J Pharm Pharmaceut Sci (www.cspscanada.org) 7(4):29-34, 2004
Preliminary results of nanopharmaceuticals used in the radioimmunotherapy of ovarian cancer.
Steve McQuarrie1, John Mercer, Alasdair Syme, Mavanar Suresh, Gerald Miller
Faculty of Pharmacy and Pharmaceutical Sciences; Noujaim Institute for Pharmaceutical Oncology Research, University of Alberta, Edmonton, Alberta, CanadaReceived 16 July 2004, Revised 17 December 2004, Accepted 19 January 2005, Published 10 February 2005
PDF Version
Abstract
PURPOSE: The treatment of late stage ovarian cancer presents an unmet clinical need for women around the world. A multistep radioimmunotherapeutic (RIT) approach, exploiting the combination of a bispecific monoclonal antibody (BsMAb) with 90Y labelled biotinylated long-circulating liposomes was tested as a potential adjuvant treatment for epithelial ovarian carcinomatosis in an attempt to meet this need. This approach was used to overcome some of the major obstacles associated with conventional strategies, in particular, to increase the amount of radioactivity delivered to the tumor site compared with conventional monoclonal antibody (MAb) radionuclide delivery. We hypothesize that sequential intraperitoneal administration of the targeting and therapeutic moieties provides the basis for an enhanced therapeutic ratio. METHODS: A BsMAb, with anti-CA 125 and anti-biotin epitopes was engineered for use with PEGylated liposomes coated with biotin to deliver the cytotoxic radionuclide 90Y to tumor sites. An in vivo therapy trial was used to test this RIT protocol with Balb/c nude mice (n=29) xenografted with the NIH:OVCAR-3 (CA 125+) human ovarian cancer cell line. RESULTS: A median tumor growth delay of 91 days for the combined treatment group versus 77.7 days for the control group was observed. CONCLUSION: An ongoing tumor growth delay/control study using this model has indicated an appreciable delay in progress of tumor and ascites development in treated vs. control populations.
Purpose
Ovarian cancer is the deadliest of the gynecologic malignancies and it accounts for an anticipated 2,300 new cases and 1,550 deaths per annum in Canada (1). If detected early, ovarian cancer is highly amenable to treatment, however, the majority of cases are not diagnosed until they have progressed to stage III or later. The prognosis for late stage ovarian cancer is poor with a five year survival of approximately 20% for stage III/IV disease (2). Cytoreductive surgery and chemotherapy are currently the main forms of treatment (3) with external beam radiotherapy used in selected instances (4). Although there is no standard treatment for recurrent ovarian cancer (5), patients may undergo further radiotherapy, chemotherapy or a combination of the two. Another option is enrollment in a clinical trial of a novel therapeutic agent. Ovarian malignancies generally spread to the peritoneal cavity and current research is directed toward effective management or cure of ovarian carcinomatosis.
Intraperitoneal (i.p.) radionuclide therapy is a treatment modality that involves the administration of an unsealed radioactive source into the peritoneal cavity for the purpose of treating malignant disease. The targets of i.p. radionuclide therapy are two-fold: single cells and clusters of cells that are floating in the peritoneal fluid and seeded tumor nodules that are found on the surfaces of the peritoneal cavity. This type of therapy was practiced for palliative purposes as early as 1945 (6) when radioactive gold (Au-198) colloid was used to treat malignant effusions in the peritoneal cavity. This radionuclide produced some successful results but also resulted in a number of serious complications and deaths (7). Investigators were subsequently drawn to 32P for its high energy (Ebmax = 1.71 MeV) beta emission and lack of gamma emissions (8). In a study comparing cisplatin chemotherapy with i.p. radionuclide therapy and whole-abdomen external beam irradiation, Vergote and co-workers observed similar survival rates in all groups (9). They concluded that cisplatin was a more appropriate choice for therapy because the 32P produced a higher incidence of bowel complications. A recent study by Young and co-workers (10) confirmed these findings. Problems with radioactivity distribution within the peritoneal cavity and small bowel perforation from the 32 P led them to conclude that chemotherapy was a more attractive treatment option. Research into i.p. administration of radioactivity for the treatment of ovarian cancer continues to evolve with the goal of destroying tumor tissue with minimal consequences for the surrounding healthy tissue. Efforts to spare healthy tissue have resulted in developments that include more appropriate radionuclide selection, improved retention of radioactivity in the peritoneal cavity and enhanced tumor selectivity.
We are currently investigating the application of nano-radiopharmaceuticals as an adjuvant therapy for late-stage ovarian cancer. Our approach uses multistep radioimmunotherapy exploiting the combination of a bispecific monoclonal antibody (BsMAb) with 90Y labelled biotinylated long-circulating liposomes (mean diameter 210nm). Several protocols were tested as potential adjuvant treatments for epithelial ovarian carcinomatosis. The BsMAb, with anti-CA 125 and anti-biotin epitopes was used with PEGylated liposomes coated with biotin to deliver the cytotoxic radionuclide to tumor sites. This approach was used to overcome some of the major obstacles associated with conventional strategies, in particular, to increase the amount of radioactivity delivered to the tumor site compared with conventional monoclonal antibody (MAb) radionuclide delivery.
We have previously reported the development of the BsMAb and liposome delivery systems as well as the results from in vitro testing of this novel strategy (11,12). Therapeutic radiation doses were calculated based on our recent work in microdosimetry and radiation dose model development for intraperitoneal targeted liposome radioimmunotherapy of ovarian cancer metastases (13). We report here the results of an in vivo therapy using our RIT approach as well as the results of our 18F-fluorodeoxyglucose (FDG) positron emission tomographic metabolic imaging protocol that was used to assess tumor burden prior to end-point euthanasia.
Methods
Liposomes
Liposomes used for this work were made from the following constituents: 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), cholesterol (Chol), 1,2-distearoyl- sn-glycero-3-phospho-ethanolamine-N-[biotinyl (polyethylene glycol) 2000] (ammonium salt) (DSPE-PEG-Biotin) and 1,2-dimyristoyl- sn-glycero-3-phosphoethanol-amine-N-DTPA (DMPE-DTPA) in molar ratios of (2: 1: 0.1: 0.1) respectively. Liposome components were purchased from Avanti Polar Lipids (Alabaster, AL, USA). The phospholipid mixture was dissolved in chloroform, evaporated to obtain a thin film and remove traces of moisture. The phospholipid film was rehydrated with 1M sodium acetate saline at pH 6 (2 mL) and the sample was passed through a Lipex Biomembranes Inc. extruder (Vancouver, BC, Canada) 8 times; the 0.2 micron filter was changed after the second passage. After extrusion, 1 mL of the liposome preparation was eluted through a PD-10 column equilibrated with 1M sodium acetate buffer, pH 6 to yield a homogenous population of liposomes. The liposome fractions were collected and their size distribution was determined using a Malvern Instruments Zetasizer 3000HSA particle sizer (Malvern, Worcestershire, UK). The mean diameter of the liposomes was 214 nm with a polydispersity of 0.07. Liposomes were passed through a 0.4 mm filter to sterilize the preparation prior to labeling with 90Y. The size exclusion chromatography columns used for this work were disposable PD-10 columns from Amersham Biosciences (Uppsala, Sweden).
Bispecific Monoclonal Antibodies
The MAb, B27.1 (Biomira Inc., Edmonton) used this study, was a murine IgG1 with k light chains, which were produced by immunizing mice with a partially purified CA-125 antigen (14). The antibody recognizes the glycoprotein CA-125 antigen on OVCAR-3 cell surfaces.
The BsMAb construct was produced by succinimidyl 3-(2-pyridyldithio) propionate (SPDP, Molecular Probes Inc., Eugene OR) cross-linking of FAb fragments of the B27.1 MAb with the IgG P54 (a murine anti-biotin MAb produced in our lab). The FAb fragments were prepared by digestion with immobilized papain, according to the Pierce (Rockford IL) protocol 20341 for the papain product. FAb fragments were purified from whole IgG and Fc fragments on a Protein A column. They were crosslinked with whole P54 MAb using SPDP (Molecular Probes Inc., Eugene OR). Activity of the construct was determined by ELISA assay.
Radiolabelling with 90Y
Yttrium-90 was purchased as 90YCl3 from PerkinElmer Life and Analytical Sciences (Billerica, MA, USA). The volume of the 90YCl3 was expanded as necessary in 0.05M HCl. A total of 10 mL each of 90YCl3, sterile saline and liposomes in Na-acetate were mixed together and left to react for 3 hours. The volume of the sample was then expanded to approximately 300 mL with sterile saline and then passed down a PD-10 column equilibrated with sterile saline to separate the labeled liposomes from the unbound 90Y. Fractions were collected in 1 mL volumes and the activity per fraction was determined by Bremsstrahlung counting in a gamma counter.
Animal Protocol
Approximately 7 x 106 human ovarian cancer cells (NIH:OVCAR-3 cells, American Type Culture Collection, Rockville, MD, USA) bearing the CA-125 ovarian tumor marker were injected into the peritoneal cavities of female Balb/c nude mice. The animals were randomly sorted into 3 groups: a) controls, n=11, (sham-treated with sterile saline); b) treatment regimen 1, n=9, (1.3 MBq of 90Y labelled immunoliposmes in 0.5 mL sterile saline i.p. administered 24 hours after inoculation) and c) treatment regimen 2, n=9, (1.3 MBq of 90Y labelled immunoliposmes in 0.5 mL sterile saline i.p. administered 96 hours after inoculation). In vitro binding assays of the immunoliposomes with CA 125 were developed and are reported elsewhere (12). The same routine production techniques were used for this batch of immunoliposomes. The radioactivity injected was based on microdosimetry radiation dose calculations (13).
The mice were monitored closely on a daily basis for signs of onset of the disease, and any disease or treatment-related illness. The endpoints of this experiment were: a) development of ascites fluid in the peritoneal cavity, with confirmation 24h subsequent to first detection; b) growth of solid tumors to a diameter of 10 mm in a single axis (using calipers); or c) euthanasia of asymptomatic animals 140 days subsequent to tumor inoculation. When ascites fluid develops in the mice, it is clearly visible, and the animals are euthanized when they reach a body mass of 28 g (end point a). In a minority of animals, solid tumors develop in the abdomen. Ascites fluid production is usually suppressed in these animals. The tumors are visible and palpable, and monitoring to avoid tumor-related discomfort was performed on a daily basis. For both clinical endpoints, the animals were euthanized before significant illness developed.
The mean time (days) to observation of 1) tumors or ascites and 2) euthanasia for the control and treatment groups was compared using a heteroscedastic t-test, which assumes that the variances of both ranges of data are unequal. This t-test was chosen a priori as the effect of therapy was unknown and variances between data sets were assumed to be equal. Subsequent analysis using the nonparametric Mann-Whitney test was performed in an attempt to take into account the large variances in the sample mean that might be due to non-normal distributions in some of the data.
In order to establish proof-of-concept that 18F-deoxyglucose-mediated PET scanning is a viable means of detecting peritoneal micrometastases in ovarian carcinomatosis, the tumor burden of a subset of the each group was assessed using positron emission tomography (PET) and 18F-flurodeoxyglucose (FDG) on a MicroPET animal scanner (Concorde Microsystems, Knoxville, TN). Animals in each group were selected when palpable tumours were observed; typically 8 to 12 weeks post inoculation. Clinical grade FDG was obtained using a Coincidence Technologies automated synthesis unit (Bioscan, Washington, DC). Approximately 50 MBq of FDG was injected under general anesthesia via the lateral tail vein 45 minutes prior to imaging; imaging time was 30 minutes.
All experimental protocols with animals were pre-approved by the University of Alberta Health Sciences Animal Policy and Welfare Committee using guidelines established by the Canadian Council on Animal Care.
Results and Discussion
Ovarian cancer usually spreads via cellular shedding into the peritoneal cavity followed by implantation on the peritoneal surface. Traditional therapy typically involves cytoreductive surgery in which the metastatic implants of ovarian cancer that typically involves the peritoneal surfaces and is often amenable to resection, are removed along with the primary tumor mass. Following surgery, platinum-based therapy--either cisplatin or carboplatin--in combination with paclitaxel has demonstrated a high anticancer activity (3,15).
The treatment strategy used in this experimental protocol was based on a potential adjuvant therapy regimen for those patients with peritoneal carcinomatosis. When used as an adjuvant to traditional therapy, our proposed multistep radioimmunotherapy is designed to reduce the population of single-cell ovarian cancer cells and micrometastases contained in the ascites or seeded in the peritoneal cavity. To mimic this strategy in our animal model, the treatment was started 24h and 96h after the mice were inoculated with the NIH:OVCAR-3 cells. As this was a limited pilot trial designed to ascertain the potential effectiveness of this form of therapy, we chose to compare two treatments against an untreated control in order to determine whether there was any treatment advantage to our approach and to confirm the estimated radiation dose effects of 90Y. The two treatment arms were chosen to reflect a possible clinical protocol in which the radioimmunotherapy would commence shortly after cytoreductive surgery and peritoneal lavage. Previous biodistribution studies with 99mTc, 125I and 188Re-labelled MAbs (anti-CA 125 and nonspecific), free radionuclide ( 188 Re-perrhenate) and non-targeted 188Re-liposomes were performed to confirm that treatment was confined to the peritoneal cavity. Our results were similar to that reported by others, free radioactivity and radiolabelled MAbs escaped the peritoneal cavity more easily and distributed throughout the body (16-19).
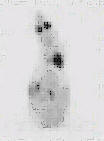
The assessment of tumor burden using 18F-FDG was tested as a potential method of identifying both peritoneal and distance metastases to aid in selected those animals scheduled for euthanasia and thereby assist in the avoidance of tumor-related morbidity. A typical scan is shown in Figure 1 and based this scan, the animal was euthanized.
Figure 1: 18F-FDG (50MBq) PET scan of a Balb/c nude mouse (control group). Tumors visible in the lower left quadrant of the abdomen were confirmed at necropsy. Note: "Click" on image to view in 3D cine mode.
The tumor-growth delay data are presented graphically in Figure 2.
Figure 2: Tumor growth delay profile in NIH:OVCAR-3 tumor bearing Balb/c nude mice. The treatment groups were combined for this figure as there was no significant difference between the treatment groups.
The experiment was terminated at 140 days, at which time the remaining mice were euthanized and sent for necropsy. At the end of the study, 3 control mice had peritoneal tumors (> 2 mm) and 2 of the 3 mice remaining mice in Regimen 2 had no visible tumors while the third had peritoneal tumors (> 2 mm). There were no mice remaining in Regimen 1 at the end of the study. The mean time to the observation of tumors greater than 2mm or the presence of ascites was 64.2 ± 44.7 days for the control group, 72.0 ± 36.7 days for treatment Regimen 1 and 92.8 ± 46.2 days for treatment Regimen 2. The mean survival time (time to euthanasia) for the control group was 77.7 ± 41.8 days, and 95.7 ± 31.9 days for treatment Regimen 1 and 106.6 ± 37.9 days for treatment Regimen 2. A heteroscedastic t-test for unequal variances was not significant (p = 0.05) for comparison between either the controls and the two treatment regimens; or between the two treatment regimens and was attributed to the wide range in the data. In an attempt to take into account the fact that the data may not be normally distributed, for example, due to variations in tumor take, a Mann-Whitney nonparametric test was performed on the same data sets; no significance was found (p = 0.05).
Conclusion
This initial tumor growth delay/control study in Balb/c mice xenografted intraperitoneally with the NIH:OVCAR-3 cell line using 90Y labelled biotinylated long-circulating liposomes indicated a delay in onset of tumor and ascites development in treated vs. control populations. There was a minor trend to reduced frequency of tumor `takes" in treated vs. control mice (83.3% vs. 90.9%, respectively). Median time to euthanasia was favorable in treated mice (104 days vs. 62 days in controls). The mean time to euthanasia was 77.7 ± 41.8 days for control animals, vs . 101.2 ± 32.9 days for treated animals. Although the difference in time to euthanasia was not significant, the results are encouraging. Further experiments are planned to monitor and optimize the therapeutic effect as a function radiation dose, timing of the multistep RIT protocol and initial tumor burden leading a delay in onset of tumor and ascites development in treated vs. control populations.
Acknowledgements
The authors wish to thank Natural Science and Engineering Research Council of Canada and the Alberta Cancer Board for their financial support of this project and the Alberta Heritage Foundation for Medical Research for their graduate student support for A Syme. Expert animal care from Gail Hipperson and Dan McGinn of the Cross Cancer Institute Vivarium is greatly appreciated.
References
National Cancer Institute of Canada 2004, Canadian Cancer Statistics p. 18, 2004.
Whitehouse C and Solomon E. Current Status of the Molecular Characterization of the Ovarian Cancer Antigen CA125 And Implications For Its Use In Clinical Screening. Gynecol. Oncol. 88:S152 – S157, 2003.
Trimble E, Berry D, Gore M, Kavanagh J, Cohen C, Pecorelli S, Creasman W, Mason P and Heinz P. Discussion: Current Issues In The Design Of Ovarian Cancer Treatment Trials. Gynecol. Oncol. 88:S122 – S123, 2003.
MacGibbon A, Bucci J, MacLeod C, Solomon J, Dalrymple C, Firth I and Carter J. Whole Abdominal Radiotherapy Following Second-Look Laparotomy For Ovarian Carcinoma. Gynecol. Oncol. 75:62 – 67, 1999.
Baker V V. Salvage Therapy For Recurrent Epithelial Ovarian Cancer. Hematol. Oncol. Clin. N. 17 977 – 988, 2003.
Croll M N and Brady L W. Intracavitary Uses Of Colloids. Semin. Nucl. Med. IX:108 – 113,1979.
Kolstad P, Davy M and Hoeg K. Individualized Treatment Of Ovarian Cancer. Am. J. Obstet. Gynecol. 128:617 – 625, 1977.
Rosenshein N B. Radioisotopes In The Treatment Of Ovarian Cancer. Clin. Obstet. Gynecol. 10:279 – 295, 1983.
Vergote I B, Vergote-De Vos L N, Abeler V M, Aas M, Lindegaard M W, Kjorstad K E, and Trope C G. Randomized Trial Comparing Cisplatin With Radioactive Phosphorus Or Whole Abdomen Irradiation As Adjuvant Treatment In Ovarian Cancer. Cancer. 69:741 – 749, 1992.
Young R C, Brady M F, Nieberg R K, Long H J, Mayer A R, Lentz S S, Hurteau J and Alberts D S. Adjuvant Treatment For Early Ovarian Cancer: A Randomized Phase III Trial Of Intraperitoneal 32P Or Intravenous Cyclophosphamide And Cisplatin – A Gynecologic Oncology Group Study. J. Clin. Oncol. 21:4350 – 4355, 2003.
McQuarrie SA, Xiao Z, Miller GG, Mercer JR and Suresh MR. Modern Trends in Radioimmunotherapy of Cancer: Pretargeting Strategies for the Treatment of Ovarian Cancer, Quart J Nucl Med, 45:160-166, 2001.
Xiao Z, McQuarrie, SA, Suresh MR, Mercer JR, Gupta S and Miller GG. A three–step strategy for targeting drug carriers to human ovarian carcinoma cells in vitro. J. Biotechnology, 94:171-184, 2002.
Syme A, McQuarrie SA and Fallone G, Beta Dose-Rate Distributions In Microscopic Spherical Tumors For Intraperitoneal Radioimmunotherapy; Int J Radiat Oncol Biol Physics, 56:1495-1506, 2003.
Krantz MJ, MacLean GD, Longenecker BM and Suresh MR. Radioimmunoassay for CA125 employing two new monoclonal antibodies. J. Cell Biochem. 12E (Suppl.) 139, 1988.
McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med; 334:1-6, 1996.
Andersson H. Palm S. Lindegren S. Back T. Jacobsson L. Leser G. Horvath G. Comparison of the therapeutic efficacy of 211At- and 131I-labelled monoclonal antibody MOv18 in nude mice with intraperitoneal growth of human ovarian cancer. Anticancer Re. 21(1A):409-12, 2001.
Breitz HB. Durham JS. Fisher DR. Weiden PL. DeNardo GL. Goodgold HM. Nelp WB. Pharmacokinetics and normal organ dosimetry following intraperitoneal rhenium-186-labeled monoclonal antibody. J Nucl Med 36(5):754-61, 1995.
Syrigos KN. Vile RG. Peters AM. Harrington KJ. Biodistribution and pharmacokinetics of 111In-dTPA-labelled pegylated liposomes after intraperitoneal injection. Acta Oncologica. 42(2):147-53, 2003.
Allen TM. Hansen CB. Guo LS. Subcutaneous administration of liposomes: a comparison with the intravenous and intraperitoneal routes of injection. Biochimica et Biophysica Acta. 1150(1):9-16, 1993.
Corresponding Author: Steve McQuarrie, Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta, Edmonton, Alberta, Canada. steve.mcquarrie@ualberta.ca
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps