J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 7(3):315-324, 2004
Artificial cell microcapsule for oral delivery of live bacterial cells for therapy: design, preparation, and in-vitro characterization.
Wei Ouyang, Hongmei Chen, Mitchell L. Jones, Terrence Metz, Tasima Haque, Christopher Martoni, Satya Prakash1
Biomedical Technology and Cell Therapy Research Laboratory, Department of Biomedical Engineering and Artificial Cells and Organs Research Centre, Faculty of Medicine, McGill University, Quebec, CanadaReceived 5 January 2004, Revised 22 July 2004, Accepted 15 September 2004, Published 24 September 2004
PDF Version
Abstract
PURPOSE: Bacterial cells can be engineered to synthesize a wide array of disease modifying substrates such as cytokines, vaccines and antibodies; however, their use as an orally delivered therapeutic is limited by poor gastrointestinal (GI) survival and instigation of immunogenic response. Artificial cell microcapsules have been well studied as a means to overcome such problems, however, presently obtainable microcapsules have limitations. This study summarizes a novel microcapsule design specifying its preparation and GI stability in-vitro. METHOD: Multilayer APPPA microcapsules were designed, prepared and characterized in-vitro for bacterial cell oral delivery using Lactobacillus reuteri cells as a model. Microcapsule structural integrity, mechanical stability, and GI survival studies were performed in simulated gastric (SGF) and intestinal (SIF) fluids in various pH conditions at 37.2°C and compared with presently available alginate/poly-l-lysine/alginate (APA) microcapsules. HPLC was used for the microcapsule membrane permeability study. RESULTS: Results show that APPPA microcapsules can be prepared for bacterial cell encapsulation and are stable in simulated GI conditions. No microcapsule damage was reported when exposed to SGF and SIF for 12 hours at 250 rpm mechanical shaking at 37.2°C. In addition, 93.2±2.3% and 98.9±0.6% of microcapsules were undamaged after 24 hours in SGF and SIF respectively. Microcapsule pH stability results show that 92.8±3.1% of microcapsules remained intact at pH 1, 3, 5, and 7 and no damage was observed at pH 9.0 when challenged for 24 hours. When exposed for 3 hours with 250 rpm shaking at 37.2°C, no damage of the microcapsules in SGF and SIF at pH, 1,3,5,7, and 9 was observed. Compared to APA microcapsules, APPPA membranes showed superior GI stability and permeability for cell encapsulation. CONCLUSION: Novel APPPA microcapsules have superior features for oral delivery of live bacterial cells and they can be used for various clinical applications. However, further study such as membrane permeability, cytotoxicity, immune protection capacity, and suitability for live bacterial cell oral delivery in-vivo is required.
Introduction
Bioencapsulation technology offers several advantages and has shown promising results for the treatment of a number of diseases (1-7). For all these applications, appropriate performance of the microcapsules is critically dependent on the properties of the capsular membrane (8-10). Considerable research interest has been dedicated to the encapsulation of bacterial cells for the growing and promising potential in therapeutic applications such as in kidney failure uremia, cancer therapy, diarrhea, cholesteremia, and other diseases (11-17). However, success of microcapsule oral delivery of live bacterial cells for therapy depends on the suitability of the microcapsule membrane for GI delivery. For example, a microcapsule can be disrupted by many different means during its intestinal passage; it may be fractured by enzymatic action, chemical reactions, heat, pH, diffusion, mechanical pressure and other related physiological and biochemical stresses. The safety of microcapsules is even more important when live cells are intended for use in the intestinal system by oral administration. This is because the live cells must be protected during the encapsulation process and microcapsules must reach the GI intact. In addition, the survival of the live cells during their passage must be ensured. Thus, the microcapsule membrane must be provided with sufficient permeability for nutrients, and secretion and excretion products, to pass through, yet prevent the entry of hostile molecules or cells from the host, for example, products of the host's immune response, which could destroy the encapsulated bacterial cells (7; 18-20). The problems inherent with oral delivery, therefore, have made the goal of oral delivery of live bacterial cells very challenging.
Several delivery systems for oral delivery of live bacterial cells have been proposed. For example, microcapsule immobilization of bifidobacteria to protect them against adverse effects of acid during oral administration has been proposed (21; 22). Rao et al. described a method to encapsulate freeze-dried B. pseudolongum using cellulose acetate phthalate (CAP) coated with beeswax, showing that encapsulated B. pseudolongum survived the simulated gastric environment in larger numbers than non-encapsulated cells (21; 22). Encapsulation of bifidobacteria in butter oil and whey based medium was proposed but was shown to be ineffective in preventing acid injury to bacteria in both low acid and high acid environments (23). Calcium alginate and k-carrageenan¯Locust bean gum gel beads are the two most commonly used polymers for immobilizing viable cells (24; 25). However, alginate beads are not acid resistant, and it has been reported that the beads undergo shrinkage and decreased mechanical strength (25-27). In order to overcome this, coating bacteria by cross-linking with a carboxyvinyl polymer career has been suggested. The carboxyvinyl polymer was, however, shown to be effective only for intestinal delivery and release (28). K-Carrageenan¯Locust bean gum gel beads are less sensitive to acid than alginate and hence used for lactic fermentation. However, 2 major limitations preclude their use. First, the formation of k-carrageenan¯Locust bean gum beads requires high potassium ions. The latter could potentially damage the cells of B. longum during lactic fermentation (29; 30). Second, it has been pointed out that, as potassium ions are important in maintaining electrolyte equilibrium, their inclusion in the diet in large amounts would not be recommended (31; 32). Gellan¯xanthan beads are not only acid resistant but are also stabilized by calcium ions (33), suggesting that they could be a good candidate for immobilizing bifidobacterial cells and protecting them against acid injury (34). However, they do not protect against immunorejection, which is a primary requirement for probiotic oral delivery of live cells for therapy. Similarly, agarose capsules prepared by emulsification/internal gelatinization for oral delivery of Bacillus Calmette-Guerin (BCG) cells, although stable for up to 12 months in-vitro, have not been shown to be suitable for oral delivery as agarose membranes do not provide immunoprotection (33; 35). Thus, agarose microspheres with various polymer coatings have been proposed (36). Among other formulations, gelatin and polymer coated gelatin capsules have been studied for oral delivery of live bacterial cells (37-40). The latter (with 20% w/v of the polymer) has shown promising results in vitro. On the other hand, concerning uncoated gelatin capsules, a radiological study among human volunteers has shown that they disintegrate within 15 minutes of ingestion (40-42). A host of other formulations using poly(lactide-co-glycolides), carrageenan, alginate-poly-L-lysine, starch polyanhydrides, polymethacrylates, polyamino acids, enteric coating polymers, etc. have been proposed, but they exhibit poor GI survival and lack immunoprotection (37-40; 43-50).
Although numerous encapsulation systems have been studied, to date, the most promising formulation is the encapsulation of calcium alginate beads with poly-L-lysine (PLL) forming alginate-poly-L-lysine-alginate (APA) microcapsules (4; 7; 51-53). The APA microcapsule has been used successfully to limit the major problem of immuno-rejection related to the use of live cells for therapy and other biomedical applications. The presently obtainable APA microcapsules, however, have posed limitations for general use, via oral administration, because of their inadequate stability in the gastrointestinal (GI) tract (4; 52; 54-57). To overcome this, APA microcapsules were modified by others using a higher concentration of alginate cross-linked with barium instead of calcium, and the alginate was fabricated as a gelled bead without solubilizing the core microcapsule (58). This modification prolonged the stability of the capsule for systemic delivery applications in canine models, but not for oral delivery. Another related limitation of the APA membranes is the potential for hydrolysis on enzymatic action during their passage within the GI tract, making them less suitable for oral delivery (59; 60). Given these limitations of the presently available APA microcapsules, and their biomedical applications, development of microcapsules suitable for oral delivery is necessary. In the present study, we use a novel approach towards the design of APPPA microcapsule formulation and test their GI stability in-vitro.
mATERIALS AND mETHODS
Preparation of Ca-alginate beads
Ca-alginate beads were first made using an Inotech Encapsulator IER-20 (Inotech Biosystems Intl. Inc.) by extruding a sodium alginate solution (Sigma-Aldrich low viscosity, 1.5% w/w,) into 120 ml stirred solution of 0.1M CaCl2. The beads were prepared using a 300 m m encapsulator nozzle at a frequency of 1160HZ, 7.93ml/min syringe pump speed and a voltage of 1.000Kv using a 60 ml syringe.
Preparation of APPPA microcapsules
Ca-alginate beads were exposed to PLL solution (Sigma, Mw=27400 d, 0.1% w/w) for 15 mins, washed with saline; then in pectin solution (Sigma, degree of esterification: 25%, 0.1% w/w) for 15 mins, washed with deionized water; and subsequently soaked in PLL solution (0.1% w/w) for 15 mins, washed with deionized water; finally put it in alginate solution (low viscosity, Sigma-Aldrich 0.05% w/w) for 10mins. The resulting microcapsules were washed with deionized water and stored in 4°C.
Preparation of APA microcapsules
APA capsules were prepared according to the standard protocol (7) with several modifications. Briefly, Ca-alginate beads were suspended in a solution of PLL (0.1% w/w) for 15 mins, washed with ion-free water and then immersed in alginate solution (0.05% w/w for 10 mins. The resulting microcapsules were washed with deionized water, stored in 4°C, and used in the experiments.
Mechanical stability test
For mechanical stability evaluations, 150±30 APPPA microcapsules were exposed to various test fluids (SGF, SIF, microcapsule storage solution, varying pH solution), in a 25 ml conical flask and exposed to various mechanical shaking speeds and stress and time periods in an Environ Shaker at 37.2°C. Samples were withdrawn and analyzed for physical damage using an optical light microscope. For comparative mechanical stability studies, similar quantities of APA microcapsule were used.
Microcapsule permeability study
Preliminary studies for APPPA microcapsule permeability were performed and compared with alginate beads and APA microcapsules. For this 4.00 mg of BSA was dissolved in 1.0 ml of 1.5% (w/w) alginate solution with a BSA/alginate ratio of 26.6 %.(w/w) and alginate beads containing BSA were obtained. Using the above-mentioned procedure, APPPA, APA microcapsule containing BSA was also prepared. The BSA leakage was analyzed by exposing BSA containing APPPA microcapsules, alginate beads, and APA microcapsules to deionized water in a 25 ml flask with 150 rpm shaking in a environ shaker at 37.2°C for 24 hours. After 24 hours of exposure, samples were withdrawn and BSA concentration was measured using an HPLC (Varian Inc. Canada) UV detector at 280nm. For this HPLC BSA permeably study, a Biosep-SEC-S3000 column, 50mM NaH2 PO4 buffer mobile phase (pH=6.8), and flow rate of 0.5ml/min were used.
Results and Discussion
Microcapsule design strategy for oral delivery of live bacterial cells: Design, and preparation of APPPA microcapsules
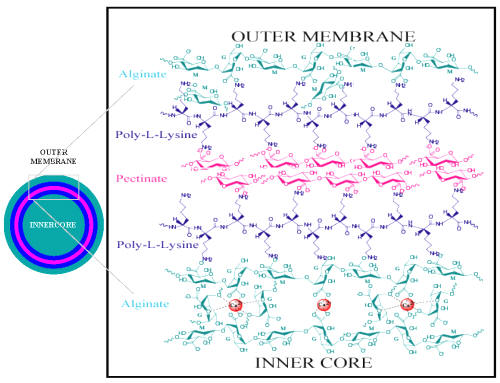
The biomatrix used for developing the microcapsules is of primary importance for addressing the complex problems associated with oral delivery. They should provide mild conditions for encapsulation, be non-toxic to the cell and host, be biocompatible, have sufficient membrane permeability, be impermeable to antibody-sized molecules, and have the ability to overcome the acidic and enzymatic environment of the stomach and GI tract. Available synthetic polymeric materials, which are excellent for the oral delivery of drugs, would not be good candidates, as they would not satisfy the above-mentioned conditions. Alginate and pectinate are potentially good candidates for designing microcapsule formulations for the delivery of live bacterial cells (61). The molecular design strategy and the schematic diagram for the APPPA microcapsule are shown in Figure 1.
Figure 1: Molecular membrane design for Alginate-Polylysine-Pectinate-Polylysine-Alginate (APPPA) membrane artificial cell microcapsule structure.
We designed and formulated novel alginate/poly-l-lysine/pectin/poly-l-lysine/alginate (APPPA) microcapsules that show special features not available in present formulations of microcapsules. To prepare these novel microcapsules we have taken advantage of the known chemistry that pectin can be cross-linked with ions and poly-l-lysine and can then be made available in the membrane formulation of alginate/poly-l-lysine microcapsules. For this, we first cross-linked sodium alginate with calcium ions through ionotropic gelation.
Then a PLL coating serves as an immunoprotective membrane layer. The subsequent pectin membrane provides better acid stability and improved strength. The additional alginate coating at the outer surface of the microcapsules ensures the optimal biocompatibility. Characteristic of a multi-layer or "sandwich" structure, this formulation is likely to make the proposed APPPA membrane stronger and more stable, and therefore may be able to solve the problems in GI applications for the current APA microcapsules.
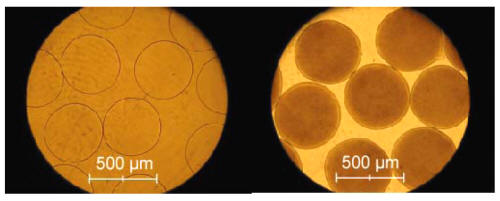
Using multi-step preparation procedures described above we can obtain APPPA microcapsules. The preparation process for the APPPA microcapsules involved the formation of calcium- alginate beads, multiple coatings of poly-l-lysine, pectin, poly-l-lysine, and alginate. We were able to obtain an extremely spherical shape, narrow size distribution and high homogeneity of the microcapsules. The size of the microcapsules obtained depends on the nozzle used; in this study, using a 300 m m nozzle resulted in the diameter of the microcapsules to be in the range of 400±25 m m (Figure 2a).
Results show that APPPA microcapsule membranes were able to retain Lactobacillus reuteri bacterial cells (Figure 2b).
Figure 2: Optical micrographs of APPPA. Left: APPPA microcapsules without bacterial cells, Right: APPPA microcapsules containing with Lactobacillus reuteri (LP80) cells.
Results show (data not shown) that bacterial cells were able to survive during the encapsulation process and grow normally when obtained supernatant was plated after breaking of the microcapsule membrane.
GI stability of APPPA microcapsules
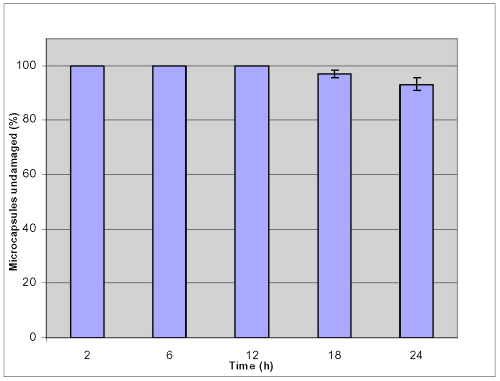
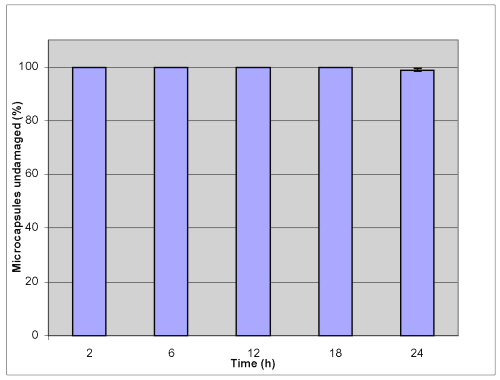
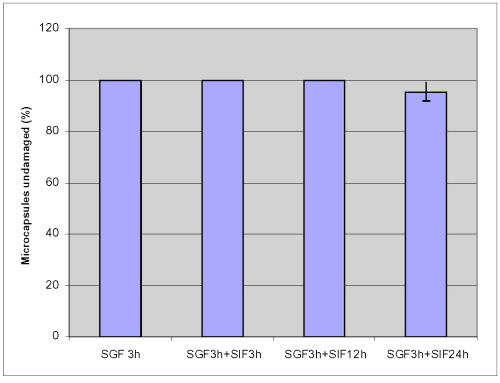
To evaluate the stability of microcapsules for oral therapy, knowledge of encapsulant dynamics is required under relevant physiological conditions that represent the different phases of digestion. In many applications, capsules are subjected to mechanical stresses exerted by their environment that induce deformation and potential break-up; thus capsular mechanical strength is believed to be paramount and should not be compromised. In this study, a shake method was used and the percentage of microcapsules undamaged was observed by microscope to provide information on the mechanical resistance of the microcapsules in various GI conditions. Figures 3 and 4 show that no damage occurs when microcapsules were challenged for 12 hour mechanical shaking at 250 rpm at 37.2°C; neither in simulated gastric fluid (SGF) nor in simulated intestinal fluid (SIF).
Figure 3: Mechanical stability of APPPA microcapsules in simulated gastric fluid (SGF) after shaking at 250 rpm (pH= 1.5, 37.2 ºC).
Figure 4: Mechanical stability of APPPA microcapsules in simulated intestinal fluid (SIF) after shaking at 250 rpm (pH= 7.5, 37.2 ºC).
93.2±2.3% of microcapsules was undamaged in simulated gastric solution and 98.9 ± 0.6 % microcapsules remained intact in simulated intestinal solution after shaking up to 24 hours. These results suggested that APPPA microcapsules were stable both in simulated gastric solution and in simulated intestinal fluid.
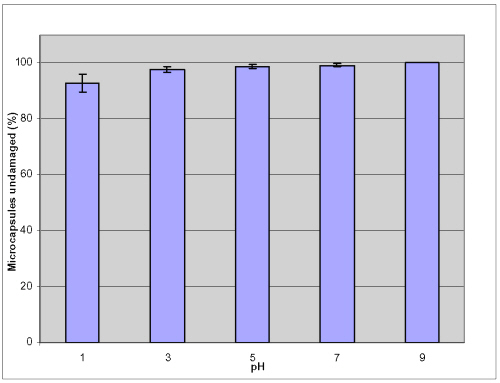
Figures 5 summarized the microcapsules undamaged percentage in various pH conditions after shaking for 24 hours at 250 rpm at 37.2°C.
Figure 5: Mechanical stability of APPPA microcapsules in various GI pH conditions after shaking at 250rpm for 24hrs at 37.2 ºC.
In all these pH values, more than 92.8 ± 3.1% of microcapsules were found undamaged or remained intact and there was no damaged observed in the solution of pH 9.0. These results further demonstrated that the membranes were stable at the different levels of pH (1, 3, 5, 7 and 9) commonly found in the human GI tract. Results (Figures 6 and 8) show that no damage occurs when microcapsules were in simulated gastric fluid (SGF) for 3 hours and in simulated intestinal fluid (SIF) for 3 hours mechanical shaking at 150 rpm at 37.2°C.
Figure 6: Mechanical stability of APPPA microcapsules in simulated GI condition after shaking at 150rpm at 37.2 ºC.
Figure 7: Mechanical stability of APPPA microcapsules in storage solution.
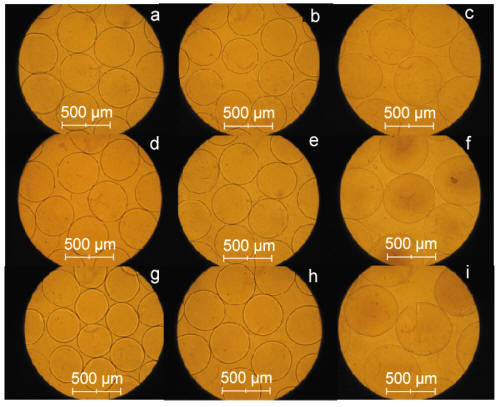
Figure 8: Optical micrographs of APPPA and APA microcapsules. a, APPPA microcapsules; b, APPPA after shaking at 150 rpm, 37.2 ºC in SGF for 3hrs. c, APPPA after shaking at 150 rpm, 37.2 ºC in SGF for 3hrs and in SIF for 12hrs. d, APPPA after shaking at 150 rpm, 37.2 ºC in SGF for 24hrs. e, APPPA after shaking at 150 rpm, 37.2 ºC in storage solution for 72hrs. f, APPPA after shaking at 150 rpm, 37.2 ºC in SGF for 3hrs and in SIF for 24hrs. g, APA microcapsules. h, APA after shaking at 150 rpm, 37.2 ºC in SGF for 24hrs. i, APA after shaking at 150 rpm, 37.2 ºC in SGF for 3hrs and in SIF for 24hrs.
However, the microcapsules were swelled in SIF. There were 95.4 ± 3.6% APPPA microcapsules undamaged after shaking up to 24 hours in SIF. These results suggested that APPPA microcapsules were stable in GI condition.
Table 1 summarizes the results of mechanical stability of the microcapsule in various GI fluids.
Table 1: Comparative mechanical stability of APPPA and APA microcapsules in simulated GI conditions after shaking at 150rpm at 37.2 ºC.
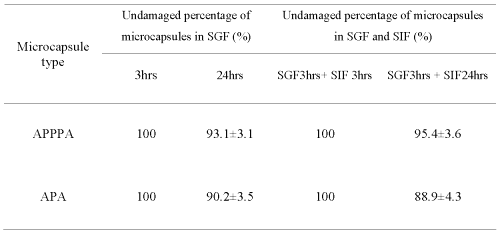
Results show that 93.1 ± 3.1% of APPPA microcapsules were undamaged or remained intact (Table 1, Figure 8d) after shaking for 24 hours in SGF solution at 150 rpm at 37.2°C compared to 90.2 ± 3.5% undamaged or intact APA microcapsules (Table 1, Figure 8h). Similar results were obtained in a GI environment; 95.4 ± 3.6% APPPA microcapsules were found undamaged or intact (Table 1, Figure 8f) when microcapsules exposed to simulated gastric fluid (SGF) for 3 hours and to simulated intestinal fluid (SIF) for 24 hours at a mechanical shaking of 150 rpm at 37.2°C compared to 88.9 ± 4.3 % undamaged or intact APA microcapsules (Table 1, Figure 8i). These in-vitro GI stability results showed that the APPPA membrane is stronger and more stable compared to the currently obtainable and most popular APA microcapsule system in SGF and SIF fluids.
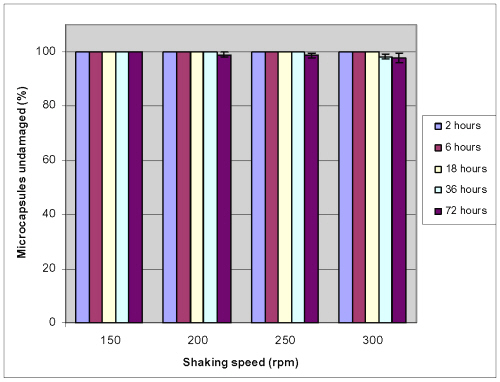
Microcapsule mechanical stability
Experiments were designed to test the microcapsule stability. For this APPPA microcapsules were immersed in it storage solution (0.1 M CaCl 2 solution) and shaken in an environ shaker at different rpm for different times at 37.2°C and then the resulting microcapsules were observed under an optical microscope. No morphological damage on microcapsule membranes was observed after mechanical shaking for up to 72 hours at 150 rpm. Moreover, at 300 rpm mechanical shaking for 72 hours 97.7 ± 1.8% microcapsules microcapsule were found to be intact (Fig. 7, Fig. 8e). These results showed that APPPA microcapsules have good mechanical stability in storage solution.
Microcapsule permeability study
In vitro studies were performed to evaluate immune protection capacity of the APPPA microcapsule membrane and compared with alginate beads and the APA microcapsule membrane using the bovine serum albumin (BSA) molecule and high-performance liquid chromatography (HPLC). Results are shown in Fig. 9.
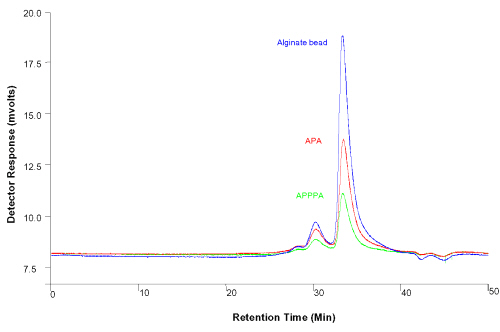
Figure 9: HPLC BSA leakage studies of the APPPA membrane microcapsule compared with alginate beads and presently available APA membrane microcapsules after 24h of shaking (150rpm) at 37.2°C. Blue: Alginate beads (top, Red: Traditional APA microcapsule (middle), Green: Proposed APPPA microcapsule (bottom), solvent used: saline.
Results show that after shaking for 24hours at 37.2°C and 150rpm, about half the amount of BSA was released from the APA microcapsules as compared to the alginate beads; and about half the amount of BSA was leaked from APPPA as compared to the APA microcapsules, suggesting the superior immuno-protective potential of the APPPA membrane. The APPPA microcapsule membrane would protect live bacterial cells form the host immune system; immune responsible molecules are bigger in molecular weight (e.g. IgM 950 KD, IgE 190 KD, IgA 170 KD, IgD 160 KD, IgG 150 KD) compared to the BSA molecule (mole. wt. 68 KD (11)) tested in this HPLC study and would be impermeable to the APPPA microcapsule membranes. These results suggest the permeability of APPPA microcapsules is lower than alginate core and APA beads giving it a competitive advantage over APA microcapsules.
Conclusion
Results show the design of multi-layer APPPA microcapsules. This multi-layer APPPA microcapsule displayed superior stability in simulated GI conditions suggesting the possibility of a GI application. These capsules also have shown to display good mechanical stability in storage solutions. Mechanical stability and permeability studies suggest that APPPA microcapsules are more stable, stronger, and display better selective permeability compared to the most popular APA microcapsule systems. It is anticipated that this research of membrane design will provide formulations that would have sufficient resistance to GI tract interactions and allow safe and effective oral delivery of live bacterial cells for various clinical applications. Further research, however, is required to substantiate these results, in particular molecular membrane permeability, immune protection property studies, and cell loading capacity, toxicology, and in-vivo affirmation of stability.
Acknowledgements
This work was supported by Canadian Institute of Health Research (CIHR). Wei Ouyang acknowledges Post Doctoral Fellowship from Fonds Québécois de la recherché sur la nature et les technologies (FQRNT).
References
Cirone, P., Bourgeois, J. M., Austin, R. C., and Chang, P. L., "A novel approach to tumor suppression with microencapsulated recombinant cells," Human Gene Therapy, Vol. 13, No. 10, 2002, pp. 1157-1166.
Orive, G., Hernandez, R. M., Gascon, A. R., Igartua, M., and Pedraz, J. L., "Development and optimisation of alginate-PMCG-alginate microcapsules for cell immobilisation," International Journal of Pharmaceutics, Vol. 259, No. 1-2, 2003, pp. 57-68.
Orive, G., Gascon, A. R., Hernandez, R. M., Igartua, M., and Pedraz, J. L., "Cell microencapsulation technology for biomedical purposes: novel insights and challenges," Trends in Pharmacological Sciences, Vol. 24, No. 5, 2003, pp. 207-210.
Prakash, S. and Chang, T. M. S., "Microencapsulated genetically engineered microorganisms for oral administration.," British Priority Patent, Vol. 960133-9., 1996.
Serp, D., Cantana, E., Heinzen, C., von Stockar, U., and Marison, I. W., "Characterization of an encapsulation device for the production of monodisperse alginate beads for cell immobilization," Biotechnology and Bioengineering, Vol. 70, No. 1, 2000, pp. 41-53.
Springer, M. L., Hortelano, G., Bouley, D. M., Wong, J., Kraft, P. E., and Blau, H. M., "Induction of angiogenesis by implantation of encapsulated primary myoblasts expressing vascular endothelial growth factor," Journal of Gene Medicine, Vol. 2, No. 4, 2000, pp. 279-288.
Sun, A. M., Goosen, M. F., and O'Shea, G., "Microencapsulated cells as hormone delivery systems," Crit Rev.Ther.Drug Carrier Syst., Vol. 4, No. 1, 1987, pp. 1-12.
Prokop, A., Hunkeler, D., DiMari, S., Haralson, M. A., and Wang, T. G., "Water soluble polymers for immunoisolation I: Complex coacervation and cytotoxicity," Microencapsulation - Microgels - Iniferters, Vol. 136, 1998, pp. 1-51.
Hunkeler, D., Prokop, A., and Wang, T., "A screening of water soluble polymers for cell encapsulation," Abstracts of Papers of the American Chemical Society, Vol. 213, 1997, pp. 258-BIOT.
Soon-Shiong, P., Lu, Z. N., Grewal, I., Lanza, R. P., and Clark, W., "An in vitro method of assessing the immunoprotective properties of microcapsule membranes using pancreatic and tumor cell targets," Transplant.Proc., Vol. 22, No. 2, 1990, pp. 754-755.
Chang, T. M. and Prakash, S., "Therapeutic uses of microencapsulated genetically engineered cells," Mol.Med.Today, Vol. 4, No. 5, 1998, pp. 221-227.
Chang, T. M. S., "Artificial cells with emphasis on cell encapsulation of genetically engineered cells," Artificial Organs, Vol. 22, No. 11, 1998, pp. 958-965.
Chang, T. M. S. and Prakash, S., "Procedures for microencapsulation of enzymes, cells and genetically engineered microorganisms," Molecular Biotechnology, Vol. 17, No. 3, 2001, pp. 249-260.
Li, L., Baumann, C. A., Meling, D. D., Sell, J. L., and Beitz, D. C., "Effect of orally administered Eubacterium coprostanoligenes ATCC 51222 on plasma cholesterol concentration in laying hens," Poult.Sci., Vol. 75, No. 6, 1996, pp. 743-745.
Prakash, S. and Chang, T. M., "Microencapsulated genetically engineered live E. coli DH5 cells administered orally to maintain normal plasma urea level in uremic rats," Nat.Med., Vol. 2, No. 8, 1996, pp. 883-887.
Prakash, S. and Chang, T. M., "Growth and survival of renal failure rats that received oral microencapsulated genetically engineered E. coli DH5 cells for urea removal," Artif.Cells Blood Substit.Immobil.Biotechnol., Vol. 26, No. 1, 1998, pp. 35-51.
McIntosh, G. H., Royle, P. J., and Playne, M. J., "A probiotic strain of L-acidophilus reduces DMH-induced large intestinal tumors in male Sprague-Dawley rats," Nutrition and Cancer-An International Journal, Vol. 35, No. 2, 1999, pp. 153-159.
Rihova, B., "Biocompatibility of biomaterials: Hemocompatibility, immunocompatibility and biocompatibility of solid polymeric materials and soluble targetable polymeric carriers," Advanced Drug Delivery Reviews, Vol. 21, No. 2, 1996, pp. 157-176.
Rihova, B., "Immunocompatibility and biocompatibility of cell delivery systems," Advanced Drug Delivery Reviews, Vol. 42, No. 1-2, 2000, pp. 65-80.
Ross, C. J. D., Ralph, M., and Chang, P. L., "Delivery of recombinant gene products to the central nervous system with nonautologous cells in alginate microcapsules," Human Gene Therapy, Vol. 10, No. 1, 1999, pp. 49-59.
Rao, A. V., Shiwnarain, N., and Maharaj, I., "Survival of Microencapsulated Bifidobacterium-Pseudolongum in Simulated Gastric and Intestinal Juices," Canadian Institute of Food Science and Technology Journal-Journal de l Institut Canadien de Science et Technologie Alimentaires, Vol. 22, No. 4, 1989, pp. 345-349.
Sun, W. R. and Griffiths, M. W., "Survival of bifidobacteria in yogurt and simulated gastric juice following immobilization in gellan-xanthan beads," International Journal of Food Microbiology, Vol. 61, No. 1, 2000, pp. 17-25.
Modler, H. W. and Vila-Gracia, L., "The growth of Bifidobacterium longum in whey-based medium and viability of this organism in frozen yogurt with low and high levels of developed acidity.," Cult.Dairy Prod., Vol. 28, 1993, pp. 4-8.
Audet, P., Paquin, C., and Lacroix, C., "Effect of Medium and Temperature of Storage on Viability of Lactic-Acid Bacteria Immobilized in Kappa-Carrageenan-Locust Bean Gum Gel Beads," Biotechnology Techniques, Vol. 5, No. 4, 1991, pp. 307-312.
Hansen, L. T., Allan-Wojtas, P. M., Jin, Y. L., and Paulson, A. T., "Survival of Ca-alginate microencapsulated Bifidobacterium spp. in milk and simulated gastrointestinal conditions," Food Microbiology, Vol. 19, No. 1, 2002, pp. 35-45.
Eikmeier, H., Westmeier, F., and Rehm, H. J., "Morphological Development of Aspergillus-Niger Immobilized in Ca-Alginate and K-Carrageenan," Applied Microbiology and Biotechnology, Vol. 19, No. 1, 1984, pp. 53-57.
Roy, D., Leduy, A., and Goulet, J., "Kinetics of Growth and Lactic-Acid Production from Whey Permeate by Lactobacillus-Helveticus," Canadian Journal of Chemical Engineering, Vol. 65, No. 4, 1987, pp. 597-603.
Baichwal, Staniforth, and John N, "Agglomerated hydrophilic complexes with multi-phasic release characteristics," US Patent, Vol. 5,670,168., 1997.
Audet, P., Paquin, C., and Lacroix, C., "Immobilized Growing Lactic-Acid Bacteria with K-Carrageenan - Locust Bean Gum Gel," Applied Microbiology and Biotechnology, Vol. 29, No. 1, 1988, pp. 11-18.
Audet, P., Paquin, C., and Lacroix, C., "Batch Fermentations with A Mixed Culture of Lactic-Acid Bacteria Immobilized Separately in Kappa-Carrageenan Locust Bean Gum Gel Beads," Applied Microbiology and Biotechnology, Vol. 32, No. 6, 1990, pp. 662-668.
Stacia, M., "Human Physiology (Deborah, ed.): ," Foundations and Frontiers, 1990, pp. 526-527.
Weisz, R. R., "Hypokalemia-Induced Clinical Neurophysiological Changes in the Human Neuromuscular-Junction," Clinical Research, Vol. 28, No. 4, 1980, pp. A752.
Norton, S., Lacroix, C., and Vuillemard, J. C., "Effect of Ph on the Morphology of Lactobacillus-Helveticus in Free-Cell Batch and Immobilized-Cell Continuous Fermentation," Food Biotechnology, Vol. 7, No. 3, 1993, pp. 235-251.
Sanderson, G. R., "Gellan gum," Food Gels, Elsevier, New York, 1990, pp. 201-233.
Esquisabel, A., Hernandez, R. M., Igartua, M., Gascon, A. R., Calvo, B., and Pedraz, J. L., "Preparation and stability of agarose microcapsules containing BCG," Journal of Microencapsulation, Vol. 19, No. 2, 2002, pp. 237-244.
Losgen, H., Brunner, G., Holloway, C. J., Buttelmann, B., Husmann, S., Scharff, P., and Siehoff, A., "Large Agarose Beads for Extracorporeal Detoxification Systems .1. Preparation and Some Properties and Applications of Large Agarose Beads in Hemoperfusion," Biomaterials Medical Devices and Artificial Organs, Vol. 6, No. 2, 1978, pp. 151-173.
Narayani, R. and Rao, K. P., "Preparation, Characterization and In-Vitro Stability of Hydrophilic Gelatin Microspheres Using A Gelatin-Methotrexate Conjugate," International Journal of Pharmaceutics, Vol. 95, No. 1-3, 1993, pp. 85-91.
Narayani, R. and Rao, K. P., "Ph-Responsive Gelatin Microspheres for Oral Delivery of Anticancer Drug Methotrexate," Journal of Applied Polymer Science, Vol. 58, No. 10, 1995, pp. 1761-1769.
Narayani, R. and Rao, K. P., "Polymer-Coated Gelatin Capsules As Oral Delivery Devices and Their Gastrointestinal-Tract Behavior in Humans," Journal of Biomaterials Science-Polymer Edition, Vol. 7, No. 1, 1995, pp. 39-48.
Narayani, R. and Rao, K. P., "Gelatin microsphere cocktails of different sizes for the controlled release of anticancer drugs," International Journal of Pharmaceutics, Vol. 143, No. 2, 1996, pp. 255-258.
Vitini, E., Alvarez, S., Medina, M., Medici, M., de Budeguer, M. V., and Perdigon, G., "Gut mucosal immunostimulation by lactic acid bacteria," Biocell, Vol. 24, No. 3, 2000, pp. 223-232.
Winzer, A. and Lutze, V., "Radiochemical Studies of the Influence of Photographically Active Substances on the Kinetics of the Mass-Transfer at Silver-Halide Crystals .9. Activation Parameters for the Mass-Transfer in the Course of the Ostwald-Ripening in the Presence of Unfolded Gelatin," Journal of Information Recording Materials, Vol. 22, No. 1, 1994, pp. 65-77.
Chen, H. M., Torchilin, V., and Langer, R., "Polymerized liposomes as potential oral vaccine carriers: Stability and bioavailability," Journal of Controlled Release, Vol. 42, No. 3, 1996, pp. 263-272.
Chen, H. M. and Langer, R., "Oral particulate delivery: status and future trends," Advanced Drug Delivery Reviews, Vol. 34, No. 2-3, 1998, pp. 339-350.
Chin, J., Turner, B., Barchia, I., and Mullbacher, A., "Immune response to orally consumed antigens and probiotic bacteria," Immunology and Cell Biology, Vol. 78, No. 1, 2000, pp. 55-66.
Kreuter, J., "Evaluation of Nanoparticles As Drug-Delivery Systems .3. Materials, Stability, Toxicity, Possibilities of Targeting, and Use," Pharmaceutica Acta Helvetiae, Vol. 58, No. 9-10, 1983, pp. 242-250.
Kreuter, J., "Nanoparticle-Based Drug Delivery Systems," Journal of Controlled Release, Vol. 16, No. 1-2, 1991, pp. 169-176.
Gao, Y. T. and Wang, B. H., "Safety comparison of insecticide microencapsulation and investigation of its mechanism," J.Microencapsul., Vol. 6, No. 4, 1989, pp. 527-533.
Kwak, H. S., Kwon, S. H., Lee, J. B., and Ahn, J., "In vitro stability of beta-galactosidase microcapsules," Asian-Australasian Journal of Animal Sciences, Vol. 15, No. 12, 2002, pp. 1808-1812.
Atkins, T. W., "Biodegradation of poly(ethylene adipate) microcapsules in physiological media," Biomaterials, Vol. 19, No. 1-3, 1998, pp. 61-67.
Sun, A. M., O'Shea, G. M., and Goosen, M. F., "Injectable microencapsulated islet cells as a bioartificial pancreas," Appl.Biochem.Biotechnol., Vol. 10, 1984, pp. 87-99.
Lanza, R. P., Kuhtreiber, W. M., Ecker, D., Staruk, J. E., and Chick, W. L., "Xenotransplantation of porcine and bovine islets without immunosuppression using uncoated alginate microspheres," Transplantation, Vol. 59, No. 10, 1995, pp. 1377-1384.
Liu, P. and Krishnan, T. R., "Alginate-pectin-poly-L-lysine particulate as a potential controlled release formulation," Journal of Pharmacy and Pharmacology, Vol. 51, No. 2, 1999, pp. 141-149.
King, A., Sandler, S., and Andersson, A., "The effect of host factors and capsule composition on the cellular overgrowth on implanted alginate capsules," J.Biomed.Mater.Res., Vol. 57, No. 3, 2001, pp. 374-383.
King, G. A., Daugulis, A. J., Faulkner, P., and Goosen, M. F. A., "Alginate-Polylysine Microcapsules of Controlled Membrane Molecular-Weight Cutoff for Mammalian-Cell Culture Engineering," Biotechnology Progress, Vol. 3, No. 4, 1987, pp. 231-240.
Ma, X. J., Vacek, I., and Sun, A., "Generation of Alginate-Poly-L-Lysine-Alginate (Apa) Biomicroscopies - the Relationship Between the Membrane Strength and the Reaction Conditions," Artificial Cells Blood Substitutes and Immobilization Biotechnology, Vol. 22, No. 1, 1994, pp. 43-69.
Quong, D., Yeo, J. N., and Neufeld, R. J., "Stability of chitosan and poly-L-lysine membranes coating DNA-alginate beads when exposed to hydrolytic enzymes," Journal of Microencapsulation, Vol. 16, No. 1, 1999, pp. 73-82.
Petruzzo, P., Cappai, A., Ruiu, G., Dessy, E., Rescigno, A., and Brotzu, G., "Development of biocompatible barium alginate microcapsules," Transplant.Proc., Vol. 29, No. 4, 1997, pp. 2129-2130.
Sefton, M. V., May, M. H., Lahooti, S., and Babensee, J. E., "Making microencapsulation work: conformal coating, immobilization gels and in vivo performance," Journal of Controlled Release, Vol. 65, No. 1-2, 2000, pp. 173-186.
Quong, D., Yeo, J. N., and Neufeld, R. J., "Stability of chitosan and poly-L-lysine membranes coating DNA-alginate beads when exposed to hydrolytic enzymes," J.Microencapsul., Vol. 16, No. 1, 1999, pp. 73-82.
Sefton, M. V., May, M. H., Lahooti, S., and Babensee, J. E., "Making microencapsulation work: conformal coating, immobilization gels and in vivo performance," Journal of Controlled Release, Vol. 65, No. 1-2, 2000, pp. 173-186.
Corresponding Author: Satya Prakash, Biomedical Technology and Cell Therapy Research Laboratory, Department of Biomedical Engineering, Faculty of Medicine, McGill University, 3775 University Street, Montreal, QC H3A 2B4 Canada. satya.prakash@mcgill.ca
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps