J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 7(4):1-6, 2004
Spectroscopic investigation of the aggregation state of amphotericin B during loading, freeze-drying, and reconstitution of polymeric micelles.
Monica Adams, Glen S. Kwon1
University of Wisconsin-Madison, School of Pharmacy, Division of Pharmaceutical Sciences, USA; Currently: Bristol-Myers Squibb Company, Pharmaceutical Research Institute, New Brunswick, New Jersey, USAReceived 18 August 2004, Revised 1 November 2004, Accepted 4 November 2004, Published 22 November 2004
PDF Version
Abstract
PURPOSE: To investigate the relative aggregation state of amphotericin B (AmB) during loading and reconstitution of polymeric micelles. METHODS. Hexanoate and stearate derivatives of PEO- b -p (L-Asp) were prepared. The polymers and AmB were dissolved in methanol (MeOH). Milli-Q water was then added slowly, and the MeOH was removed via rotary evaporation. The solutions were freeze-dried in the presence of trehalose. During micelle preparation, the aggregation state of AmB was assessed using absorption spectroscopy. Upon reconstitution, the samples were analyzed using vapor-pressure osmometry, size-exclusion chromatography (SEC), and absorption spectroscopy. The absorption spectrum of AmB in the presence of the block copolymers was compared to that of AmB alone under the same conditions. RESULTS. AmB was loaded into micelles prepared from acyl derivatives of PEO- b -p (L-Asp). Absorption spectroscopy indicated that the aggregation state was preserved during the loading process. AmB exists in a self-aggregated state in polymeric micelles containing hexanoate ester cores and in a relatively monomeric state in polymeric micelles containing stearate ester cores. Vapor-pressure osmometry confirmed the isotonicity of the formulations, while SEC indicated that the micelles were approximately 106 g/mol. CONCLUSIONS. Depending on the polymer structure and assembly conditions, it is possible to encapsulate AmB in a relatively nonaggregated or aggregated state in micelles prepared from acyl derivatives of PEO- b -p (L-Asp). In polymeric micelles containing stearate side chains, AmB was loaded in a nearly monomeric state, possibly due to interaction with the stearate side chains. The final aggregation state of the drug is preserved during lyophilization and reconstitution of polymeric micelles prepared by a novel solvent evaporation procedure..
Introduction
Polymeric micelles have attracted considerable attention as drug carriers (1-3). In water, amphiphilic block copolymers (ABCs) based on poly (ethylene oxide)-block-poly (L-aspartate), PEO-b-p (L-Asp), assemble into micellar structures capable of encapsulating hydrophobic drugs. The carboxyl functionality of the p (L-Asp) block offers the possibility of post-polymerization chemical modification (4, 5). Thus, it is possible to chemically tailor the core-forming region of the polymer backbone, thereby altering the micelle properties (6). Previously, we demonstrated the utility of acyl conjugates of poly (ethylene oxide)-block-poly (N-hexyl-L-aspartamide), PEO-b-p (N-HA), for encapsulation of AmB (7). AmB is a potent, yet toxic and poorly water-soluble, antifungal agent and the therapy of choice for treating disseminated candidiasis. Due to the amphiphilic nature and limited solubility of AmB, it poses a formulation challenge, particularly with respect to controlling the aggregation behavior of the drug.
AmB is usually given parenterally as Fungizone®, which contains AmB solubilized by sodium deoxycholate and allows no control over the aggregation state of the drug. In contrast, encapsulation of AmB in highly substituted poly (ethylene oxide)-block-poly (N-hexyl-L-aspartamide)-stearic acid ester, PEO-b-p (HASA), micelles results in nonaggregated drug and sustained release (7, 8). In addition, administration of relatively nonaggregated AmB encapsulated in highly substituted PEO-b-p (HASA) micelles is as efficacious as Fungizone® against disseminated candidiasis in a neutropenic murine model (9). Utilization of this formulation may decrease the toxicity of AmB by enabling delivery of nonaggregated drug, which could increase the selectivity of AmB toward fungal cells (10). However, physical loading of drugs into polymeric micelles in good yield can be quite challenging, and depends on the chemical and physical nature of both the polymer and the solubilizate.
For the PEO-block-poly (N-HA)-acyl ester series, reconstitution of dehydrated micelles becomes increasingly more difficult as the length of the attached acyl chain and the degree of esterification are increased (11). To obtain a reconstitutable solid, AmB-loaded polymeric micelles were prepared by a modified solvent evaporation procedure in the presence of a lyoprotectant, trehalose, as described previously (7, 9). The polymers used in the present studies contained stearate and hexanoate side chains in order to obtain relatively nonaggregated and aggregated AmB, respectively, upon encapsulation. The present work explores the effects of the drug-loading process on the aggregation behavior of AmB. In this manuscript, we demonstrate that the aggregation state of AmB loaded into polymeric micelles comprised of the stearate and hexanoate esters is preserved during the freeze-drying and reconstitution steps.
Materials and Methods
Materials
Dichloromethane (CH2 Cl2) and trehalose dihydrate were purchased from Sigma (USA). Methanol (MeOH) was purchased from Fisher (USA). Opti-Mole osmolality standards were purchased from Wescor (USA). Amphotericin B (AmB) was obtained from Chem-Impex (USA). Dicyclohexylcarbodiimide (DCC) was purchased from Lancaster (USA). All other chemicals were from Aldrich (USA).
Polymer Preparation
Poly (ethylene oxide)-block-poly (N-hexyl-L-aspartamide)-hexanoic acid ester, PEO-b-p (HAHA), and methoxypoly (ethylene oxide)-block-poly (6-hydroxylhexyl-L-aspartamide), PEO-b-p (6-HHA), were prepared previously (6). Poly(ethylene oxide)-block-poly(N-hexyl-L-aspartate)-stearic acid ester, PEO-b-p(HASA), was prepared as previously described except for the following modifications in reaction conditions. Presently, 200 mg of PEO-b-p (6-HHA) was reacted for 36 h at ambient temperature with 3 eq. of stearic acid in 5.0 mL of CH2 Cl2 in the presence of 5 eq. DCC and 3 eq. DMAP. The product was characterized using 1H NMR (Varian, 500 MHz, USA) as previously described (6). The improved reaction conditions resulted in 82 % yield and an esterification level of 96.9 ± 1.1 % (approximately 24.3 stearate chains per 25 L-Asp repeat units).
Micelle Assembly and Drug Loading
To obtain different aggregation states of AmB, 5 mg of PEO-b-p (HAHA) or 10 mg of PEO-b-p (HASA) was dissolved in 2 mL of MeOH containing 0.32 mg/mL of AmB. Milli-Q water (H2O) was added dropwise to the stirring solutions to obtain a 50:50 MeOH:H2O mixture. To further drive micellization and enhance lyoprotectant solubility, an additional 1 mL of H2O was added prior to the addition of 0.75 g of trehalose dehydrate. MeOH was removed, and the volume was reduced to around 1.5 mL via rotary evaporation. The aqueous solutions were collected and diluted to 5 mL with H2O. To control tonicity, an additional 0.36 g of trehalose dihydrate was added and dissolved by stirring. The solutions were then filtered (0.22 mm, ISC BioExpress; USA). Aliquots (0.5 mL) were placed in 5-mL serum vials, immersed in N2 (l) until frozen, and freeze-dried (Labconco, p < 133 x 10-3 mbar, condenser T = 49°C). Following lyophilization, the samples were shielded from light and stored over desiccant at 5°C until use. At key time points during assembly, the aggregation state of AmB was determined by absorption spectroscopy. As a control, the aggregation state of AmB in the absence of polymer was also monitored.
Absorption Spectroscopy
The formulations were reconstituted with 1 mL of H2O. Next, each was diluted two-fold with DMF in order to break up the reversible aggregates, and then as necessary into the linear range using 50:50 DMF:H2O. AmB content was determined by absorbance at 412 nm (Amersham Pharmacia Biotech Ultraspec 4000, USA). To assess the aggregation state of AmB, samples were reconstituted in H2O and diluted as necessary with H2O. As a comparison, the aggregation state of AmB in MeOH/water mixtures was also monitored. To track the aggregation state of AmB in MeOH/water, solutions were prepared by diluting 900 mL aliquots of 0.6 mg/mL AmB in 1 - 13 mL of MeOH and then diluting to 25 mL with H2O. Spectra were acquired from 320 to 450 nm at 405 nm/min. Quantitative measurements were performed with a 1 cm path length cell. Dilution of the samples during micelle assembly was avoided by choosing an appropriate path length, b = 0.1 mm - 1.0 cm, for evaluation of the aggregation state.
Vapor-Pressure Osmometry
The reconstituted solutions contained 10 % (w/v) trehalose. Tonicity of the samples (20 mL aliquots) was determined using a Wescor 5500 vapor-pressure osmometer (USA) calibrated with Wescor Opti-Mole osmolality standards.
Size Exclusion Chromatography (SEC)
Measurements were performed on an Agilent 1100 series HPLC system with a refractive index detector and SEC software (Agilent, USA). The flow rate and column compartment temperature were set to 0.8 mL/min and 37°C, respectively. The solids were reconstituted with 1 mL of phosphate-buffered saline (PBS, pH 7.2), and injected (100 mL) in triplicate onto a Shodex SB-806M HQ OHpak column equipped with a Shodex OHpak SB-G guard column (Showa Denko, USA). The calibration was performed using dextran standards, Mw = 1 - 7 x 106 g/mol (JM Science, USA).
Results and Discussion
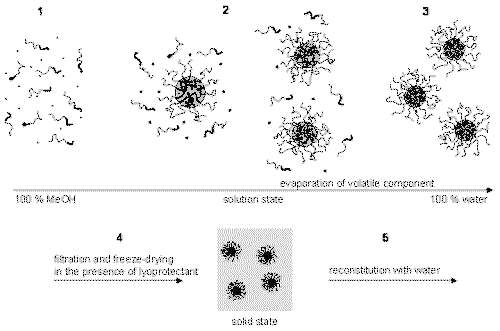
The first step in the drug-loading process involves dissolving the drug and polymer in a common volatile, organic solvent (Figure 1, step 1). Then, water is gradually added to induce micellization (Figure 1, step 2). As the water content increases, it is likely that the partitioning of AmB into the polymeric micelles also increases. At this step, trehalose, a lyoprotectant, is added to facilitate reconstitution. The volatile component is removed via rotary evaporation (Figure 1, step 3). Then, the volume is adjusted to control the polymer concentration, and additional lyoprotectant is added to control the tonicity of the reconstituted formulations. The aqueous solution is then sterile-filtered, aliquoted, immersed in N2 (l), and freeze-dried (Figure 1, step 4). The solid may then be reconstituted with water to yield a clear solution (Figure 1, step 5).
Figure 1: Schematic representation of drug loading conditions. Thin and thick lines represent the hydrophilic and hydrophobic blocks of the polymer, respectively. Circles represent drug molecules.
Table 1 describes the properties of the reconstituted formulations.
Table 1: Characteristics of AmB-loaded polymeric micelles determined by absorption spectroscopy.
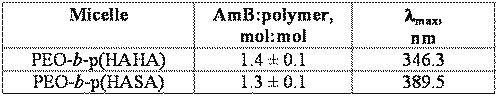
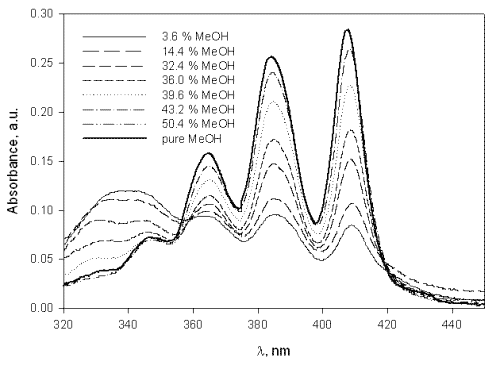
AmB molecules are large, ring-like structures characterized by a hydrophobic heptaene region, which is chemically conjugated to a relatively hydrophilic chain containing numerous hydroxyl groups. In pure methanol, AmB exists in an unaggregated, or monomeric, state. However, AmB aggregates in water at concentrations above 1 m M due to the amphiphilic nature of the molecule (12, 13). Self-aggregation leads to strong absorption in the 330 - 340 nm region of the absorption spectrum (Figure 2).
Figure 2: Absorption spectrum of 24 m M AmB in methanol/water mixtures expressed as v/v % MeOH.
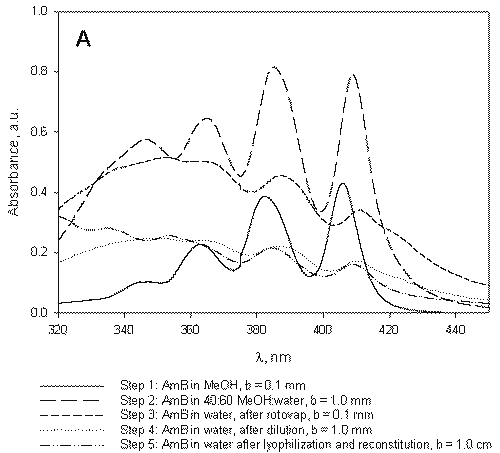
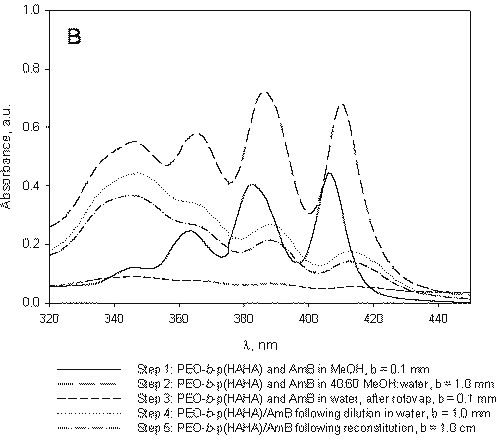
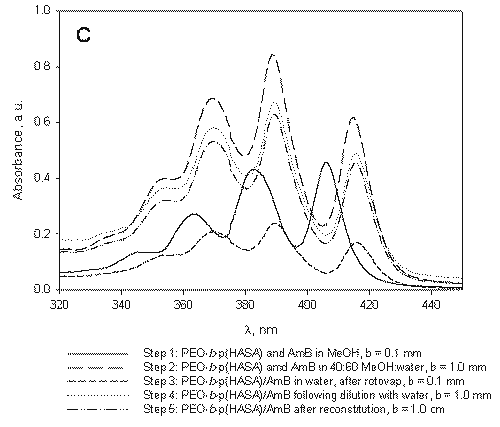
Figure 3: A) B) C) Absorption spectrum of AmB under micelle assembly conditions. The steps listed correspond to those designated in Figure 1 and represent sampling points. A) Absorption spectrum of AmB in the absence of polymers. B) Absorption spectrum of AmB under processing conditions in the presence of PEO-b-p (HAHA). C) Absorption spectrum of AmB under processing conditions in the presence of PEO-b-p(HASA).
The aggregation behavior of AmB in the presence of PEO-b-p(HAHA) (Figure 3B) is similar to that of AmB in the absence of polymer (Figure 3A).
Analysis of the absorption spectrum of free AmB under the micelle assembly conditions indicates that the drug begins to aggregate upon the addition of water (Figure 3A, step 2).
However, the PEO-b-p(HAHA)/AmB formulation readily reconstitutes following lyophilization while, in the absence of polymer, free AmB precipitates from solution in the form of irreversible aggregates. Following the formation of these aggregates, monomeric AmB could not be obtained even by dilution in large quantities of DMF. Therefore, it was not possible to accurately determine the concentration of free AmB after lyophilization in the absence of polymer. In contrast, the rapid reconstitution of PEO-b-p(HAHA)/AmB at high drug concentrations suggests that, while aggregated, AmB was contained inside the micelles.
At 40:60 MeOH:water in the presence of PEO-b-p(HAHA), the spectrum of AmB contains four bands at 346.3, 365.7, 386.4, and 410.3 nm (Figure 3B, step 2).
This spectrum is nearly identical to that observed for free AmB under the same conditions (Figure 3A, step 2), which is characterized by bands at 346.4, 364.8, 385.2, and 409.1 nm. The similarity between the two spectra may indicate poor partitioning of AmB into PEO-b-p(HAHA) micelles, likely due to weak hydrophobic interaction between AmB and PEO-b-p(HAHA).
In the presence of PEO-b-p(HASA), the aggregation behavior of AmB is quite different. In pure MeOH, the absorption spectrum of AmB is the same as in the other cases with absorption bands at 346.9, 363.4, 382.6, and 406.0 nm (Figure 3C, step 1).
At 40:60 MeOH:water, the absorption spectrum of AmB in the presence of PEO-b-p(HASA) contains four bands at 353.9, 369.2, 382.7, and 414.8 nm (Figure 3C, step 2), indicating favorable hydrophobic interaction between AmB and PEO-b-p(HASA). Upon removal of MeOH and encapsulation at a drug to polymer ratio of approximately 1.3 in PEO-b-p(HASA) micelles, a unique absorption spectrum is observed (Figure 3C, step 3). The spectrum of the encapsulated species is characterized by a narrowing and red shifting of the bands. In addition, the λmax (389.5 nm) of the encapsulated species is well out of the range observed for the aggregated species. Taken together, these spectral features indicate that AmB self-aggregation does not predominate when the drug resides in PEO-b-p(HASA) micelles. Instead, AmB exists in a nearly monomeric state, presumably due to interaction with the stearate side chains.
AmB has been loaded into PEO-b-p(HASA) micelles previously at the same drug to polymer ratio, yet this unique absorption spectrum was not observed (7). Presently, the drug to polymer ratio in the initial dissolution step was lowered relative to previous studies. The present work clearly indicates that, in addition to the chemical nature of the hydrophobic block, the micelle assembly/drug loading conditions play an important role in controlling the aggregation state of AmB. Therefore, the final drug to polymer ratio, while important, is not the only factor affecting the aggregation state of AmB encapsulated by PEO-b-p(HASA) micelles.
The large hydrophobic block of the PEO-b-p(HASA) used in the present study required the addition of a lyoprotectant to aid in reconstitution. Trehalose was chosen as it has been successfully employed for lyoprotection and reconstitution of PEO-stabilized association colloids (7, 14, 15). Because interactions between PEO and water are critical to micelle formation and stabilization, a similar mechanism to that proposed for proteins may be responsible for the lyoprotecting activity of trehalose toward polymeric micelles. De Jaeghere et al. proposed that a trehalose matrix might maintain a pseudo-hydrated state of the PEO chains of polymeric micelles, thereby preventing PEO crystallization (14). Under the conditions described here, it is feasible that micelle integrity might be preserved during the freeze-drying process and in the solid-state, enabling the relative aggregation state of encapsulated AmB to be maintained following reconstitution.
In the presence of trehalose, the micelles reconstitute rapidly and completely into isotonic solutions. Although initial screening studies were performed using various disaccharide lyoprotectants/bulking agent combinations, no benefit was observed beyond that of adding trehalose alone as a lyoprotectant and tonicity adjuster. The quantity of trehalose was chosen in order to impart isotonicity following the addition of 1 mL of water. The composition of reconstituted, AmB-loaded PEO-b-p(N-HA)-acyl micelles are given in Table 1. Vapor-pressure osmometry indicated that the osmolality upon reconstitution was 283.4 ± 3.5 mmol/kg for PEO-b-p(HASA)/AmB and 277.2 ± 2.7 mmol/kg for reconstituted PEO-b-p(HAHA)/AmB micelles.
SEC evidenced the formation of micelle structures, which were stable toward dilution on the column. Following injection on to the column, PEO-b-p(HASA) micelles eluted at 7.9 min., while micelles prepared from PEO-b-p(HAHA) eluted at 8.0 min. PEO-b-p(HAHA) and PEO-b-p(HASA) micelles had a weight-averaged molecular weight (Mw) of 4.50 ± 1.60 x 106 g/mol and 6.92 ± 1.15 x 106 g/mol, respectively. This finding is in line with published results (7, 9, 16).
Conclusions
The studies presented here provide insight into controlling the aggregation state of AmB by physical loading into polymeric micelles based on PEO-b-p(L-Asp) derivatives. Entrapment of AmB into PEO-b-p(HAHA) and PEO-b-p(HASA) micelles via the described assembly conditions yields a solid polymeric micelle formulation, which can be readily reconstituted to an isotonic solution. AmB can be loaded in a nearly monomeric state into PEO-b-p(HASA) micelles due to hydrophobic interaction with the stearate side chains. However, loading AmB into micelles prepared from the hexanoate esters results in little, if any, control of the aggregation state of the drug. These examples illustrate the importance of core/drug compatibility for effective solubilization. In addition, the examples given here demonstrate the need for chemically tailored polymeric micelles as well as the development of effective assembly conditions based on drug/polymer interactions.
Under the described conditions, it is possible to maintain the aggregation state of encapsulated AmB during lyophilization and reconstitution. A less self-aggregated form of AmB may ultimately lead to decreased toxicity toward mammalian cells, while maintaining potent antifungal activity. To this end, the preparation of a reconstitutable polymeric micelle formulation of nonaggregated AmB may be a viable strategy for treating systemic fungal disease.
Acknowledgements
This research was by supported by NIH grant AI43346-01. The authors wish to thank Professor Joseph Robinson of the University of Wisconsin-Madison School of Pharmacy for the use of his osmometer. The authors also wish to thank Dr. Ichiro Nakatomi of NanoCarrier, Inc. (Japan) for kindly donating the starting material for PEO-b-p(6-HHA).
References
Adams, M.L.; Lavasanifar, A. and Kwon, G.S. Amphiphilic block copolymers for drug delivery. J Pharm Sci, 92:1343-1355, 2003.
Allen, C.; Maysinger, D. and Eisenberg, A. Nano-engineering block copolymer aggregates for drug delivery. Col Surf B: Biointerfaces, 16:3-27, 1999.
Kataoka, K.; Harada, A. and Nagasaki, Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Del Rev, 47:113-131, 2001.
Li, Y and Kwon, G.S. Methotrexate esters of poly(ethylene oxide)-block-poly(2-hydroxyethyl-L-aspartamide). Part I: Effects of the level of methotrexate conjugation on the stability of micelles and on drug release. Pharm Res, 17:607-611, 2000.
Yokoyama, M.; Satoh, A.; Sakurai, Y.; Okano, T.; Matsumura, Y.; Kakizoe, T. and Kataoka, K. Incorporation of water-insoluble anticancer drug into polymeric micelles and control of their particle size. J Contr Rel, 55:219-229, 1998.
Adams, M.L. and Kwon G.S. The effects of acyl chain length on the micelle properties of poly(ethylene oxide)-block-poly(N-hexyl-L-aspartamide)-acyl conjugates. J Biomater Sci, Polym Ed, 13:991-1006, 2002.
Adams M.L. and Kwon G.S. Relative aggregation state and hemolytic activity of amphotericin B encapsulated by poly(ethylene oxide)-block-poly(N-hexyl-L-aspartamide)-acyl conjugate micelles: effects of acyl chain length. J Contr Rel, 87:23-32, 2003.
Lavasanifar, A.; Samuel, J.; Sattari, S. and Kwon, G.S. Block copolymer micelles for the encapsulation and delivery of amphotericin B. Pharm Res, 19:418-422, 2002.
Adams, M.L.; Andes, D.R. and Kwon, G.S. Amphotericin B encapsulated in micelles based on poly(ethylene oxide)-block-poly(L-amino acid) derivatives exerts reduced in vitro hemolysis, but maintains potent in vivo antifungal activity. Biomacromol, 4:750-757, 2003.
Gruda, I. and Dussault, N. Effects of the aggregation state of amphotericin B on its interaction with ergosterol. Biochem Cell Bio, 66:177-183, 1988.
Nielsen, L.J.L. Effect of fatty acid chain length of poly(ethylene oxide)-block-poly(hexyl-aspartamide amino acid) micelles on the encapsulation and aggregation state of amphotericin B. Master’s thesis, The Royal Danish School of Pharmacy, Copenhagen, DK, 63, 2001.
Szoka, F.C.T. and Tang, M. Amphotericin B formulated in liposomes and lipid-based systems: a review. J Liposome Res, 3:363-375, 1993.
Abu-saleh, K.M. Amphotericin B: an update. Brit J Biomed Sci, 53:122-133, 1996.
De Jaeghere, F.; Allemann, E.; Leroux, J.C.; Stevels, W.; Feijen, J.; Doelker, E. and Gurny, R. Formulation and lyoprotection of poly(lactic acid-co-ethylene oxide) nanoparticles: influence on physical stability and in vitro cell uptake. Pharm Res, 16:859-866, 1999.
De Jaeghere, F.; Allemann, E.; Feijen, J. ; Kissel, T. ; Doelker, E. and Gurny, R. Freeze-drying and lyopreservation of diblock and triblock poly(lactic acid)-poly(ethylene oxide) (PLA-PEO) copolymer nanoparticles. Pharm Devel Tech, 5:473-483, 2000.
Lavasanifar, A.; Samuel, J. and Kwon, G.S. Micelles self-assembled from poly(ethylene oxide)-block-poly(N-hexyl stearate L-aspartamide) by a solvent evaporation method: effect on the solubilization and haemolytic activity of amphotericin B. J Contr Rel, 77:155-160, 2001.
Corresponding Author: Glen S. Kwon, University of Wisconsin-Madison, School of Pharmacy, Division of Pharmaceutical Sciences, 777 Highland Avenue, Madison, WI 53705-2222, USA. gsk@pharmacy.wisc.edu
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps