J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 8(1):10-17, 2005
Sodium citrate cross-linked chitosan films: optimization as substitute for human/rat/rabbit epidermal sheets.
Vikas Rana
Department of Pharmaceutical Sciences, Government Polytechnic for Women, Patiala, IndiaKumar Babita
Department of Pharmaceutical Sciences and Drug Research, Punjabi University, Patiala, IndiaDinesh Goyal
Department of Biotechnology and Environmental Sciences, Thapar Institute of Engineering and Technology, Patiala, IndiaAshok Tiwary1
Department of Pharmaceutical Sciences and Drug Research, Punjabi University, Patiala, IndiaReceived 18 August 2004, Revised 24 November 2004, Accepted 24 November 2004, Published 23 December 2004
PDF Version
Abstract
PURPOSE. To prepare chitosan films that shall be capable of simulating in vitro permeation of polar (5-FU) and non polar (indomethacin) drugs across rat/rabbit/human epidermis. METHOD: Statistical designs were utilized to identify the formulation and process variables that significantly influenced permeation of both drugs across chitosan films cross-linked with sodium citrate (Nacit). In addition, atomic absorption spectroscopy for Na+ and differential scanning calorimetry of these films were performed for understanding the cross-linking behaviour. RESULTS: Concentration of chitosan, cross-linking time and concentration of cross-linking agent significantly influenced the in vitro flux of both drugs. Na+ content in films cross-linked with Nacit solutions without adjustment of pH was found to increase whereas, DH of cross-linking endothermic transition decreased. However, after dipping these films in phosphate buffer (pH 7.4) both Na+ and DH was found to decrease. Such a decrease was not observed when films cross-linked with sodium citrate solutions after adjustment to pH 5 were dipped in phosphate buffer (pH 7.4). The in vitro permeation of both drugs across latter films was significantly less. CONCLUSION: Adjustment of Nacit solution pH to 5 produced ionic complexes that were resistant to alkaline pH. Chitosan films can be cross-linked with Nacit to simulate in vitro permeation of polar and non polar drugs across animal/human epidermis.
Introduction
Development of a transdermal drug delivery system requires data from in vitro permeation experiments for assessing the permeation of drug molecules and predicting their in vivo performance in humans.
These studies require liberal use of animal skin. However, animal skin differs significantly from human skin in terms of thickness, nature of stratum corneum, density of hair follicles and sweat glands (1). Therefore, the obtained data often cannot be meaningfully extrapolated to human skin. Further, inter-species and intra-species (normal vs diseased skin) differences in skin microconstituents influence reliability of the data generated (2). In addition, the role of race, sex, age, anatomical site etc. in influencing the reproducibility of data cannot be neglected. Also, restricted availability of animal skin and ethico-legal issues associated with the use of human skin makes it imperative to search for their substitutes.
Artificial membranes possess distinct advantage over biological membranes due to controlled composition, ease of preparation and reproducibility of results (3). Therefore, such membranes have a great potential to be developed as substitutes of animal and human cadaver skin.
Chitosan is a bio-polysaccharide derived from chitin, a polymer from bio-waste of shellfish industry. It is reported to form complex with sodium carboxymethylcellulose, citrates, pectin, acacia, agar, sodium caprylate, stearic acid, glutaraldehyde, sodium tri-polyphosphate, lactic acid, malic acid and alginic acid (4-8). This property of complexed chitosan has been utilized in preparing beads (9, 10) and microspheres (11, 12). Chitosan citrate complex has been reported to delay the release of drugs from matrix (4, 13), microcapsule (14) and produced a pH dependent release of propranolol from coated tablets (15). Therefore, it can be envisaged that the permeation of drug molecules can be modulated across chitosan films cross-linked with sodium citrate.
The present investigation aimed at formulating chitosan films by cross-linking with sodium citrate that shall be capable of simulating the in vitro permeation of modal polar drug, 5-fluorouracil (5-FU) and non polar drug, indomethacin (INDO) across rat, rabbit, and human epidermis. Statistical design was employed to identify and optimize various process and formulation variables that significantly influenced permeation of both drugs across chitosan films. Chitosan films prepared by employing optimized process and formulation variables were found to simulate in vitro permeation of both drugs across animal and human epidermis.
Materials and Methods
Materials
Chitosan, 95% deacetylation (Central Institute of Fisheries Technology, Cochin, India), 5-fluorouracil (Dabur Research Foundation, Delhi, India), indomethacin (Crystal Pharmaceuticals, Ambala, India), sodium citrate and glacial acetic acid (Loba Chemie, Mumbai, India) were used as received. All other reagents were of analytical grade.
Methods
Preparation of epidermal sheets
Animal (rat/rabbit) skin was obtained after sacrificing them by administration of excess chloroform inhalation. Human cadaver skin (male) was obtained post-mortem within 6 h of death from the local medical college after obtaining consent of relatives of the deceased. The respective institutional ethical committees approved these protocols. Dorsal skin portion of albino Wistar rats or rabbits was shaved with electrical hair clipper and excised after sacrificing. Human cadaver skin was excised from the abdominal / chest portion. Epidermal sheet was separated by soaking the excised whole skin in phosphate buffer saline (PBS, pH 7.4) containing trypsin (0.1% w/v) at 60°C for 2 min.
Freshly prepared epidermal sheets were washed with PBS and conditioned by stirring in receptor solution for 4 h before commencing in vitro permeation experiments.
Formulation design and preparation of cross-linked chitosan films
Plackett Burman screening design was used to formulate chitosan films (P1-P8) for screening the effect of selected process and formulation variables on permeation parameters of 5-fluorouracil and indomethacin (Tables 1 and 2).
Table 1: Screening design for identifying active formulation and process variables influencing flux of indomethacin (INDO) and 5-fluorouracil (5-FU) across chitosan films cross-linked with sodium citrate without adjustment to pH 5.
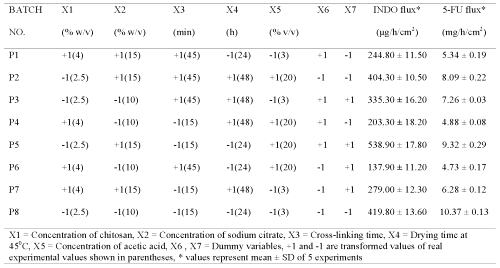
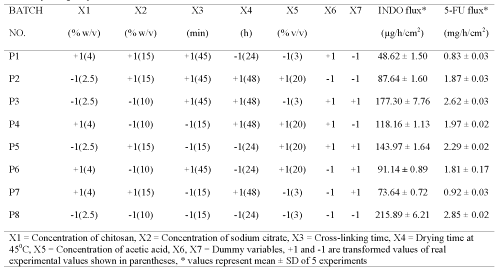
Table 2: Screening design for identifying active formulation and process variables influencing flux of indomethacin (INDO) and 5-fluorouracil (5-FU) across chitosan films cross-linked with sodium citrate after adjusting its pH to 5.
Additional films (1F-8F, 1S-6S, 1C-4C; Table 3) were prepared according to central composite design (CCD) using variables (concentration of chitosan, concentration of sodium citrate and cross-linking time) that were found to significantly influence the permeation of both drugs during initial screening studies (16).
Table 3: Central composite design using active formulation and process variables influencing flux of 5-fluorouracil (5-FU) and indomethacin (INDO) across chitosan films cross-linked with sodium citrate after adjusting the pH to 5.
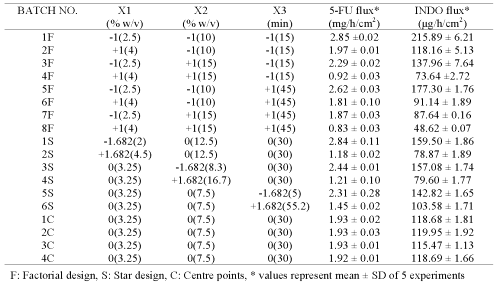
The permeation of both drugs was studied across these films and compared with that obtained across rat/rabbit/human epidermal sheets. Multiple linear regressions between active variables obtained from CCD and in vitro flux of both drugs were performed using Statistica software-5.0 (Sta Soft Inc., Tulsa, USA).
All the chitosan films were prepared by the following method:
Chitosan (2.5-4% w/v) solutions were homogenized in acetic acid (3-20% w/v) at 2000 rpm using a homogenizer (ROT-134S, Remi, Mumbai, India). These solutions were subjected to vacuum filtration (10 psi) through muslin cloth to remove debris. A portion (15 mL) was poured in a glass ring (cross sectional area 3.14 cm2) fitted on polycarbonate petri plate and subjected to drying. Dried films (45°C for 24-48 h) were stored in polyethylene bags till use. Cross-linking of chitosan films was done by dipping in a 10 mL solution of sodium citrate (1-20% w/v, without pH adjustment or after adjustment to pH 5) for 15-45 min. These films were washed with water to remove excess sodium citrate. Films that were insoluble in receptor solution (phosphate buffer pH 7.4) for more than 48 h were used for in vitro permeation experiments.
Physicochemical characteristics of films
Atomic absorption spectroscopy for Na+ in chitosan films
Chitosan films were prepared by dissolving chitosan (4% w/v) in 3% v/v acetic acid (15 mL) followed by drying at 45°C for 24 h. Cross-linking was done by dipping in 10 mL solution (1, 5, 10, 15 or 20% w/v sodium citrate) for 45 min. Each film was dissolved in aqua regia (2 mL) and evaporated to dryness on water bath. The residue was cooled, dissolved in 50% v/v HCl (10 mL) and filtered through G3 filter. The filtrate obtained was subjected to atomic absorption spectroscopy (932AAS, GBC, Victoria, Australia). The same procedure was employed for determining Na+ in cross-linked films obtained after dipping in PBS (pH 7.4) for 48 h.
Differential scanning calorimetric (DSC) analysis
Cross-linked chitosan films (as prepared for atomic absorption spectroscopy) were stored in a desiccator at RH 50% for 48 h. Then they were subjected to DSC studies (821E, Mettler Toledo Star System, Switzerland). A heating rate of 100C /min was used for all the samples.
In vitro permeation studies
An indigenously fabricated vertical Franz diffusion cell apparatus was used for studying in vitro permeation of both drugs. Chitosan films or epidermal sheets were clamped between donor and receptor compartments. The receptor compartment containing phosphate buffer (pH 7.4), sodium azide (0.5% w/v) and 5% v/v of PEG 400 was maintained at 37°C and stirred at 300 rpm. Either drug was suspended in propylene glycol (4 mL) and loaded in the donor compartment. Aliquots (1 mL) withdrawn at various intervals were immediately analyzed for 5-FU or INDO by HPLC (515W, Waters, Milford, USA) using spherisorb C18 column (4.6 ∞ 250 mm) and UV detector (2487 Dual wavelength). Sodium acetate (0.1% w/v) or methanol:10 mM citrate buffer (75:25) at flow rates of 0.6 mL/min or 1.0 mL/min were used as mobile phases for 5-FU and INDO, respectively. The respective detection wavelengths were 265 and 240 nm as reported by Sasaki et al. (17).
Results and Discussion
It has been reported that chitosan films cross-linked with sodium citrate solutions (pH 5.5-6.5) exhibited lowest swelling and released less than 40% model drugs from the matrix in simulated intestinal fluids over 24 h. This was suggested to be due to significant electrostatic attraction between chitosan and citrate because of increased ionization of sodium citrate in this pH range. In addition, both adjustment of sodium citrate solution pH below 4.5 and above 6.5 was found to produce very weak ionic bonds due to which the films swelled and exhibited faster drug release (18). Hence, adjustment of sodium citrate solution to pH 5 can be envisaged to only influence the ionization of sodium citrate unlike in solutions without pH adjustment where both degree of ionization and pH shall change with change in sodium citrate concentration. Therefore, chitosan films were prepared by cross-linking with different concentration of sodium citrate solutions both without pH adjustment and after adjustment to pH 5.
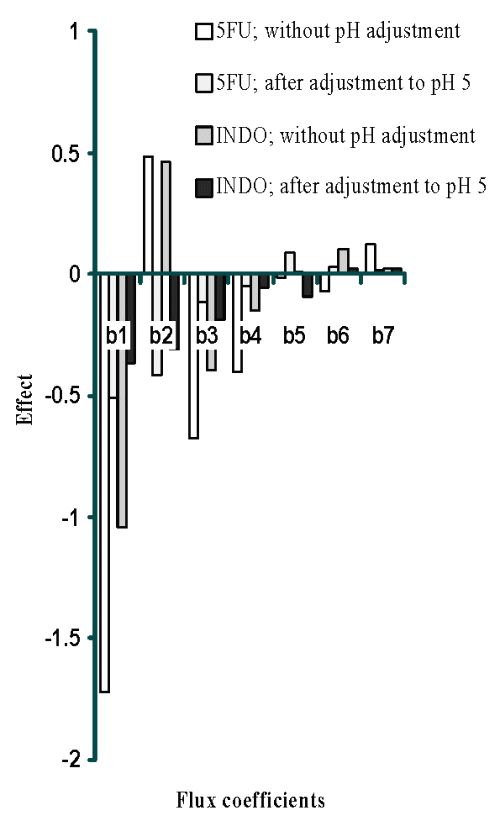
Plackett-Burman design (PBD) was employed to formulate chitosan films for screening the process and formulation variables that produced a significant (at 5% level of confidence) effect on flux (slope of linear portion of cumulative amount permeated vs time) of both drugs. The in vitro permeation of 5-FU and INDO across chitosan films was significantly influenced by concentration of chitosan, concentration of sodium citrate and cross-linking time when chitosan films were cross-linked by sodium citrate solutions either without pH adjustment (Table 1) or after adjustment to pH 5 (Table 2). This indicates that the influence of active process and formulation variables on in vitro permeation of both drugs remained unaltered even when chitosan films were cross-linked after pH adjustment of sodium citrate solutions. The flux of 5-FU across rat, rabbit and human epidermal sheets was found to be 2.05 ± 0.15, 2.47 ± 0.03 and 0.72 ± 0.03 mg/h/cm2, respectively. For INDO these values were 92.6 ± 2.5, 217.6 ± 7.5 and 34.2 ± 3.2 mg/h/cm2, respectively. The in vitro permeation of both drugs across chitosan films cross-linked by sodium citrate without pH adjustment (Tables 1 and 2) was significantly more than that across both rat and human epidermal sheets. Therefore, additional films were prepared by cross-linking chitosan films with sodium citrate solutions after adjusting them to pH 5. The coefficients (b1...b7) associated with the effect of various formulation and process variables on flux of both drugs across chitosan films are shown in Figure 1.
Figure 1: Effect of formulation and process variables on flux of 5FU and INDO [X10-2 ] across chitosan citrate films.
It is evident that increasing the concentration of chitosan, cross-linking agent or cross-linking time had a negative influence and reduced the permeation of both drugs across films cross-linked by dipping in sodium citrate solutions of pH 5. However, permeation of both drugs was found to increase with increase in concentration of cross-linking agent across films cross-linked with sodium citrate solutions when the pH was not adjusted to 5. This suggests an overwhelming role of pH adjustment of sodium citrate solutions in decreasing permeation of both drugs across cross-linked films possibly through modulation of the cross-linking behaviour.
Permeation of both drugs across additional films (Table 3) prepared according to central composite design (CCD) was studied and the data obtained was used for generating equations for optimizing the film formulation (Table 4) in order to mimic the flux of both drugs across animal and human epidermal sheets (Table 5).
Table 4: Equations for relating influence of concentration of chitosan (X1), concentration of cross-linking agent (X2) and cross-linking time (X3) on flux of 5-FU (Y1) and INDO (Y2) across sodium citrate (pH adjusted to 5.0) cross-linked chitosan films.
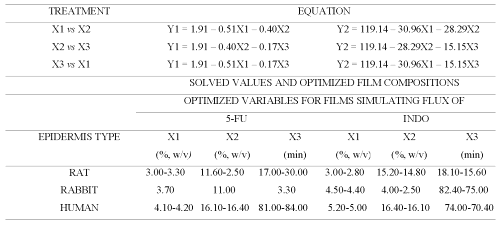
Table 5: Optimized composition of cross-linked chitosan films capable of mimicking 5-FU and INDO flux across rat, rabbit and human epidermis.
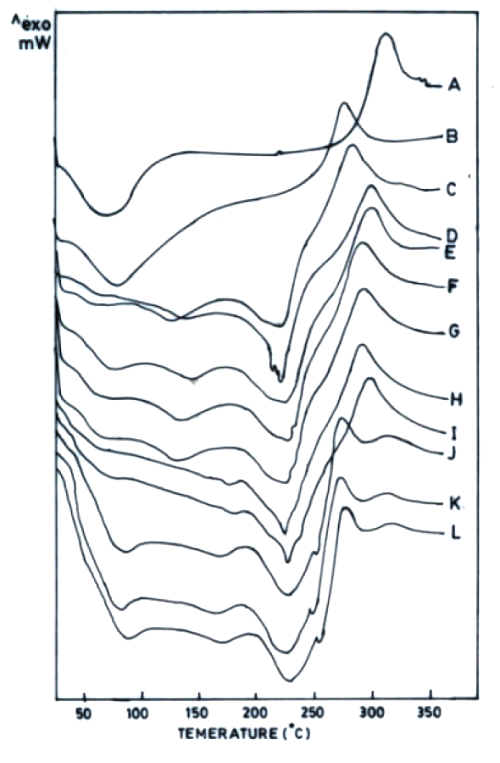
The thermograms of films cross-linked with sodium citrate solutions either after adjustment to pH 5 or without pH adjustment exhibited one exothermic and three endothermic peaks (Figure 2).
The first endotherm (68 ± 5.6°C) can be ascribed to loss of water as the films were hydrated (37°C, RH 45%). This endotherm was broader in chitosan acetate films probably due to combined influence of moisture and acetic acid (Figure 2B). Chitosan citrate films are reported to exist in crystalline form (19). Therefore, the second endotherm appears to be due to some sort of transition of chitosan citrate from crystalline to amorphous form. The third endotherm can be ascribed to melting of chitosan citrate (Figures 2C- 2F).
Figure 2: DSC thermograms of chitosan powder (A), chitosan acetate film (B) and chitosan (4% w/v) films cross-linked by various concentrations of sodium citrate (% w/v) without adjustment to pH 5 (C-1%, D-5%, E-10%, F-15%, G-20%) and sodium citrate solutions after adjustment to pH 5 (H-1%, I-5%, J-10%, K-15%, L-20%). All films were hydrated at 37°C (RH 45%).
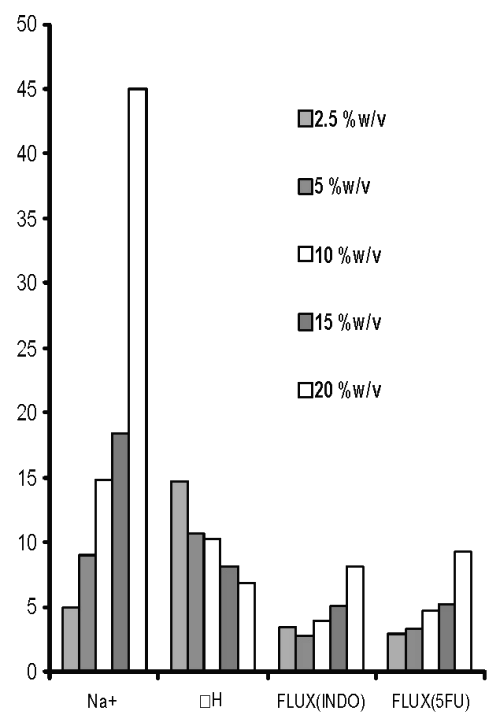
As varying the concentration of cross-linking agent altered the ΔH of the third endothermic transition, this peak seems to be indicative of the cross-linking behaviour of chitosan citrate films. In addition to the endotherms, all these films were observed to produce only one exotherm that was also obtained in chitosan powder / chitosan acetate, indicating it to be characteristic of chitosan degradation. Increased Na + content in chitosan films cross-linked with increasing concentration of sodium citrate was accompanied with decrease in ΔH of endothermic transition around 220°C (third endotherm). The permeation of both drugs across films was enhanced when cross-linking sodium citrate solutions were not adjusted to pH 5 (Figure 3).
Figure 3: Sodium content (Na+, mg/film), third endothermic DH (J/g) and permeation of 5-FU (mg/h/cm2 X 10-2 ) and INDO (mg/h/cm 2 X 10-1 ) across chitosan films prepared with different concentrations of sodium citrate solutions without pH adjustment.
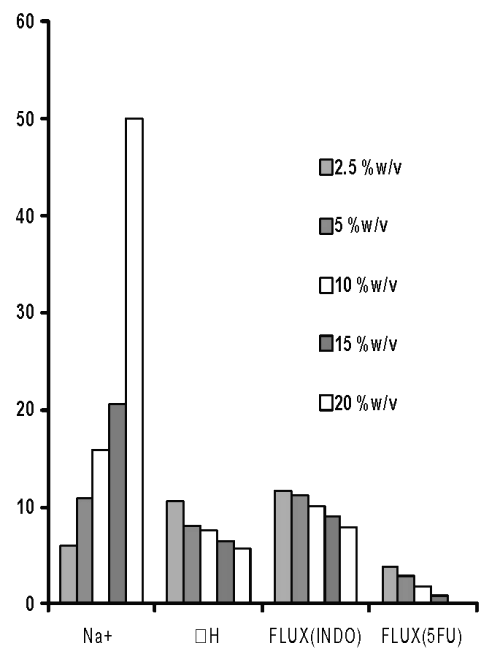
However, when the pH of these solutions was adjusted to pH 5, the permeation of both drugs was found to decrease (Figure 4).
On the other hand, the Na + content was invariably found to increase with an increase in concentration of cross-linking agent irrespective of the fact whether it was adjusted to pH 5 or not. Theoretically, this can be expected to be due to formation of more ionic complexes with increasing concentration of sodium citrate that shall enhance cross-linking.
Figure 4: Sodium content (Na+, mg/film), third endothermic DH (J/gm) and permeation of 5-FU (mg/h/cm2 X 10-2) and INDO (mg/h/cm 2 X 10 -1 ) across chitosan films prepared with different concentrations of sodium citrate solutions after adjustment to pH 5.
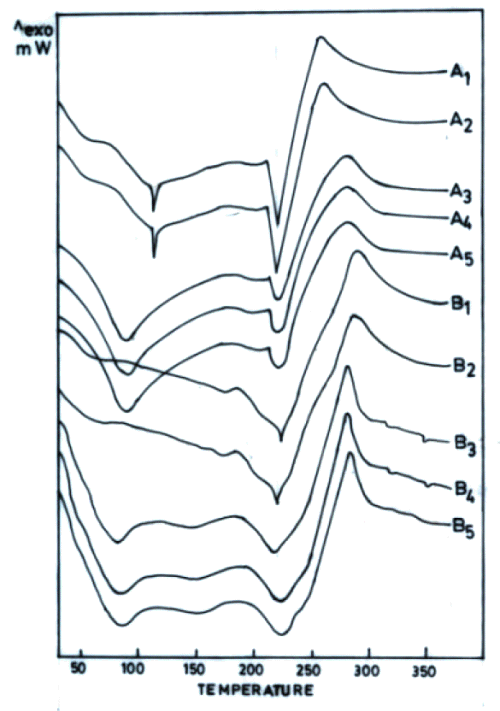
However, the increased permeation of both drugs across films cross-linked with increasing concentration of sodium citrate without adjustment of its pH to 5 cannot be explained on the basis of this hypothesis. DSC thermograms of these films after dipping in phosphate buffer (pH 7.4) revealed a 78.7 % to 88.5 % reduction in DH of the third endotherm (Figure 5).
This indicates that during in vitro permeation experiments the cross linking ionic complexes would have broken down and this accounted for enhanced permeation of both drugs. Moreover, the Na+ content in these films (dipped in phosphate buffer, pH 7.4) was also found to decrease significantly as compared to the freshly prepared films thereby, substantiating the above contention. Therefore, the cross-linking of chitosan with sodium citrate in films prepared by utilizing sodium citrate solutions without adjustment to pH 5 appears to be too weak to restrict the in vitro permeation of either drug till 48 h.
However, the DH of the third endothermic transition in films prepared by cross-linking with sodium citrate solutions adjusted to pH 5 was found to decrease by only 23.3 % to 13.5% after dipping in phosphate buffer. This indicated that the ionic complexes formed by cross-linking chitosan films with sodium citrate solutions of pH 5 were stable. Hence, stronger cross-linking of chitosan by increasing concentration of sodium citrate solutions seems to restrict the permeation of both drugs (Figure 4).
Figure 5: DSC thermograms of chitosan (4% w/v) films cross-linked with various concentrations sodium citrate (% w/v) without pH adjustment (A) and after adjustment to pH 5 (B) followed by dipping in phosphate buffer pH 7.4 for 48 h. [A1, B1-1%, A2, B2-5%, A3, B3-10%, A4, B4-15%, A5, B5-20%].
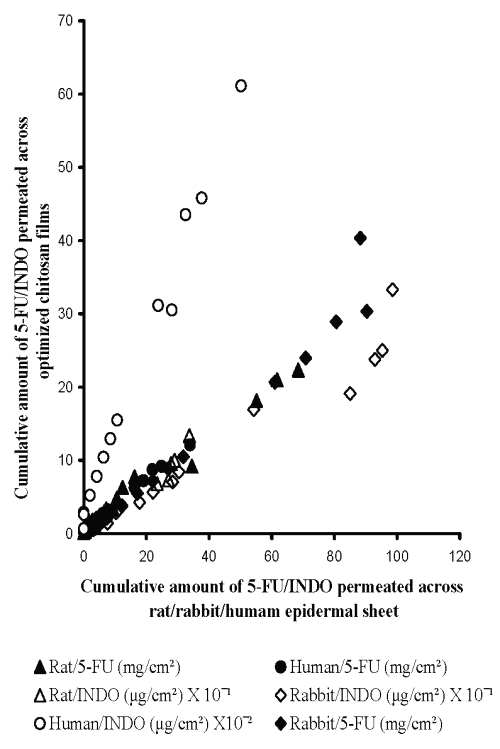
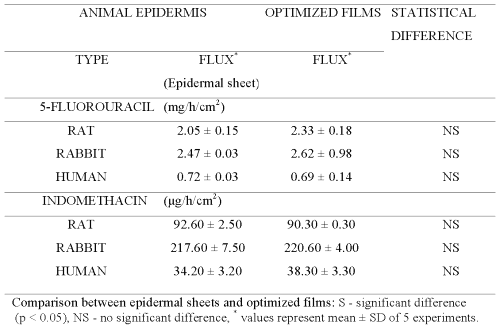
Therefore, central composite design (CCD) was employed to prepare chitosan films by cross-linking with sodium citrate solutions adjusted to pH 5 (Table 3). The equations generated from correlation between flux of 5-FU or INDO and three active variables of CCD (Table 3) were solved for calculating optimum values of X1 (concentration of chitosan), X2 (concentration of cross-linking agent) and X3 (cross-linking time). These calculated optimized values were used for preparing chitosan films that shall exhibit flux comparable to that across epidermal sheets (Table 5). It is evident from Figure 6 that cross-linked chitosan films prepared by using the calculated optimized values of active variables were capable of mimicking in vitro flux of 5-FU and INDO comparable to that across rat, rabbit and human cadaver epidermal sheets.
Figure 6: Correlation between in vitro permeation of 5-FU across optimized chitosan films and rat epidermal sheet (Y = 0.31X +1.07, R2 = 0.98, n=5), rabbit epidermal sheet (Y = 0.39X - 1.17, R2 = 0.96, n=5) or human cadaver epidermal sheet (Y = 0.35X + 0.59, R2 = 0.99, n=5) and INDO across optimized chitosan films and rat epidermal sheet (Y = 0.33X + 0.25, R2 = 0.96, n=5), rabbit epidermal sheet (Y = 0.28X - 0.37, R2 = 0.95, n=5) or human cadaver epidermal sheet (Y = 1.16X + 0.25, R2 = 0.99, n=5).
It is important to note that the optimized values for preparing chitosan films capable of simulating the flux of either drug are different for epidermal sheets of different animals. This indicates that a single chitosan film with a particular optimized composition cannot be used for simulating drug permeation across all types of epidermis. This can be attributed to the different inherent permeability of epidermis of different animals due to variation in their biochemical constituents, anatomical ultra structure, etc. Nevertheless, a high correlation evident from Figure 6 suggests that sodium citrate cross-linked chitosan films prepared by using their optimized composition can be used to simulate the in vitro permeation of 5-FU and INDO across rat, rabbit and human cadaver epidermal sheets.
Conclusion
The present investigation revealed overwhelming influence of pH and concentration of sodium citrate solutions in influencing the permeation of both 5-FU (polar) and INDO (non polar) across cross-linked chitosan films. The ionic complexes formed upon cross-linking chitosan with sodium citrate solutions adjusted to pH 5 were found to be resistant to breakdown by phosphate buffer (pH 7.4) that resulted in restricted permeation of both drugs. The optimized film composition was found to closely mimic the permeation of both drugs across these sodium citrate cross-linked chitosan films. Further, the results suggest that the formulation and process variables can be modified to prepare sodium citrate cross-linked chitosan films that simulate the in vitro permeation of polar and non polar drug molecules across animal and human epidermal sheets. This knowledge has a great potential for exploitation in transdermal dosage form research for making films that shall serve as an alternative to animal and human skin. This is expected to reduce the widespread use of natural skin during preliminary in vitro investigations during development of transdermal dosage forms.
References
Barry, B. W., Dermatological Formulations, Percutaneous Absorption. Marcel Dekker Inc, New York, pp. 138-150, 1983.
Nardo, A. D., Wertz, P., Giannetti, A. and Seidenari, S., Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm Venereol 78:27-30, 1985.
Feldstein, M. M., Raigorodskii, I. M., Iordanskii, A. L. and Hadgraft, J., Modeling of percutaneous drug transport in vitro using skin-imitating carbosil membrane. J Control Rel, 52:25-40, 1998.
Adusumilli, P. S. and Bolton, S. M., Evaluation of chitosan citrate complexes as matrices for controlled release formulations using a 32 full factorial design. Drug Dev Ind Pharm, 17:1931-1945, 1991.
Akbuga, J. and Bergisadi, N., 5-Fluorouracil loaded chitosan microspheres: Preparation and release characteristics. J Microencap 13:161-168, 1996.
Suheyla, H. K., Chitosan: Properties, preparation and application to micro particulate system. J Microencap 14:689-711, 1997.
Dureja, H., Tiwary, A. K. and Gupta, S., Simulation of skin permeability in chitosan membranes. Int J Pharm, 213:193-198, 2001.
Wang, L., Khor, E. and Lim, L. Y., Chitosan-alginate-CaCl2 system for membrane coat application. J Pharm Sci, 90:1134-1142, 2001.
Bodmeier, R., Oh, K. H. and Parmar, Y., Preparation and evaluation of drug containing chitosan beads. Drug Dev Ind Pharm, 15:1475-1494, 1989.
Sezer, A. D. and Akbuga, J., Controlled release of piroxicam from chitosan beads. Int J Pharm, 121:113-116, 1995.
Genta, I., Perugini, P., Conti, B. and Pavanetto, F., A multiple emulsion method to entrap a lipophilic compound in to chitosan microsphere. Int J Pharm, 152:237-246, 1997.
Aiedeh, K., Gianasi, E., Orienti, I. and Zecchiv, Chitosan microcapsules as controlled release system for insulin. J Microencap, 14:567-575, 1997.
Nigalaye, A. G., Adusumilli, P., Bolton, S., Investigation of prolonged drug release from matrix formulations of chitosan. Drug Dev Ind Pharm, 16:449-467, 1990.
Lin, S. Y. and Lin, P. C., Effect of acid type, acetic and sodium carboxymethlycellulose concentrations on the formulation, micromeritics, dissolution and floating properties of theophylline chitosan microcapsules. Chem Pharm Bull, 40:2491-2497, 1992.
Phaechamud, T., Koizumi, T. and Ritthidej, G. C., Chitosan citrate as film former: compatibility with water-soluble anionic dyes and drug dissolution from coated tablet. Int J Pharm, 198:97-111, 2000.
Lewis, G. A., Mathieu, D. and Luu, R. P., Pharmaceutical Experimental Design, Marcel Dekker Inc. New York, pp. 23-78, 1999.
Sasaki, H., Kojima, M., Mori, Y., Nakamura, J. and Shibasaki, J., Enhancing effect of pyrrolidone derivatives on transdermal penetration of 5-fluorouracil, Triamcinolone acetonide, indomethacin, and flurbiprofen. J Pharm Sci, 80:533-538, 1991.
Shu, X. Z., Zhu, K. J. and Song, W., Novel pH-sensitive citrate cross-linked chitosan film for drug controlled release. Int J Pharm, 212:19-28, 2001.
Ritthidej, G. C., Phaechamud, T. and Koizumi, T., Moist heat treatment on physicochemical change of chitosan salt films. Int J Pharm, 232:11-22, 2002.
Corresponding Author: Ashok K. Tiwary, Department of Pharmaceutical Sciences and Drug Research, Punjabi University, Patiala, 147002, India. aktiwary2@rediffmail.com
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.cspscanada.org