J Pharm Pharmaceut Sci (www.cspscanada.org) 8(1):107-114, 2005
Anti-inflammatory effects of Podophyllum hexandrum (RP-1) against lipopolysaccharides induced inflammation in mice.
Hridayesh Prakash
Department of Radiation Biology, Institute of Nuclear Medicine and Allied Sciences, Brig. S. K. Mazumdar Marg, Delhi, IndiaArif Ali
Department of Biosciences, Faculty of Natural Sciences, Jamia Millia Islamia, Delhi, IndiaMadhu Bala
Department of Radiation Biology, Institute of Nuclear Medicine and Allied Sciences, Brig. S. K. Mazumdar Marg, Delhi, IndiaHarish Chandra Goel1
Department of Microbiology, Faculty of Science, C.C.S. University, Meerut, (UP), IndiaReceived 15 November 2004, Revised 7 December 2004, Accepted 3 February 2005, Published 30 April 2005
PDF Version
Abstract
PURPOSE: Down-regulation of lipopolysaccharide (LPS) induced hyper-inflammatory response by non-toxic pharmacological agents acquires paramount importance for countering bacterial sepsis. Anti-inflammatory potential of aqueous extract of Podophyllum hexandrum, a plant well documented in Ayurvedic literature for various therapeutic purposes, was investigated. METHODS: In vivo studies were performed on Balb/c mice pre-treated with supra-lethal dose of LPS endotoxin (E.coli 055:B5) with or without treatment with P. hexandrum extract (RP-1). Mouse peritoneal macrophage cultures were used to understand ex vivo effects of RP-1 on LPS generated nitric oxide (NO), secretion of IFN-γ , IL-6 and TNF- α. Griess assay and sandwich ELISA method were used to quantify inducible NO and cytokines respectively. RESULTS: Minimal dose of LPS that rendered 100% mortality to mice was found to be 450 μg/kg b.w. Administration of RP-1 (200 mg/kg b.w., i.p.) one hour before lethal LPS treatment (0.5 mg/kg b.w.) rendered maximum (78%) survival. Ex vivo study revealed that RP-1 (50 μg/ml) treatment to peritoneal macrophages inhibited LPS (5 μg/ml) induced nitrite generation to 37%, IFN-γ secretion to 5%, IL-6 secretion to 50% and TNF-α secretion to 50 % of LPS treated control values. CONCLUSION: This study has demonstrated anti-inflammatory potential of aqueous extract of P. hexandrum.
Introduction
Lipopolysaccharide (LPS) from gram-ve bacteria are well known to cause bacterial sepsis mediated through activation of monocytes, neutrophils and macrophages (1).
Sometimes, activation of these cells may induce hyper-secretion of various pro-inflammatory and toxicity mediating molecules such as TNF-a, IFN-g, IL-6, eicosonoids and nitric oxide (NO) free radicals. The excessive inflammatory responses, in turn, may manifest respiratory failure and multiple organ dysfunction syndrome (MODS) i.e. loss of capillary integrity, multiple organ dysfunction (2-4), distributive shock, septic shock and mortality. For recovery from these shocks, down-regulation of hyper-inflammatory response by non-toxic or less toxic pharmacological agents acquires immense importance.
Plants are known to produce plethora of secondary metabolites showing wide array of pharmacological properties and therefore have been used in both the traditional and modern systems of medicine. Isolated compounds or the active principle from a plant extract or the molecular drugs could regulate a particular biochemical pathway involved in the inflammatory response but may interfere with many other sub-cellular and cellular pathways and thereby causing toxicity. The natural combination of compounds present in the whole plant extract often contained several molecules, which nullify the side effects produced by the active biomolecules or their combinations.
Podophyllum hexandrum a Himalayan herb, has been extensively exploited in traditional Ayurvedic system of medicine for treatment of a number of ailments like Condyloma acuminata, Taenia capitis , monocytoid leukemia, Hodgkins disease, non-Hodgkin's Lymphoma, cancer of brain, lung, bladder (5) and venereal warts (6). Utility of P. hexandrum has also been reported against constipation, cold, biliary fever, septic wound, burning sensation, erysipelas insect bite, mental disorders, rheumatism, plague (7) and to provide symptomatic relief in some of the allergic and inflammatory conditions of skin. P. hexandrum has also been employed in treatment of cancer (8). In view of these facts immunomodulatory potential of P. hexandrum was investigated by studying whole body survival subsequent to LPS induced shock. Various pro-inflammatory mediators such as NO, IFN-g, TNF-a and IL-6 were studied ex vivo in LPS treated peritoneal macrophages in Balb/c mice.
Materials and Methods
Experimental Animals
6-8 weeks old inbred Balb/c female mice weighing 25 ± 3 g, were maintained under controlled environment (25 ± 2° C) and provided standard animal food pellet (Amrut laboratory animal feed, India) and water ad libitum . Animal experiments were conducted strictly according to INSA-Ethical guidelines for use of animals in scientific research as prescribed by Central Drug Research Institute, CSIR, Lucknow, India and animals were sanctioned and issued after the approval of the Animal Experimentation Ethics Committee of the Institute.
Reagents
RPMI 1640 medium and Foetal Bovine Serum (FBS) were procured from Hyclone, USA; Brewer's thioglycolate medium from Becton Dickinson, USA; BSA, o-phenylene-diamine (OPD), LPS ( E. coli B55:B5) and ConA from Sigma USA; TNF-a, INF-g, IL-6 OptEIATM ELISA kits from Pharmingen, USA; N-[naphthyl]ethyl-ene-diamine dihydro chloride, Sulphanilamide, HEPES and H2 O2 buffer from E-Merck, Germany; Streptomycin, Gentamycin, Penicillin, Trypan blue dye, PBS, PHA, H2 SO4, NaNO2, from Himedia, India.
Preparation of herbal extract
The aqueous extract of whole rhizome of P. hexandrum (family Berberidaceae ) was prepared as described earlier (8). Briefly, the dried rhizome of P. hexandrum was powdered, extracted in triple distilled water, filtered and lyophilised yielding a brownish black resinous material (RP-1) which was stored at 4° C. For experiments, RP-1 was resuspended in triple distilled water and sterilised by passing through 0.2 mm filter (Minisart® NML). For ex vivo experiments the extract was diluted in serum free RPMI 1640 medium.
HPLC characterization
HPLC of RP-1 was performed with Simadzu HPLC system using C-18 column and UV detector. Methanol:water :: 70:30; v/v, were used as eluents and the flow rate was maintained at 1 ml/min. The HPLC fingerprint revealed nine prominent peaks (Fig. 1).
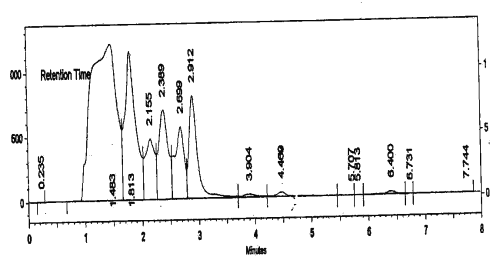
Figure 1: HPLC fingerprint of RP-1. Conditions: C-18 column and UV detector, methanol:water:: 70:30, v/v, was used as eluent and the flow rate was maintained at 1 ml /min, injection volume was 10 ml.
This HPLC spectrum was used as a fingerprint to monitor batch-to-batch variations in the herbal extract. Any batch showing gross variations in the HPLC spectrum was rejected.
Generation of septic shock
Female Balb/c mice (6-8 weeks old) were grouped and maximum five animals were housed in a polyvinyl cage. Each mouse was administered a lethal dose of LPS ( E. coli 055:B5, 0.5 mg/kg b.w., i.p.) with or without RP-1 treatment. Control group received saline treatment. To detect the septic shock, onset of shivering leading to death within 96 h was recorded as per procedure described (9, 10).
Peritoneal macrophage cell culture
A standard and established method for peritoneal macrophage cell culture (11) was adopted. Briefly, the mouse peritoneal macrophages were elicited by injecting 4% brewer's thioglycolate medium (Becton Dickinson, MD, USA) into female Balb/c mice. Peritoneal cells were exudated 72 h after injection by flushing peritoneal cavity with ice-cold RPMI 1640 incomplete medium using a 22G1 needle. Peritoneal lavage was pooled and collected in 50 ml conical centrifuge tube and centrifuged at 1500 rpm, (10 min, 4°C). The pallet was resuspended in complete RPMI 1640 medium supplemented with 10% heat inactivated FBS containing 15 mM HEPES buffer, 2 mM L-glutamine, 100 U/ml penicillin, 100 U/ml streptomycin. Cells were counted and their viability was checked by trypan blue dye exclusion method. The peritoneal macrophages were examined microscopically for their morphological identification. Their identification was confirmed based on membrane marker (Mac-1), stained with anti-Mac-1-FITC conjugated antibody (12). 1 x 10 5 cells/well in a final volume of 200 μl were seeded in flat bottom 96-well polystyrene microtiter plate (Costar, Corning, NY) and incubated at 37°C, 5% CO2 and 85% humidity chamber for 3 h to achieve adherence. The non-adherent cells were removed by repeated washing with ice-cold serum RPMI 1640 medium while adherent cells were cultured for nitric oxide and cytokine assay.
Measurement of total nitrite
The macrophage culture supernatant was used for estimating the stable NO metabolite, nitrite (NO2-) by Griess assay as described (13). Equal volume of the culture supernatant treatment groups and Griess reagent (1% sulphanilamide and 0.1% N-[naphthyl] ethylenediamine dihydrochloride; 1:1) were mixed and the absorbance was measured at 550 nm by ELISA reader (BIO-TEK instrument INC, Canada.). The amount of nitrite was calculated from a NaNO2 standard curve.
ELISA for cytokines
Different pro-inflammatory cytokines in the cell supernatant were measured by standard, sensitive and reliable sandwich ELISA method (14) using standard instruction protocol supplied by (BD Pharmingen, USA). Briefly, polystyrene plates (Nunc, USA) were coated with monoclonal anti-cytokine antibody and incubated overnight at 4°C. The plates were washed thereafter with wash buffer (PBS + 0.05 % Tween 20) three times. The reaction was blocked further by treating with PBS + 10 % FBS for 1 h. Thereafter, for developing standard graph, serially diluted recombinant mouse cytokine standards were added. To quantify treatment-induced changes in the level of cytokines, the cell supernatants from different experimental groups were added. All the plates were incubated for 2 h at room temperature. The plates were again washed with wash buffer three times and incubated for 1 h at 25°C with biotinylated detection antibody. After washing with wash buffer, 3% H2O2 with o-phenylene diamine (Sigma, USA) chromogem in 0.1 M citrate buffer was added and plates were incubated for 10-30 minutes at room temperature. The reaction was stopped by adding 2 N H2SO4 and the absorbance was measured at 492 nm. Cytokine concentration was calculated against recombinant cytokine standard curve using KC4 software (BIO-TEK, Canada).
Analysis of data
The results have been expressed as mean S.D. of three repeats. Data were subjected to student-t test and significance was assessed at p < 0.05 confidence level.
Results
Effect on whole body survival
Doses of LPS from 50 mg/kg. b.w. onwards administered to Balb/c mice rendered increasing mortality. LPS dose (450 mg/kg, b.w.) to mice induced 100% mortality as compared to control group (Fig. 2).
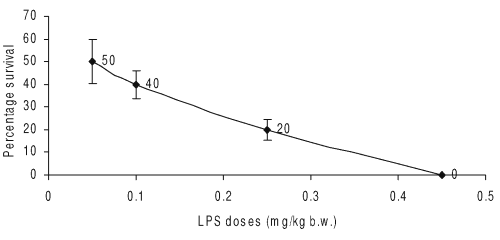
Figure 2: Effect of different doses of LPS on induction of septic shock in female Balb/c mice (n=5). The whole body survival (percentage) was observed 96 h after LPS shock to Balb/c mice (n=5).
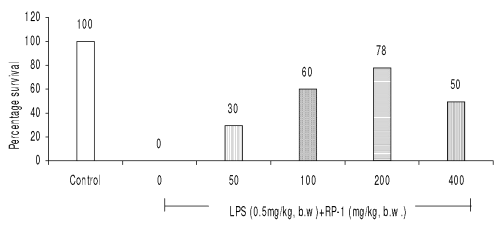
RP-1 (200 mg/kg b.w., i.p.) administered 1 h before LPS (0.5 mg/kg b.w.) challenge rendered maximum survival (78%) (Fig 3).
Figure 3: Effect of different doses of RP-1 (mg/kg b.w.) administered one hour before lethal dose of endotoxin, LPS (0.5 mg/kg b.w., i.p.). Whole body survival (percentage) was observed 96 h after LPS shock to Balb/c mice (n=5).
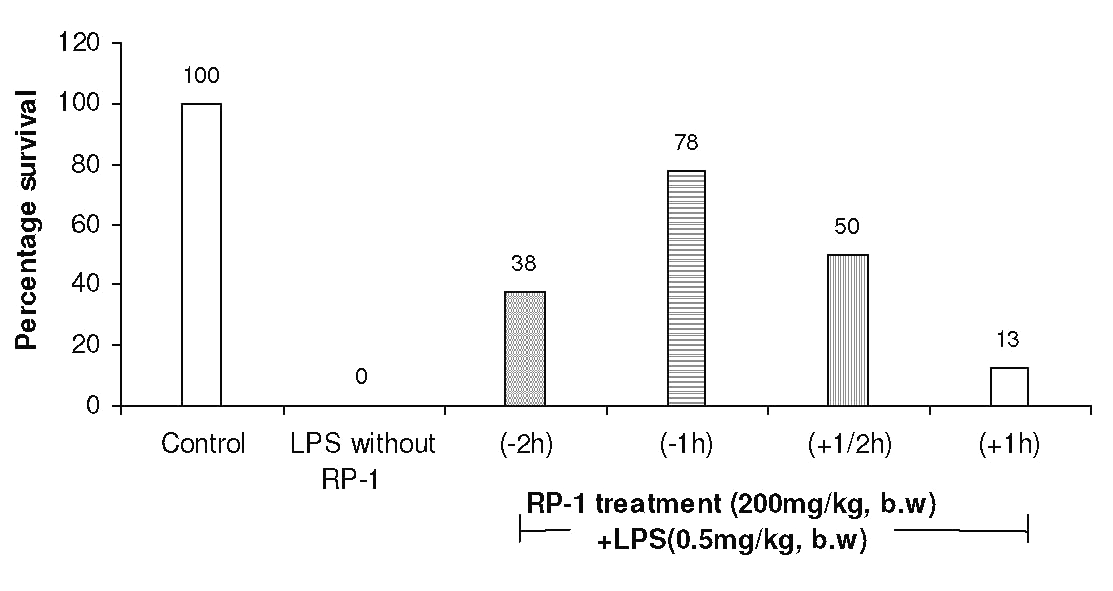
The other doses of RP-1 were, however, less effective (Fig. 3). RP-1 treatment (200 mg/kg b.w., i.p.) administered one hour before LPS challenge rendered maximum survival (Fig. 4).
Figure 4: Protection of mice from endotoxin shock by RP-1 (200 mg/kg, b.w., i.p.) given at different time intervals either before (-) or after (+) LPS (0.5 mg/kg b.w.) challenge. Whole body survival (percentage) was observed at 96 h after treatment.
RP-1 treatment given half an hour after LPS challenge was also appreciably effective. However, other intervals between RP-1 treatment and LPS challenge remained little effective.
Effect on IFN-g
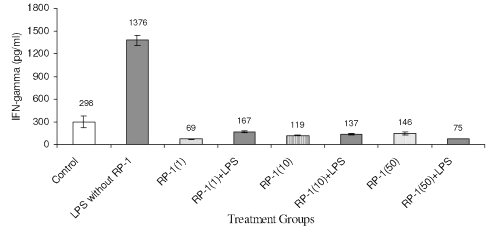
LPS challenge to peritoneal macrophage cultures enhanced IFN-g titre (Fig. 5) significantly (1376+68 pg/ml; p<0.05) as compared to untreated control (298+81 pg/ml; p<0.05). Treatment of peritoneal macrophage with RP-1 (1 g/ml) alone reduced IFN-g levels significantly (69+4 pg/ml; p<0.05) as compared to both untreated and LPS challenged groups. However, RP-1 treatment one hour before LPS challenge inhibited IFN- g generation significantly ( p< 0.05 ) as compared to LPS challenged group. The inter se differences in IFN- g level in macrophage cultures administered different doses of RP-1 remained insignificant.
Figure 5: Effect of different doses of RP-1 (mg/ml; mentioned in parenthesis) administered one hour before LPS (5 mg/ml) treatment to peritoneal macrophages (ex vivo culture from female Balb/c mice). The supernatant was investigated for IFN-g secretion 48 h after treatment. Each bar represented mean+S.D of three repeats.
Effect on TNF-a generation
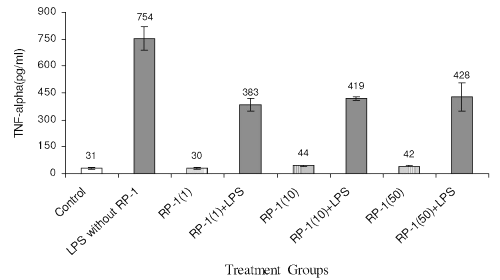
LPS treatment to peritoneal macrophage enhanced TNF-a generation (Fig. 6) significantly (754+66 pg/ml; p<0.05) as compared to control (31+6 pg/ml; p<0.05) RP-1 treatment alone did not influence TNF-a production as compared to the control. However, RP-1 treatment inhibited the TNF-α production (383 +34 pg/ml; p<0.05) in LPS challenged groups significantly (p<0.05) as compared to LPS challenged control group.
Figure 6: Effect of different doses of RP-1 (mg/ml; mentioned in parenthesis) administered one hour before LPS (5 mg/ml) treatment to peritoneal macrophages (ex vivo culture from female Balb/c mice). The supernatant was investigated for TNF-a secretion 48 h after treatment. Each bar represented mean+S.D of three repeats.
Effect on IL-6
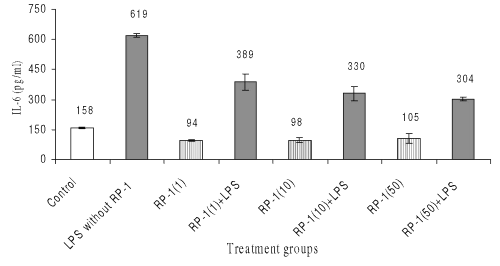
LPS treatment also enhanced IL-6 generation in peritoneal macrophages significantly (619+10 pg/ml; p<0.05) as compared to untreated control (159+5 pg/ml; p<0.05). Treatment of peritoneal macrophage with various doses of RP-1 alone decreased IL-6 production significantly (p<0.05) as compared to both untreated and LPS challenged control groups.
However administration of various doses of RP-1 one hour before LPS challenge inhibited IL-6 secretion significantly ( p< 0.05 ) as compared to LPS challenged control group, though inter se difference in IL-6 levels between the groups given different doses of RP-1 remained insignificant. (Fig. 7).
Figure 7: Effect of different doses of RP-1 (mg/ml; mentioned in parenthesis) administered one hour before LPS (5 mg/ml) treatment to peritoneal macrophages (ex vivo culture from female Balb/c mice). The supernatant was investigated for IL-6 secretion 48 h after treatment. Each bar represented mean + S.D of three repeats.
Effect on nitrite production
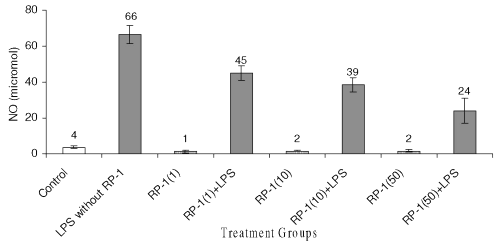
Ex vivo studies revealed that, LPS treatment enhanced the level of nitrites in peritoneal macrophage cell culture supernatant significantly ( p< 0.05 ) as compared to untreated control (Fig. 8).
Figure 8: Effect of different doses of RP-1 (vg/ml; mentioned in parenthesis) administered one hour before LPS (5 mg/ml) treatment to peritoneal macrophages (ex vivo culture from female Balb/c mice). The supernatant was investigated for NO secretion at 48 h after treatment. Each bar represented mean + S.D of three repeats.
RP-1 treatment to peritoneal macrophage alone reduced nitrite level significantly ( p< 0.05 ) as compared to untreated control group, yet the inhibition of nitrite among groups treated with different doses of RP-1 remained insignificant. However, increasing doses of RP-1 administered one hour before LPS challenge reduced nitrite levels significantly ( p< 0.05 ) in a dose dependent manner.
Discussion
For anti-inflammatory studies, standard endotoxin shock model was generated in Balb/c mice by non-infectious molecule LPS. This method eliminates the risk of handling contagious microbial cultures that are required for such studies and is one of the standard experimental models adopted widely (9). Whole body survival was recorded as the initial parameter. A dose of 450 mg/kg b.w of LPS administered to mice rendered 100% mortality (Fig. 2). However, to ensure 100% mortality, a supra-lethal dose of 500 mg/kg, b.w. of LPS was adopted as a challenge dose for whole body survival experiments. LPS has been reported to activate macrophages and induce subsequent release of massive amount of pro-inflammatory cytokines and nitric oxide free radicals during the generation of endotoxin shock. These pro-inflammatory molecules are known to manifest oxidative stress (10), vascular anomalies like intravascular coagulation, vascular perfusion and hypotension (15) and ultimately the mortality. RP-1 treatment before LPS challenge rendered 78% survival as against 100% mortality in LPS control group (Fig. 3). RP-1 has been reported to have antioxidant property (16), which may be attributed to presence of several molecules in the extract like quercetin and flavanoides, the known inhibitors of oxidative stress (17) and inflammatory response (18). Protective action of RP-1 against LPS induced septic shock could be attributed to the presence of such molecules in RP-1. The optimum time required to nullify the endotoxic effect of LPS was one hour (Fig. 4). It may be explained based on pharmaco-dynamics of the bioactive molecules, which could have concentrated at the inter- and intra-cellular target sights maximally within one hour. Shorter intervals could achieve the concentration sub-optimally while the higher time intervals permitted the metabolisation of the bioactive molecules and thus might have resulted in sub-optimal concentration. Such time related bio distribution and concentration of active molecules is a well-known biological phenomenon (19).
LPS treatment has been shown to enhance IFN-γ secretion (Fig. 5) which is a strong activator of macrophages and subsequent inducer of TNF-α, IL-6 and NO during various phases of inflammation (20). RP-1 treatment alone inhibited secretion of IFN- γ in peritoneal macrophages. Such activity has been reported for many other herbal extracts also (21). LPS challenge to peritoneal macrophages that had already received RP-1 treatment, did not demonstrate any increase in IFN-γ secretion (Fig. 5). This could be achieved if molecules present in RP-1 interacted with LPS directly thereby rendering them ineffective in inducing signal transduction pathways responsible for IFN- γ production. It is also possible that the bioactive molecules in RP-1 could directly down-regulate the signal transduction pathways leading to IFN- γ production. This aspect however needs further investigation. The effect of RP-1 on stimulation of IFN - γ secretion was not dose dependent though with respect to other parameters like whole body survival, dose dependence was explicitly evident. This indicates that anti-inflammatory molecules responsible for nullifying IFN- γ production even at lowest concentration of RP-1 studied here were in more than the required quantity. It implies that the IFN- γ mediated inflammatory response may not be solely responsible for LPS induced mortality, which is counteracted by RP-1 in a dose dependent manner.
RP-1 treatment to macrophage also inhibited TNF-a (Fig. 5) and IL-6 (Fig. 6) secretions. These cytokines are well known for activation of phagocytes (1) and their inflammatory response related functions (22). The inhibition of these cytokines due to RP-1 treatment could lead to anti inflammtory action. The dose dependent effect of RP-1 on the generation of these cytokines has not been revealed during this study. Different doses of RP-1 alone used here did not induce TNF-a, IFN-γ and IL-6 as compared to control. This indicated that the doses studied here were not inflammatory by themselves and therefore the use of this agent as anti-inflammatory agent is promising.
Enhanced nitrite levels suggested induction of nitric oxide (NO) generation in supernatant of peritoneal macrophage by LPS treatment, which in turn indicated enhanced oxidative burst activity (Fig. 8). These findings corroborate the already reported literature (10) RP-1 pre-treatment to macrophages in cultures decreased nitrite level even on challenge by LPS. This indicates the antioxidant potential of RP-1. This could be due to inhibition of either inducible nitric oxide synthase (NOs) activity or inhibition of transcriptional activation of iNOs mRNA by the presence of flavanoides and iso-flavonoides molecules present in RP-1. Flavonoids and iso-flavonoides from several plant extracts have been reported to exhibit such activity (23-26). Fe (+3) has been reported to act as a co-factor for transcription of iNOs gene (27), which is responsible for nitric oxide production. RP-1 treatment has been demonstrated to chelate iron (28) and thus could affect inhibition of nitric oxide generation. RP-1 treatment also enhanced level of cellular antioxidant molecules like GST and SOD (16). These antioxidant molecules seem to play an important role in preserving various functions of leukocytes by protecting them from oxidative stress. RP-1 treatment induced decrease in pro-inflammatory cytokines in LPS treated group could also be responsible for inhibition of nitrite in the cell supernatant (20). This study reports for the first time the anti-inflammatory potential of RP-1, which may be exploited to ameliorate LPS induced toxicity.
Acknowledgments
The authors acknowledge Director INMAS for supporting this study and also S. Singh., J. Prasad., P. K. Agrawala and I. Premkumar, for their help during the experiments. This work was supported by DRDO grant.
References
Veckman, V., Miettinen, M., Matikinen, S., Lande, R., Giacomini, E., Coccia, E.M. and Julkunen, I., Lactobacilli and streptococci induce inflammatory chemokines production that stimulate Th1 cell chemotaxis. Journal of Leukocyte Biology, 74: 395-402, 2003.
Pinsky, M.R., Vincent, J.L., Deviere, J., Alegre, M., Khan, R.J. and Dupont, E., Serum cytokine level in human septic shock: Relation to multiple organ failure and mortality. Chest, 103: 565-575, 1993.
Tracey, K.J. and Lowry, S.F., The role of cytokine mediator in septic shock. Advance Surgery 23, 21-56, 1998.
Deitch, E.A., Multiple organ failure: Pathophysiology and potential failure therapy. Annals of Surgery, 216:117-120, 1992.
Blasko, G.; Cordell, G.A., Economic and Medicinal Plants. Research. Vol. II, Academic Press, London, p. 163, 1988.
Beutner, K. R. and von Krogh, G., Current status of podophyllotoxin for treatment of genital warts. Semin. Dermatol., 9:148-152, 1990.
Chatterjee, A.; Prakashi S.C., The Treatise on Indian Medicinal Plants Vol.VI. Publication & Information Directorate, CSIR Publication, New Delhi, p.130-135, 1995.
Goel, H.C., Prasad, J. and Sharma, A., Antitumor and radioprotective action of Podophyllum hexandrum. Indian Journal of Experimental Biology, 36:583-587, 1998.
Villa, P. and Ghezzi, P., Animal models of endotoxin shock. Methods of Molecular Medicine 98: 199-206, 2004.
Victor, V.M., Minano, M., Guayerbas, N., Del Rio, M., Medina, S. and De la Feunte, M., Effect of endotoxic shock in several functions of murine peritoneal macrophage. Molecular and Cellular Biochemistry, 189: 25-31, 1998.
Edelson P.J. and Cohn Z.A., Purification and cultivation of monocytes and macrophage in In vitro Methods in Cell Mediated and Tumor Immunity, Bloom, B.A., and Davis, J.R. (eds). Academic Press, San Diego, 15: 333-339, 1976.
Vyctor, V.M. and De la Fuente, M., Comparative study of peritoneal macrophage functions in mice receiveing lethal and non-lethal doses of LPS. J Endotoxin Res. 6: 235-241, 2000.
Green, L.C., Wagner, D.A., Gloglowski, J., Skipper, P.L., Wishnok, J.S. and Tannenbaum, S.R., Analysis of nitrate, nitrite and [15N] nitrate in biological fluids. and quantification of cytokines. J Immunol Methods 284: 99-106, 2004.
Gogos, C.A., Lekkou, A., Papageorgiou, O., Siagris, D., Skoutelis, A. and Bassaris, H.P., Clinical prognostic markers in patients with severe sepsis: a prospective analysis of 139 consecutive cases. Anal. Biochem., 126: 131-138, 1982.
Copeland, S., Siddiqui, J. and Remick, D., Direct comparison of traditional ELISAs and membrane protein arrays for detection Journal of Infection, 47: 300–306, 2003.
Mittal, A., Pathania, V., Agrawala, P.K., Prasad, J., Singh, S.and Goel, H.C., Influence of P. hexandrum on endogenous antioxidants defence system in mice: possible role in radioprotection. Journal of Ethanopharmacology, 76: 253-262, 2001.
Georgetti, S.R., Casagrande, R., Di Mambro, V.M., Azzoloni, A.E. and Fonseca, M.J., Evaluation of the antioxidant activity of different flavonoids by the chemiluminescence method. AAPS Pharmacology Science, 5: E20, 2003.
Ferrandiz, M., Nair, A.G. and Alcaraz, M.J., Effect of flavonoids on arachidonate metabolism in rat peritoneal leukocytes. Pharmazie, 45: 444-445, 1990.
Ohnishi, N., Okada, K., Yoshioka, M., Kuroda, K., Nagasawa, K., Takara, K. and Yokoyama, T., Studies on interactions between traditional herbal and western medicines. V. effects of Sho-saiko-to (Xiao-Cai-hu-Tang) on the pharmacokinetics of carbamazepine in rats. Biological Pharmacology Bulletin 25: 1461-1466, 2002.
Klimp, A.H., De Vries, E.G., Scherphof, G.L., Daemen, T., A potential role of macrophage actvation in the treatment of cancer. Critical Review of Oncology and Hematology, 44: 143-161, 2002.
Lin, A.P., Tsai, W.J., Fan. C.Y., Lee, M.J. and Kuo, Y.C., Vandellia cordifolia regulated cell proliferation and cytokines production in human mononuclear cells. American Journal of Chinese Medicine, 28: 313-323, 2000.
Kuroiwa, T., Schlimgen, R., Illei, G.G.and Boumpas, D.T., Monocytes response to Th1 stimulation and effector function towards human mesengial cells are not impaired in patient with lupus nephritis. Clinical Immunology, 106: 65-72, 2003.
Olaszanecki, R., Gebska, A., Kazlovski, V.I. and Gryglewski, R.J., Flavonoids and nitric oxide synthase. Journal of Physiology and Pharmacology 53: 571-584, 2002.
Pavanto, A., Tunon, M.J., Sanchez-Campo, S., Marroni, C.A., Llesuy, S., Gonzalez- Gallego, J.and Marroni, N., 2003. Effect of quercetin on liver damage in rats with carbon tetrachloride induced cirrhosis. Digestive Discipline Science, 48: 824-829.
Miyamoto, M., Hashmoto, K., Minagava, K., Satoh, K., Komatsu, N., Fujimaki, M., Nakashima, H., Yokote, Y., Akahane, K., Guputa, M., Sarna, D.N., Mitra, S.K. and Sakagami, H., Effect of poly-herbal formula on NO production by LPS stimulated mouse macrophage-like cells. Anti Cancer Research, 22: 3293-3301, 2002.
Hong, C.H., Hur, S.K., Oh, O.J., Kim, S.S., Nam, K.A. and Lee, S.K., Evaluation of natural products on inhibition of cyclooxegenase (COX-2) and nitric oxide synthase (iNOs) in cultures mouse macrophage cells. Journal of Ethnopharmacology, 83: 153-159, 2002.
Weiss, G., Werner, F.G., Werner, E.R., Grunewald, K., Wachter, H. and Hentze, M.W., Iron regulates nitric oxide synthase activity by controlling nuclear transcription. Journal of Experimental Medicine, 180: 969-976, 1994.
Premkumar, I. and Goel, H.C., Iron chelation and related properties of Podophyllum hexandrum, a possible role in radioprotection. Indian Journal of Experimental Biology, 38: 1003-1006, 2000.
Corresponding Author: Harish Chandra Goel, Department of Microbiology, C. C. S. University, Meerut, (UP), India. goelharish@hotmail.com, facinations@vsnl.net
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.cspscanada.org