., Takaishi, M., Haire, Y., Fukuda, M., Mizukami, K. and Ishii, H., Oxidative stress on mitochondria and cell membrane of cultured rat hepatocytes and perfused liver exposed to ethanol. Gastroenterol, 112: 1331-1343, 1997.
Lieber, C.S., Ethanol metabolism, cirrhosis and alcoholism. Clin Chim Acta, 257: 59-84, 1997.
Niemela, O., Aldehyde protein adducts in the liver as a result of ethanol induced oxidative stress. Front Biosci, 4: 506-513, 1999.
Sircar, S. and Kansra, V., Choice of cooking oils – myths and realities. J Ind Med Assoc, 96: 304-307, 1998.
Alexander, J.C., Chemical and biological properties related to toxicity of heated fats. J Toxicol Environ Health, 7: 125-138, 1981.
Aruna, K., Kalpana, C., Viswanathan, P. and Menon VP., Toxic effects of sunflower oil on ethanol treated rats. Hepatol Res, 24: 125-135, 2002.
Rukkumani, R., Sribalasubashini, M., Viswanathan, P. and Menon VP., Comparative effects of curcumin and photo-irradiated curcumin on alcohol and polyunsaturated fatty acid induced hyperlipidemia. Pharmacol Res, 46: 257-264, 2002.
Dinkova-kostova, H. and Talalay, P., Relation of structure of curcumin analogs to their potencies as inducers of phase-2 detoxification enzymes. Carcinogenesis,20: 911-914, 1999.
Sreejayan, R. and Rao, M.N., Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol, 49: 105-107, 1997.
Anto, R.J., George, J., Dinesh Babu, K.V., Rajasekaran, K.N. and Kuttan, R., Antimutagenic and anticarcinogenic activity of natural and synthetic curcuminoids. Mutat Res, 17: 127-131, 1996.
Devasena, T., Rajasekaran, K.N., Gunasekaran, G., Viswanathan, P. and Menon, V.P., Anticarcinogenic effect of bis-1,7-(2-hydroxy phenyl)-hepta-1,6-deine – 3,5-dione a curcumin analog on DMH-induced colon cancer model. Pharmacol Res, 47: 133-140, 2003.
Anusuya, S., Menon, V.P., Viswanathan, P. and Rajasekaran, K.N., Protection of pancreate b-cell by the potential antioxidant Bis-O-hydroxycinnamoyl methane, analogue of natural curcuminoid in experimental diabetes. J Pharm Pharmaceut Sci, 6: 327-333, 2003.
Dinesh Babu, K.V. and Rajasekaran, K.N., Simplified conditions for the synthesis of curcumin 1 and other curcuminoids. Org Prep Procedure Int, 26: 674-677, 1994.
Rajakrishnan, V., Vishwanathan, P. and Menon, V.P., Hepatotoxic effect of alcohol on female rats and siblings: Effects of n-acetyl cysteine. Hepatol Res, 9: 37-50, 1997.
15. King, E.J. and Armstrong, A.R, Calcium, phosphorus and phosphatase. In: Practical clinical Biochemistry. New Delhi: CBS Publishers, 458, 1988.
Fiala, S., Fiala, A.E. and Dixon, B., Gamma glutamyl transpeptidase in chemically induced rat hepatomas and spontaneous mouse hepatomas. J Natl cancer Inst, 48: 1393-1409, 1972.
17. Niehaus, W.G. and Samuelsson, B., Formation of malondialdehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem, 6: 126-130, 1968.
Jiang, Z.Y., Hunt, J.Y. and Wolff, S.P., Detection of lipid hydroperoxides using the ‘fox method’. Anal Biochem, 202: 384-389, 1992.
Ellman, G.L., Tissue sulphydryl groups. Arch Biochem Biophys, 82: 70-77, 1959.
Baker, H., Frank, O., De Angelis, B. and Feingold, S., Plasma tocopherol in man at various times after ingesting free or acetylated tocopherol. Nutr Res Int, 21: 531-536, 1980.
Roe, K.M. and Kuether, C.A., Detection of ascorbic acid in whole blood and urine through 2, 4 DNPH derivative of dehydro ascorbic acid. J Biol Chem, 147: 399-407, 1943.
Kakkar, P., Das, B. and Viswanathan, P.N., A modified spectrophotometric assay of superoxide dismutase (SOD). Ind J Biochem Biophys, 21: 130-132, 1984.
Sinha, K.A., Colorimetric assay of catalase. Anal Biochem, 47: 389-394, 1972.
Lieber, C.S., Alcoholic liver disease: new insights in pathogenesis lead to new treatments. J Hepatol, 32: 113-128, 2000.
Tuma, D.J., Thiele, G.M., Xu, D., Klassen, L.W. and Sorrel, M.F., Acetaldehyde and malondialdehyde react together to generate distinct protein adducts in the liver during long-term ethanol administration. Hepatol, 23: 872-880, 1996.
Gouillon, Z., Lucas, D., Li, J., Hagbjork, A.L., Freuch, B.A., Fu, P. and Fang, C., Inhibition of ethanol induced liver disease in the intragastric feeding rat model by chlormethiazole. Proc Soc Exp Biol Med, 224: 302-308, 2000.
Knecht, K.T., Thurman, R.G. and Mason, R.P., Role of superoxide and trace transition metals in the production of alpha-hydroxy ethyl radical from ethanol by microsomes from alcohol dehydrogenase – deficient deermice. Arch Biochem Biophys, 303: 339-348, 1993.
Bailey, S.M., Pietsch, E.C. and Cunningham, C.C., Ethanol stimulates the production of reactive oxygen species of mitochondrial complexes I and III. Free Radic Biol Med, 27: 891-900, 1999.
Yin, M., Wheeler, M.D., Kono, H., Bradford, B.U., Gallucci, R.M., Luster, M.I. and Thurman, R.G., Essential role of tumor necrosis factor alpha in alcohol induced liver injury in mice. Gastroenterol, 117: 942-952, 1999.
Farrel, S.O. and Jackson, M.J., Dietary PUFAs vitamin E and hypoxia/ reoxygenated – induced damage to cardiac tissue. Clin Chem Acta, 267: 197-211, 1997.
Jethmalani, S.M, Viswanathan, G., Bandyopadhyay, C. and Noronha, J.M., Effects of ingestion of thermally oxidized edible oils on plasma lipids, lipoproteins and postheparin lipolytic activity of rats. Ind J Exp Biol, 27: 1052-1055, 1989.
Schlorff, E.C., Husain, K. and Somani, S.M., Dose and time dependent effects of ethanol on plasma antioxidant system in rat. Alc, 17: 97-105, 1999.
Gonthier, B., Jeunet, A. and Barret, L., Electron spin resonance study of free radicals produced from ethanol and acetaldehyde after exposure to a fenton system or to brain and liver microsomes. Alc, 8: 369-375, 1991.
Mira, L., Maia, L., Barreira, L. and Manso, C., Evidence for free radical generation due to NADH oxidation by aldehyde oxidase during ethanol metabolism. Arch Biochem Biophys, 318: 48-58, 1995.
Liber, C.S. Cytochrome P4502E1: its physiological and pathological role. Physiol Rev, 77: 517-544, 1997.
Nordey, A. and Goodnight, S.H., Dietary lipids and thrombosis, relationships to atherosclerosis: A review. Arteriosclerosis, 10: 149-163, 1990.
Jaya, D.S., Augustine, J. and Menon, V.P., Role of lipid peroxides, glutathione and antiperoxidative enzymes in alcohol and drug toxicity. Ind J Exp Biol, 31: 453-459, 1993.
Alexander, J.C., Chemical and biological properties related to toxicity of heated fats. J Toxicol Environ Health, 7: 125-138, 1981.
Ray, G., Hussain, S.A., Oxidants, antioxidants and carcinogenesis. Ind J Exp Biol, 42: 1213-1232, 2002.
Suresh, M.V., John, J.L., Kumar, C.V.S. and Indira, M., Interaction of ethanol and ascorbic acid on lipid metabolism in guinea pigs. Ind J Exp Biol, 35: 1065-1069, 1997.
Gerster, H., b-carotene, vitamin E and vitamin C in different stages of experimental carcinogenesis. Eur J Clin Nutr, 49: 155-168, 1995.
Ookhtens, M. and Kaplowitz, N., Role of the liver in interorgan homeostasis of glutathione and cysteine. Semin Liver Dis,18: 313-329, 1998.
Fernandez, V. and Videla, L.A., Effect of acute and chronic ethanol ingestion on the content of reduced glutathione on various tissues of the rat. Experientia 37: 392-394, 1981.
Chance, B., Sies, H. and Boveris, A., Hydroperoxides metabolism in mammalian organ. Physiol Rev 59: 72-77, 1979.
Dinkova-kostova, A.T., Protection against cancer by plant phenyl propenoids: induction of mammalian anticarcinogenic enzymes. Mini Rev Med Chem, 2: 595-610, 2002.
Husain, K. and Somani,S.M., Interaction of exercise and ethanol on hepatic and plasma antioxidant system in rat. Pathophysiol 4: 69-74, 1997.
Okado-Matsumoto, A. and Fridovich, I., Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu, Zn-SOD in mitochondria. J Biol Chem, 276: 38388-38393, 2001.
Huang, T.T., Yasunami, M., Carlson, E.J., Gillespie, A.M., Reaume, A.G., Hoffman, E.K. and Chan, P.H., Superoxide mediated cytotoxicity in superoxide dismutase deficient fetal fibroblasts. Arch Biochem Biophys, 344: 424-434, 1997.
Pigeolot, E., Corbisier, P., Houbion, A., Lambert, D., Michiels, C., Raes, M., Zachary, M.O. and Ramacle, J., Glutathione peroxidase, superoxide dismutase and calatase inactivation by peroxides and oxygen derived radicals. Mech Age Dev, 51: 283-297, 1990.
Soudamini, K.K., Unnikrishnan, M.C., Soni, K.B. and Kuttan, R., Inhibition of lipid peroxidation and cholesterol levels in mice by curcumin. Ind J Physiol Pharmacol 36: 239-243, 1992.
Unnikrishnan, M.K. and Rao, M.N.A., Curcumin inhibits nitrite induced methemoglobin formation. FEBS, 301: 195-196, 1992.
Sreejayan. and Rao, M.N., Curcuminoids as potent inhibitors of lipid peroxidation. J Pharm Pharmacol 46: 1013-1016, 1994.
Schroeter, H., Williams, R.J., Martin, R., Iversen, L. and Rice Evans. Phenolic antioxidants attenuate neuronal cell death following uptake of oxidised low density lipoprotein. Free Rad Biol Med 29: 1222-1233, 2000.
Castelluccio, C., Bolwell, P., Gerrish, C. and Rice Evans, C., Differential distribution of ferulic acid to the major plasma constituents in relation to its potential as an antioxidant. Biochem J 316: 691-694, 1996.
Pan, G.X., Spencer, L. and Leary, G.J., Reactivity of ferulic acid and its derivative towards hydrogen peroxide and peracetic acid. J Agric Food Chem 47: 3325-3331, 1999.
Corresponding Author: Venugopal P. Menon, Professor and Head, Department of Biochemistry, Annamalai University, Annamalainagar-608 002, Tamil Nadu, India. cmrana@sify.com
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps