J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 7(3):325-331, 2004
In vitro studies on guar gum based formulation for the colon targeted delivery of Sennosides.
Munira Momin, K. Pundarikakshudu1
Department of Pharmacognosy, S.K.Patel College of Pharmaceutical Education and Research, Ganapat Vidyanagar, Mehsana, Kherva, Gujarat, IndiaReceived 18 April 2004, Revised 29 June 2004, Accepted 16 September 2004, Published 24 September 2004
PDF Version
Abstract
PURPOSE: The objective of the present study is to develop colon targeted drug delivery systems for sennosides using guar gum as a carrier. METHODS: Matrix tablets containing various proportions of guar gum were prepared by wet granulation technique using starch paste as a binder. The tablets were evaluated for content uniformity and in vitro drug release study as per BP method. T50 % value from the dissolution studies was taken for selecting the best formulation. RESULTS: Guar gum matrix tablets released 4-18% sennosides in the physiological environment of gastrointestinal tract depending on the proportion of the guar gum used in the formulation. The matrix tablets containing 50% of guar gum were found to be suitable for targeting of sennosides for local action in the colon. Compared to tablets having 30% and 40% of guar gum, those with 50% guar gum gave better T50% (11.7 h) le and fewer amounts (5-8%) of drug release in upper GIT. These tablets with 50% guar gum released 43% and 96% sennosides with and without rat caecal fluids. This suggests the susceptibility of matrix to the colonic micro flora. The similarity factor ( f2 value) for drug release with and without rat caecal fluids was found to be less than 30. When hydroxy propyl methylcellulose phthalate (10%) was used as a coat material on the matrix tablets, the initial loss of 5-8% sennosides in stomach could be completely averted. These tablets showed no change in physical appearance, content and dissolution profile upon storage at 45°C / 75% relative humidity for 3 months. CONCLUSION : The results of our study indicates that matrix tablets containing 50% guar gum and coated with 10% hydroxy propyl methylcellulose phthalate are most suitable for drugs like sennosides which are mainly active in the lower GIT.
Introduction
Several polysaccharides like, pectin and its salts, chondroitin sulphate, amylose and guar gum are being investigated as carriers for colon specific drug delivery. In pharmaceutical formulations, guar gum is used as a binder, disintegrant, suspending agent, thickening agent and stabilizing agent. (1,2) Guar gum and pectin are reported to be potential carriers for colon specific drug delivery. Colon specific drug delivery systems for 5-ASA and mebendazol have been developed using guar gum as a carrier (3,4). Guar gum in the form either of a matrix tablet or as a compression coat over a core tablet of drug is reported to target the drug to colon (5-9). The guar gum matrix tablets of albendazole were found degraded by colonic bacteria of rat caecal contents and released about 44% of albendazole in simulated colonic fluids (control study) at the end of 24 h indicating the susceptibility of the guar gum formulations to the rat caecal contents. Compression coated tablets of 5-ASA and matrix tablets of mebendazole have been prepared using guar gum as a carrier. Matrix tablets containing various proportions of guar gum were prepared by wet granulation technique using starch paste as a binder. The tablets were evaluated for drug content uniformity, and were subjected to in vitro drug release studies. The results of the study revealed that matrix tablets containing either 20% or 30% of guar gum are most likely to provide targeting of mebendazole for local action in the colon. A novel colon-specific drug delivery system based on guar gum matrix tablets was evaluated by conducting gamma scintigraphy studies using technitium-99m-DTPA as a tracer, in six healthy male human volunteers. Scintigraphs taken at regular intervals showed that some amount of tracer present on the surface of the tablets was released in stomach and small intestine and the bulk of the tracer present in the tablet mass delivered to the colon. These studies have shown the drug release retarding property of guar gum in the upper GIT and its degradation by the anaerobic bacteria in the colon (10-11).
Senna is a well-known drug in Ayurvedic system of medicine and has been included in most of the pharmacopoeias of the world. In India, this drug is known as Tinnevelly Senna, family Leguminoseae. The active constituents' sennosides A and B are of medicinal interest due to their strong laxative properties. The action of sennosides is mainly upon the large intestine and is, therefore, especially suitable in habitual constipation. The glycosides are absorbed from the intestinal tract and the active anthraquinones are excreted into the colon, where they stimulate and increase the peristaltic movements of the colon, decrease absorption of water from colon and produce a bulky softer fecal mass. This suggests their action is in lower bowel and have no effect in stomach and small intestine (12-14).
In the light of this information, it is planned to develop novel sennosides matrix tablets for colon targeting to provide effective safe therapy of laxation.
Experimental
Materials
Calcium sennosides (20%w/w) was gifted by Dishman Pharmaceuticals, Ahmedabad, India. Guar gum (viscosity of 1% aqueous dispersion is 4000 cps, particle size < 75 m ) was obtained from H.B. Gum industries, Kalol, Gujarat, India, and was of pharmacopoeial grade (USNF). Ferric chlorides (AR), sodium bicarbonate (AR), chloroform, hydrochloric acid, magnesium acetate and solvent ether were obtained from S.D. fine Chemicals, Mumbai, India. Double distilled water (DD water) was used. Other materials in the study such as microcrystalline cellulose (Avicel PH 101), starch, magnesium stearate and talc were of pharmacopoeial grade.
Preparation of sennosides matrix tablets
Matrix tablets of calcium sennosides were prepared by wet granulation method. Microcrystalline cellulose (MCC) was used as a diluent and compression promoting agent and a mixture of talc- magnesium stearate (2:1) was added as lubricant. Guar gum was included in the formulations in various proportions (30%-70%). The composition of different formulations used in the study containing 100 mg of calcium sennosides (20%w/w) in each formulation is shown in Table 1.
Table 1: Composition and characteristics of calcium sennosides matrix tablets.
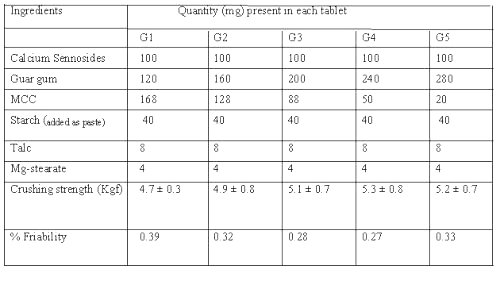
In all the formulations, guar gum was sieved separately and mixed with calcium sennosides and MCC. The powders were blended and granulated with 10%w/v starch paste. The wet mass was passed through a mesh (10#) and the granules were dried at 60°C for 1 h. The dried granules were passed through a mesh (20#), lubricated with a mixture of talc-magnesium stearate (2:1) and compressed using 12 mm round, concave and plain punches on a Rotary tablet press (Cadmach, India). The tablets were evaluated for their physical characteristics as per standard methods. The crushing strength and friability of matrix tablets was determined by Pfizer Hardness tester and Roche Friabilitor respectively.
Determination of drug content in tablet formulations
Sennosides were analyzed by British pharmacopoeial (BP) method. Ten tablets were finely powdered and transferred to 100 ml volumetric flask. Initially about 50 ml of double distilled water was added to the volumetric flask and allowed to stand for 1 h with intermittent sonication, volume was made up to 100ml with double distilled water, and the mixture was centrifuged. Ten ml of supernatant liquid was taken for further analysis of sennosides as per BP method. Ten ml of the supernatant liquid transferred to 100 ml round bottomed flask fitted with ground glass stopper. Twenty ml ferric chloride solution (10.5%w/v) was added and mixed. It was then heated in a water bath under reflux condenser for 20 min. one ml hydrochloric acid was added and heating was continued for 20 min, with frequent shaking until the precipitate is dissolved. Mixture was cooled and extracted with 100ml (25 ml X 4) ether. Ten ml ether extract was evaporated carefully to dryness and dissolved the residue in 10.0 ml of a 0.5% w/v solution of magnesium acetate in methanol. Absorbance of the resulting solution was measured at the maximum at about 515nm, using methanol as the blank. Content of sennosides B was calculated.
In-Vitro drug release studies
The ability of guar gum matrix tablets of sennosides to remain intact in the physiological environment of stomach and small intestine was assessed by mimicking mouth to colon transit. Drug release studies were carried out using USP XXIII dissolution apparatus (Apparatus 1, 100 rpm, 37± 0.5°C) in 900 ml 0.1 N HCl for 2 h as the average gastric emptying time is ~2 h.. The dissolution medium was replaced with 900 ml pH 7.4 phosphate buffer saline (PBS) and the dissolution was continued for 24 h. Ten ml of the sample was taken at the end of the specified time period (2 h, 5 h, 8 h, 12 h, 16 h, 18 h and 24 h) and analyzed for sennosides. A 10 ml volume of filtered, fresh dissolution medium was added to make the volume after each sample withdrawal (15).
The susceptibility of the matrix tablets to the enzymatic action of colonic bacteria was assessed by continuing the drug release studies in simulated colonic fluids prepared using rat caceal content as described by Sinha et al. (16).
Preparation of rat caecal content medium
Wistar rat weighing 150-200 g and maintained on a normal diet (soaked gram) were used. Forty-five minutes before the commencement of drug release studies, seven rats were killed by spinal traction. The abdomen were opened, the cecal were traced, ligated at both the ends, dissected, and immediately transferred into pH 7.4 buffer previously bubbled with nitrogen. The cecal bags were opened, their contents were individually weighed, pooled, and suspended in the buffer continuously bubbled with carbon dioxide. These were finally added to the dissolution media to give a final cecal dilution of 4% w/v, respectively. All the above procedures were carried out under carbon dioxide in order to maintain anaerobic conditions.
The drug release studies were continued with 500 ml of rat caecal content medium (4%). A continuous supply of CO2 was ensured, as the caecum is naturally anaerobic. At specified time intervals, 10 ml of the sample was withdrawn, filtered and replaced with 10 ml of fresh PBS containing 4% w/v rat ceacal fluids, bubbled with CO2, and the experiment was continued for 24 h as the usual colonic transit time is 20-30 h. Three sample samples for each data point were analyzed for sennoside content using BP method.
Optimized batch was enteric coated with 10% w/v solution of hydroxy propyl methylcellulose phthalate (HPMCP) in order to retard the loss of sennosides in stomach. Weight gain of the coated tablet was found to be 6.7 %. Dissolution study was carried out for the coated tablets with and without colonic fluids.
Stability studies
To assess the stability of sennosides matrix tablets, tablets of batch G3 were stored at 45°/75% RH for 3 months. At the end of the study period, the formulation was observed for changes in physical appearance, color, drug content and drug release characteristics. Drug release studies in rat caecal content medium were also carried out on sennosides matrix tablets of batch G3 after storage at 45°/75% RH for 3 months (17).
Statistical analysis
The drug release profiles of guar gum matrix tablets containing sennosides (n=3) in the dissolution medium at 24 h with and without rat caecal fluids (Control study) was compared using USP dissolution specification, f2 value, a similarity factor (18). A value less than 30 was considered significant value indicating dissimilarity in dissolution profiles. Drug release kinetics was also studied for all matrix formulations.
Results and Discussion
The present study was aimed at developing novel matrix tablet of sennosides for colon targeting using guar gum as a matrixing agent. Guar gum matrix tablets released decreased amount of sennoside in the physiological environment of stomach and small intestine. Majority of drug was released in the physiological environment of colon.
Since guar gum is found to have poor flow characteristics and is to be incorporated in large amount in matrix tablets, sennoside tablets were prepared by wet granulation technique using starch paste as a binder. The small samples of powder mixture of guar gum and sennoside drawn randomly were analyzed for assessing uniformity of mixing. The crushing strength of the tablet was found to be 4.7 kgf to 5.3 kgf (Table 1). The matrix tablets were found to contain 98.83% to 103.8% of the sennosides in each batch.
The matrix tablets were subjected to in-vitro drug release study in 0.1 M HCl (2 h), pH 7.4 PBS (3 h) and simulated colonic fluids (rat caecal content medium at 4% w/v level after 7 days of enzyme induction). It is reported that rat caecal content medium at the 4% w/v level after 7 days of enzyme induction provides a satisfactory condition for assessing the susceptibility of guar gum to colonic bacterial degradation (16).
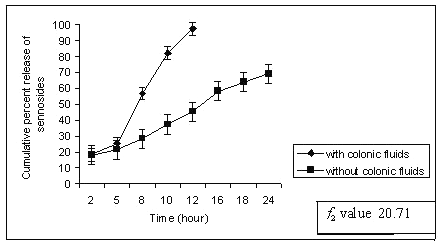
Dissolution studies were extended up to 24 h wherever necessary. When the matrix tablets were subjected to in-vitro drug release study, sennoside tablets containing 30 % of guar gum (G1) released 18% sennosides in first 2 h and most of the sennoside was released within 12 h with complete erosion of tablet. This might be due to lower guar gum content and higher quantity of MCC in matrix tablet (Table 1). The dissolution study was conducted without rat caecal content in dissolution medium (control). Sennosides tablets containing 40-60% of guar gum (G2-G5) showed better results compared to batch G1 (30% guar gum). The percent of sennosides released from the tablets of G1 is shown in Figure 1.
Figure 1: Percent sennosides released from matrix tablets containing G1 (30% guar gum) in dissolution study with and without rat ceacal content.
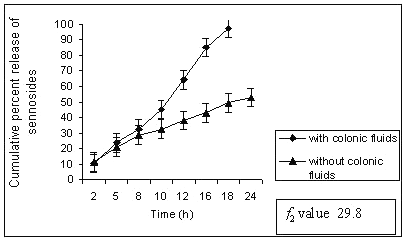
In control study batch G1 showed 70% cumulative percent release of sennoside at the end of 24 h. Tablet remained intact during control study but showed very loose gel. Matrix tablets containing 40% guar gum (G2) remain intact until 20 h. The amount of sennoside released from G2 matrix tablets at the end of 18 h was found to be 97 ± 1.32 % whereas in control study (without rat ceacal content in dissolution medium) only 53.01 ± 1.06 % of sennoside was released even after 24 h. (fig. 2).
Figure 2: Percent sennosides released from matrix tablets containing G2 (40% guar gum) in dissolution study with and without rat ceacal content.
On exposure to the dissolution fluids, the guar gum is hydrated and forms a viscous gel layer that slows down further entry of dissolution fluids towards the core of tablets. The hydration of guar gum is independent of the pH of the dissolution medium (19). The study shows that the release of sennosides in the physiological environment of colon is due to the microbial degradation of guar gum matrix tablets in the presence of rat ceacal content.
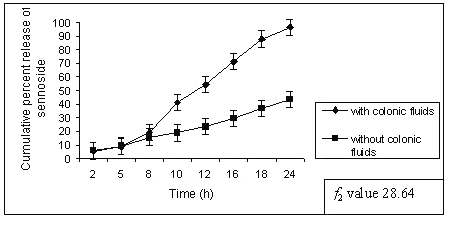
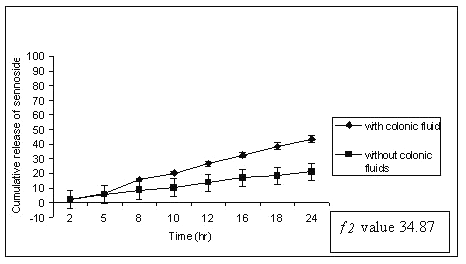
Similar observation was also noted by increasing the amount of guar gum in the matrix tablets, the release of sennosides decreased at the end of 24 h of dissolution study. Tablets of G3 (50% guar gum) released about 96 ± 1.12 % of sennoside in the presence of rat caecal content as against 43 ± 0.97 % of sennoside in control study (fig.3).
Figure 3: Percent sennosides released from matrix tablets containing G3 (50% guar gum) in dissolution study with and without rat ceacal content.
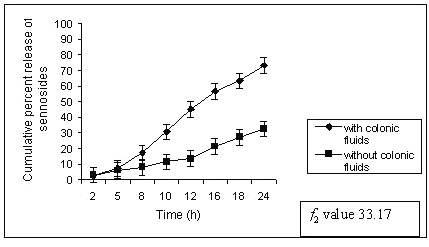
A significant difference was noted in release data obtained with and without rat ceacal content. The matrix tablets containing 60% of guar gum (G4) released only 73.19 ± 1.32 % of sennoside in rat ceacal content medium at the end of 24 h whereas in control study it was only 32.54 ± 0.89 % (fig. 4).
Figure 4: Percent sennosides released from matrix tablets containing G4 (60% guar gum) in dissolution study with and without rat ceacal content.
Batch G5 (70% guar gum) showed 43.57± 1.0 % and 21.08 ± 0.94 % drug release at the end of 24h, with and without colonic fluids respectively (fig 5). The results show that G3 and G4 formulations were degrading slowly in simulated colonic fluids indicating that 50% and 60% of guar gum in the matrix formulation is good enough for the colonic enzyme to act upon the formulation and degrade it. On further increasing the proportion of guar gum from 60 to 70%, a significant decline in the percent of drug release was noticed (73% and 43% for G4 and G5 respectively).
Figure 5: Percent sennosides released from matrix tablets containing G5 (70% guar gum) in dissolution study with and without rat ceacal content.
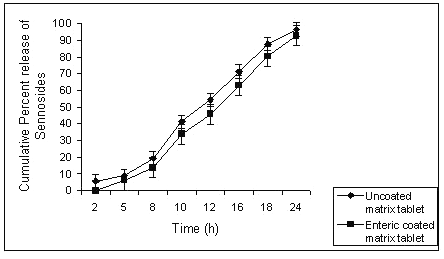
The gel strength of the swollen matrix formulation might be too high to release the drug from the formulation. The action of colonic bacteria was not sufficient to degrade such a high strength gel barrier of the swollen G5 matrix formulation. Applying an enteric coat to the matrix tablets of guar gum could retard the initial loss of sennosides, releasing major portion of drug in colon. Batch G3 released almost 8% of sennosides in physiological environment of stomach; this could be further retarded by giving an enteric coat with 10% w/v HPMCP to the matrix tablets. (Fig 6).
Figure 6: Comparison of percent sennosides released from uncoated and enteric coated matrix tablet of batch G3.
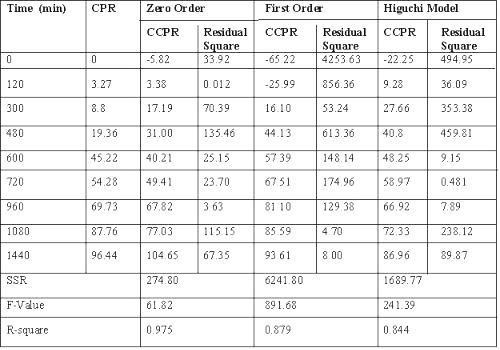
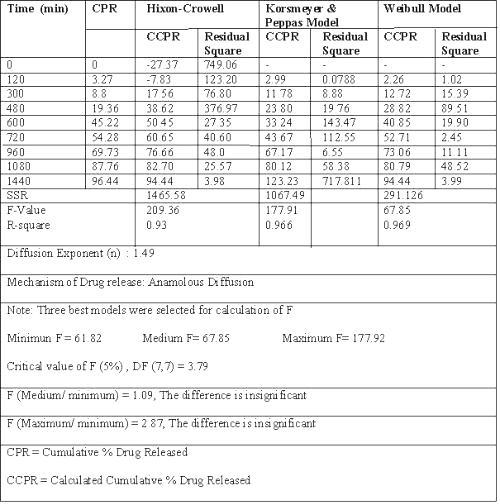
Dissolution data of batch G3, a potential candidate for 24 h drug release, fitted best to zero order release kinetics, giving the least residual sum of square as compared to Weibull model or Korsemeyer and Peppas equation. (Table 2) (20-24).
Table 2: Results of model fitting of batch G3 (with rat caecal content).
Tablets from batch G3 could target sennosides in physiological environment of colon. Stability studies were carried out at 45°c / 75%RH for 3 months to assess their stability. After storage, the tablets were subjected to assay of drug and in-vitro dissolution study. No changes in physical appearance, drug content and dissolution profiles were noted. When dissolution study was carried out in physiological environment of stomach, small intestine and colon, no significant difference was observed in cumulative percent release of sennosides from formulation (Table 3).
Table 3: Percent of sennoside released from G3 matrix formulation before and after storage at 45°c / 75%rh for 3 months.
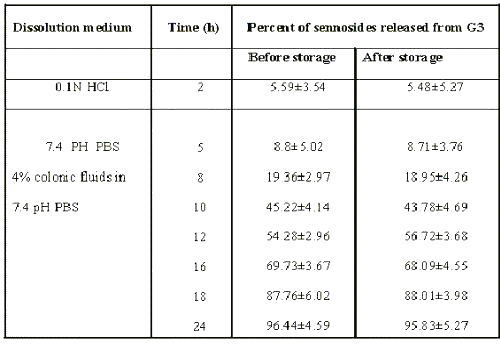
These data suggest the formulation of G3 to be stable.
Conclusions
The present study was carried out to develop colon-targeted delivery system for sennosides using guar gum as a carrier. Guar gum matrix tablet containing various proportions of guar gum were prepared and subjected to in vitro drug release studies. Sennoside matrix tablets containing 30 % of guar gum are not suitable for colon targeting as they released most of the drug within 10 h. In addition, matrix tablets containing 60% of guar gum are considered unsuitable for colon targeting as they released only 73% of sennoside after 24 h of dissolution study. Tablets containing 40% and 50% of guar gum completely degraded in the presence of rat ceacal contents thereby releasing almost all the sennosides. Batch G3 containing 50% guar gum released entire drug in colon by zero order kinetics. The optimized batch, after storing at 45°c / 75%RH for 3 months, showed no change in physical appearance, drug content or dissolution pattern.
References
Latchman, L., Lieberman, H. A., Kanig, J. L., The Theory and Practice of Industrial Pharmacy. 3rd Indian edition, Varghese Publishers, India, 1990. 592.
Goldstein, A.M., Alter, E.N., Seaman, J.K., Guar Gum. In: Whistler RL (Ed). Industrial Gums, Polysaccharides and their derivatives. Academic Press, New York, 1993. 303-321.
Krishnaiah Y.S.R., Satyanarayan. S., Rama Prasad Y. V., Studies of guar gum compression coated 5-aminosalicylic acid tablets for colon-specific drug delivery. Drug Dev. Ind. Pharm., 25: 651-657, 1999.
Krishnaiah Y.S.R., Raju P. V., Kumar B. D., Bhaskar P., Satyanarayan V., Development of colon targeted drug delivery systems for mebendazole. J Control release, 77: 87-95, 2001.
Krishnaiah, Y.S.R., Muzib, Y. I., Shrinivasa Rao, G., Bhaskar, P., Satyanarayana, V., Design and In Vitro Evaluation of oral colon Targeted Drug Delivery system for Tinidazole. Journal of Drug Targeting, 10 (8): 579-584, 2002
Krishnaiah, Y.S.R., Satyanarayana, V., Seetha Devi, A., Nageswar rao, L., Bhaskar P., Karthikeyan, R.R.S., Guar Gum as a Carrier for colon specific delivery; Influence of Metronidazole and Tinidazole on In Vitro release of Albendazole from Guar gum Matrix tablets. J Pharm Pharmaceui Sci, 4(3): 235-243, 2001.
Rama Prasad, Y.V., Krishnaiah Y.S.R., Satyanarayana, S., In vitro evaluation of guar gum as a carrier for colon-specific drug delivery. J Control Rel, 51:281-287, 1998.
Krishnaiah, Y.S.R., Satyanarayana, S., Rama Prasad Y.V., Narasimha Rao, S., Gamma scintigraphic studies on guar gum matrix tablets for colonic drug delivery in healthy subjects. J Control Rel, 55: 245-252, 1998.
Krishnaiah, Y.S.R., Satyanarayana, S., Rama Prasad Y.V., Narasimha Rao, S., Evaluation of guar gum as a compression coat for drug targeting to colon. Int J Pharm, 171: 137-146, 1998.
G.L. Simon and S.L. Gorbach, Intestinal flora in health and disease, Gastroenterology, 86: 174-193, 1984.
Macfarlane, G.T., Hay, S., Macfarlane, S., Gibson, G.R., Effect of different carbohydrates on growth, polysaccharidase and glycosidase production of Bacteroides ovatus in batch and continuos culture. J Appl Bacteriol, 68:179-187, 1990.
Leng-Peschlow E. Dual effect of orally administered Sennosides on large intestine transit and fluid absorption in the rat. J Pharm parmacol, 38: 606-610, 1986.
British Pharmacopoeia, HMSO, London, UK, 1993
Martindale: the extra Pharmacopoeia, 32nd ed. Pharmaceutical Press, London, UK.
United States Pharmacopoeial Convention (USP). 1999, USP 24-NF-19, Rockwille, USA.
Sinha, V. R., Mittal, B. R., Bhutani K. K., Rachna Kumari, Colonic drug delivery of 5-fluorouracil: an in vitro evaluation. Int. J Pharm, 269: 101-108, 2004.
J.T. Carstesen. Drug Stability: Principle and Practices, 2nd ed, Marcel Dekker, Inc., New York, 1995.
Guidance for Industry, Dissolution Testing of Immediate release Solid oral Dosage Forms, U. S. Health and Human Services, Food and Drug Administration, CDER, 1997.
Gohel M. C., Amin A., Panchal M.K., Momin M., Bajaj S., Lalwani A., Preliminary investigations in matrix based tablet formulations of diclofenac sodium containing succinic acid treated guar gum. Bollettino Chimico Farmaceutico, 137: 198-203, 1997.
Bamba M., Puisieux F., Marty J. P., Cartesen J. T. Int. J. Pharmaceutics, 2: 307, 1979.
Lindner W. D., Lippold B. C., Drug release from hydrocolloid embeddings with high or low susceptibility to hydrodynamic stress, Pharm. Res., 12 (11): 1781-1785, 1995.
Higuchi T., Mechanism of sustained action medication. J Pharm. Sci., 52 (12): 1145-1149, 1963.
Nelson K. G., Wang L. Y., Determination of time course of tablet disintegration II: Method using continuous functions. J Pharm. Sci. 67 (1): 86-89, 1961.
Peck G. E., Johnson A. D., Anderson V. L. A statistical approach for the development of an oral controlled release matrix tablet, Pharm. Res. 7: 1092-1097, 1990.
Corresponding Author: Dr. K. Pundarikakshudu, Department of Pharmacognosy, S.K.Patel College of Pharm. Edu. & Res., Ganapat Vidyanagar, Kherva- 382711, Mehsana, Gujarat, India. momin_munira@hotmail.com
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps