J Pharm Pharmaceut Sci (www.cspscanada.org) 8(1):76-93, 2005
A review of co-processed directly compressible excipients.
M. C. Gohel
Lallubhai Motilal College of Pharmacy, Navarangpura, Ahmedabad, IndiaPranav D Jogani1
USV Limited, B. S. D. Marg, Govandi, Mumbai, IndiaReceived 16 November 2004, Revised 28 January 2005, Accepted 31 January 2005, Published 16 April 2005
PDF Version
Abstract
Direct compression is the preferred method for the preparation of tablets. The present review outlines the importance of the functionality of the directly compressible adjuvants in the formulation of tablets. The co-processing is the most widely explored method for the preparation of directly compressible adjuvants because it is cost effective and can be prepared in-house based on the functionality required. Hence, the present review focuses on the properties of the co-processed directly compressible adjuvants available in the market.
Introduction
Over the past hundred years tablet manufacturers have developed materials and processes that can produce compressed tablets containing a precise amount of an active pharmaceutical ingredient (API) at high speed and at relatively low cost. The development in the field of APIs, excipients and tableting machines during the past decades has made tablet manufacturing a science and the tablets the most commonly used dosage form (1, 2). The ease of manufacturing, convenience in administration, accurate dosing, and stability compared to oral liquids, tamper-proofness compared to capsules, safe compared to parental dosage forms makes it a popular and versatile dosage form. Experts in the art of tableting are aware with the basic art of tableting by the three well-known methods, i.e. wet granulation, roller compaction and direct compression. The pros and cons of wet granulation and roller compaction are well documented in the literature (3, 4, 5).
Prior to the late 1950s, the literature contained few references on the direct compression of pharmaceuticals. A great deal of attention has been given to both product and process development in the recent years. The availability of new materials, new forms of old materials and the invention of new machinery has allowed the production of tablets by simplified and reliable methods (1). In early 1960's, the introduction of spray-dried lactose (1960) and Avicel (1964) had changed the tablet manufacturing process and opened avenues of direct compression tableting.
Shangraw (6) conducted a survey of 58 products in United States of America for the preference for the granulation process. The results were in favour of direct compression. Of the five processes listed in the survey, the average score (1.0 being the perfect score) for direct compression was 1.5 compared to wet massing and fluid bed drying (2.0), wet massing and tray drying (2.5), all-in-one (3.3) and roller compaction (3.6). About 41% of the companies indicated that direct compression was the method of choice, and 41.1% indicated that they used both direct compression and wet granulation. Only 1.7% of the respondents indicated that they never used direct compression and 15.5% indicated that the process was not recommended.
Previously, the word "direct compression" was used to identify the compression of a single crystalline compound (i.e. sodium chloride, potassium chloride, potassium bromide, etc.) into a compact form without the addition of other substances.
Current usage of the term "direct compression" is used to define the process by which tablets are compressed directly from the powder blends of active ingredient/s and suitable excipients. No pre-treatment of the powder blends by wet or dry granulation is involved (5). The simplicity of the direct compression process is apparent from a comparison of the steps involved in the manufacture of tablets by wet granulation, roller compaction and direct compression techniques (4) (See Table 1). It has been estimated that less than 20 percent of pharmaceutical materials can be compressed directly into tablets (4). The rest of the materials lack flow, cohesion or lubricating properties necessary for the production of tablets by direct compression. The use of directly compressible adjuvants may yield satisfactory tablets for such materials.
Table 1: Comparison of major steps involved in the granulation methods.

Directly compressible adjuvants
The International Pharmaceutical Excipients Council (IPEC) defines excipient as "Substances, other than the API in finished dosage form, which have been appropriately evaluated for safety and are included in a drug delivery system to either aid the processing or to aid manufacture, protect, support, enhance stability, bioavailability or patient acceptability, assist in product identification, or enhance any other attributes of the overall safety and effectiveness of the drug delivery system during storage or use" (7). Solvents used for the production of a dosage form but not contained in the final product are considered to be excipients, i.e. the granulation fluids, which might be dried off later, should comply with relevant requirements of pharmacopoeia unless adequately justified (7). Excipients no longer maintain the initial concept of "inactive support" because of the influence they have both over biopharmaceutical aspects and technological factors. The desired activity, the excipients equivalent of the active ingredient's efficacy, is called its Functionality (7). The inherent property of an excipient is its functionality in the dosage form. Determination of an excipient's functionality is important to the excipient manufacturer in its assessment of the proper level of GMP, and yet the drug manufacturer may withhold this information until well into the development process (8).
In order to deliver a stable, uniform and effective drug product, it is essential to know the properties of the active ingredient alone and in combination with all other ingredients based on the requirements of the dosage form and processes applied. Excipients are usually produced by batch process; hence, there is a possibility of batch-to-batch variation from the same manufacturer. Excipients obtained from the different sources may not have identical properties with respect to use in a specific formulation. To assure interchangeability in such circumstances, users may wish to ascertain equivalency in final performance or determine such characteristics before use. Such tests are thus related to the functionality, that the excipient impart to a specific formulation (9).
In order to manufacture any finished product with consistent quality, standardization of raw materials in the drug formulation is necessary for its acceptance by regulatory authorities and pharmaceutical formulators. Unfortunately, such performance standards have not been included in pharmacopoeia primarily because their specifications have always been based on chemical purity and because it is not possible to standardize performance criteria (10). Pharmacopoeial standards do not take into account particle characteristics or powder properties, which determine functionality of excipients (11).
Control of functionality is important as a control of identity and purity. The following reasons can be cited (10):
- Many excipients have multiple functions (e.g. microcrystalline cellulose, starch).
- There is lack of awareness that the excipients behave differently, depending upon the vendor (i.e. microcrystalline cellulose).
As a consequence, excipients with optimal functionality are needed to ensure smooth tablet production on modern machines (11). The introduction of special force feeder to improve flow of granules from hopper marked a significant advancement in direct compression technology (4).
Ideal requirements of directly compressible adjuvants
The directly compressible adjuvant should be free flowing. Flowability is required in case of high-speed rotary tablet machines, in order to ensure homogenous and rapid flow of powder for uniform die filling. During the short dwell-time (milliseconds), the required amount of powder blend should be transferred into the die cavities with reproducibility of + 5%. Many common manufacturing problems are attributed to incorrect powder flow, including non-uniformity in blending, under or over dosage and inaccurate filling (14).
Compressibility is required for satisfactory tableting, i.e., the mass must remain in the compact form once the compression force is removed. Few excipients can be compressed directly without elastic recovery. Hence, the directly compressible diluent should have good compressibility, i.e. relation between compaction pressure and volume (3, 4).
Dilution potential can be defined as the amount of an active ingredient that can be satisfactorily compressed in to tablets with the given directly compressible excipient. A directly compressible adjuvant should have high dilution potential so that the final dosage form has a minimum possible weight. The dilution potential is influenced by the compressibility of the active pharmaceutical ingredient. A directly compressible adjuvant should be capable of being reworked without loss of flow or compressibility. On recompression, the adjuvant should exhibit satisfactory tableting characteristics. The adjuvant should remain unchanged chemically and physically. The directly compressible adjuvant should not exhibit any physical or chemical change on ageing and should be stable to air, moisture and heat.
A directly compressible adjuvant should have a particle size equivalent to the active ingredients present in the formulation (12). The particle size distribution should be consistent from batch to batch. Reproducible particle size distribution is necessary to achieve uniform blending with the active ingredient(s) in order to avoid segregation.
Filler-binders should not accelerate the chemical and/or physical degradation of the API(s) or excipients (12). It should not interfere with the biological availability of active ingredient/s (13). It should be compatible with all the adjuvants present in the formulation. It should be physiologically inert (5). It should not interfere with the disintegration or dissolution of the active ingredient. It should be colourless and tasteless. It should be relatively cost effective and available in desired time. It should accept colorants uniformly. It should show low lubricant sensitivity. It should show batch-to-batch reproducibility of physical and physico-mechanical properties. It should possess proper mouth fill, which is defined as the feel or the sensation in the mouth, produced when the excipient is used in chewable tablets (13). The pros and cons with reference to direct compression are discussed in following section and brief description is given in Table 2.
Table 2: Ideal requirements, advantages and limitations of direct compression.

Advantages of direct compression
The prime advantage of direct compression over wet granulation is economic since the direct compression requires fewer unit operations. This means less equipment, lower power consumption, less space, less time and less labor leading to reduced production cost of tablets. Direct compression is more suitable for moisture and heat sensitive APIs, since it eliminates wetting and drying steps and increases the stability of active ingredients by reducing detrimental effects. Changes in dissolution profiles are less likely to occur in tablets made by direct compression on storage than in those made from granulations (5). This is extremely important because the official compendium now requires dissolution specifications in most solid dosage forms (10).
Disintegration or dissolution is the rate-limiting step in absorption in the case of tablets of poorly soluble API prepared by wet granulation. The tablets prepared by direct compression disintegrate into API particles instead of granules that directly come into contact with dissolution fluid and exhibits comparatively faster dissolution. The high compaction pressure involved in the production of tablets by slugging or roller compaction can be avoided by adopting direct compression. The chances of wear and tear of punches and dies are less. Materials are "in process" for a shorter period of time, resulting in less chance for contamination or cross contamination, and making it easier to meet the requirement of current good manufacturing practices (15). Due to fewer unit operations, the validation and documentation requirements are reduced. Due to the absence of water in granulation, chance of microbial growth is minimal in tablets prepared by direct compression (16).
Limitations of direct compression
Direct compression is more prone to segregation due to the difference in density of the API and excipients (15). The dry state of the material during mixing may induce static charge and lead to segregation. This may lead to the problems like weight variation and content uniformity. Directly compressible excipients are the speciality products produced by patented spray drying, fluid bed drying, roller drying or co-crystallization. Hence, the products are relatively costly than the respective raw materials. Most of the directly compressible materials can accommodate only 30-40 % of the poorly compressible active ingredients like acetaminophen that means the weight of the final tablet to deliver the 500 mg of acetaminophen would be more than 1300 mg. The large tablets may create difficulty in swallowing.
All the spray-dried directly compressible adjuvants show poor reworkability since on preparation of tablets the original spherical nature of the excipient particles is lost. API that has poor flow properties and/or low bulk density is difficult to process by direct compression. Lubricants have a more adverse effect on the filler, which exhibit almost no fracture or shear on compression (e.g. starch 1500). The softening effects as well as the hydrophobic effect of alkaline stearates can be controlled by optimising the length of blending time to as little as 2-5 min (5).There is a lack of awareness in some situations that the excipient behave differently, depending upon the vendor so much so that substitution from one source to that of another is not possible (10). Hence, there is a need for greater quality control in purchasing of raw material to assure batch uniformity.
Methods of preparing directly compressible excipients
Directly compressible adjuvant can be prepared by various methods. The outline and main features of the methods are depicted in Table 3 (11, 17, 18). Co-processing is the one of the most widely explored and commercially utilized method for the preparation of directly compressible adjuvants. Hence, co-processing is discussed in more depth in the present review.
Table 3: Summary of various methods used to prepare directly compressible adjuvant (11, 17, 18).

Co-processing
Co-processing is another way that new excipients are coming to market without undergoing the rigorous safety testing of a completely new chemical (19). It can be defined as combining two or more established excipients by an appropriate process (11). Co-processing of excipients could lead to the formation of excipients with superior properties compared to the simple physical mixtures of their components. The main aim of co-processing is to obtain a product with added value related to the ratio of its functionality/price. Development of co-processed directly compressible adjuvant starts with the selection of the excipients to be combined, their targeted proportion, selection of preparation method to get optimized product with desired physico-chemical parameters and it ends with minimizing avoidance with batch-to-batch variations. An excipient of reasonable price has to be combined with the optimal amount of a functional material in order to obtain integrated product, with superior functionality than the simple mixture of components.
Co-processing is interesting because the products are physically modified in a special way without altering the chemical structure. A fixed and homogenous distribution for the components is achieved by embedding them within minigranules. Segregation is diminished by adhesion of the actives on the porous particles making process validation and in process control easy and reliable (20).
The randomized embedding of the components in special minigranules minimizes their anisotropic behaviour. So, deformation can occur along any plane and multiple clean surfaces are formed during the compaction process. Thus, the use of the co-processed excipient combines the advantages of wet granulation with direct compression (20). The use of one-body components is justified if it results in a potentiation of the functionalities over that of the mere dry blend of the components prepared by gravity mixture. This synergistic effect should improve the quality of the tablet equally in all aspects ranging from hardness to dissolution and/or stability. Excipient mixtures in co-processing are produced to make use of the advantages of each component and to overcome specific disadvantages, if any. Most important characteristics are the binding and blending properties of the co-processed excipients, which must be better than those of a physical mixture of the starting materials. Cost is another factor to be considered in the selection of co-processed product.
Major limitation of co-processed excipient mixture is that the ratio of the excipients in a mixture is fixed and in developing a new formulation, a fixed ratio of the excipients may not be an optimum choice for the API and the dose per tablet under development (18). Co-processed adjuvant lacks the official acceptance in pharmacopoeia. For this reason, a combination filler-binder will not be accepted by the pharmaceutical industry until it exhibits significant advantages in the tablet compaction when compared to the physical mixtures of the excipients. Although the spray-crystallized dextrose-maltose (Emdex) and compressible sugar are co-processed products, they are commonly considered as single components and are official in USP/NF. Table 4 shows examples of co-processed directly compressible adjuvants.
Table 4: Co-processed directly compressible excipients (4, 5, 11, 16, 18, 19, 20).
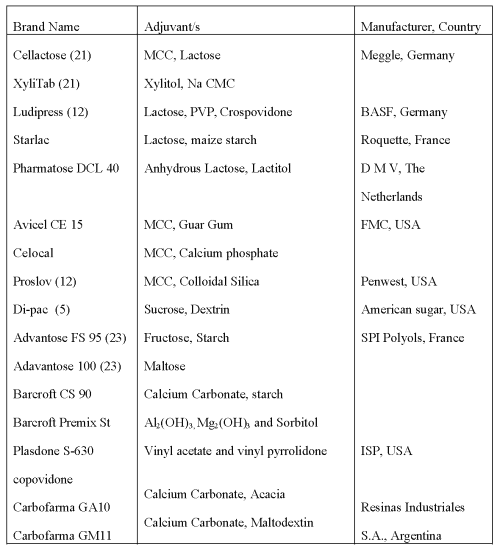
Examples of Directly Compressible Adjuvants
Lactose
It is one of the main constituents of human and mammalian milk. Lactose is produced from whey, as a by-product of cheese and casein production. Lactose may appear in different polymorphs depending on the crystallization conditions. Each polymorph has its specific properties. α-lactose monohydrate has very hard crystals and is non-hygroscopic. Lactose is the most widely used filler-diluent in tablets. The general properties of lactose that contribute to its popularity as an excipient are cost effectiveness, easy in the availability, bland taste, low hygroscopicity, excellent physical and chemical stability and water solubility (26). Lactose from different suppliers exhibits different properties and therefore could not be treated as interchangeable in direct compression formulations. The compaction profile of the lactose samples depends on the machine speed (27). Crystalline lactose mainly consolidates by fragmentation and amorphous lactose by plastic deformation. Tablets containing amorphous lactose show high crushing strength with increasing water content (28). Lactose based tablets exhibit better stability than mannitol and cellulose containing tablets at 40° C and 90% RH over a 10 week period (29). The amorphous lactose yields tablets of higher tensile strength than crystalline lactose. Tensile strength increases with reduced particle size (30).
a-Lactose Monohydrate
Coarse sieved fraction of α-lactose monohydrate (100 mesh) is used in direct compression due to its flowability. It contains about 5% w/w water. Compared to other filler-binders, a-lactose monohydrate exhibits relatively poor binding properties. It consolidates mainly by fragmentation. It has higher brittleness compared to spray-dried lactose and anhydrous b-lactose (31). α-lactose monohydrate (100 mesh) is often combined with microcrystalline cellulose. This combination results in a stronger synergistic effect on disintegration time, whereas the crushing strength increases as the percentage of microcrystalline cellulose in the blend is increased. The strength of tablets compressed from α-lactose monohydrate increases with a decrease in particle size of the excipient (32).
Gohel et al. prepared and evaluated lactose based directly compressible diluents. The preparation method consisted of controlled freezing and thawing of lactose solution. They concluded that the concentration of lactose and controlled nucleation are the most important parameters. In another method, the saturated solution of lactose was used for preparation of free-flowing agglomerates of lactose, where the volume of saturated lactose solution was found to be the most significant processing parameter. The developed products exhibited satisfactory flowability and compressibility essential for directly compressible diluent (33). Gohel et al. attempted to improve the flow and compressibility of lactose using a freeze-thaw method. They tried three binders like polyethylene glycol 6000, polyvinyl pyrrolidone and gelatin at 0.5, 1, 1.5, or 2% concentration. The agglomerates containing 1% PEG 6000 exhibited good direct compression characteristics. They compared its tableting performance with Microcelac using diclofenac sodium as a model drug candidate. The developed adjuvant exhibited satisfactory flowability, compressibility, granular friability and crushing strength of the tablets (34). Michoel reported that the MicroceLac 100 has superior flow and binding properties compared to three different lactose mixed with microcrystalline cellulose. It also showed good adhesion of folic acid particles, which could decrease demixing and segregation. The improved characteristics of co-processed material are attributed to spray drying (35). Gohel and Jogani developed and evaluated multifunctional co-processed directly compressible adjuvant containing lactose, polyvinylpyrrolidone, and croscarmellose sodium. This product has comparatively better flowability, compressibility and disintegration of the tablets than lactose monohydrate (36).
Anhydrous a-Lactose
Binding capacity of a-lactose monohydrate increases dramatically by thermal or chemical dehydration. During dehydration, a-lactose monohydrate changes from single crystals into aggregates of anhydrous a-lactose particles. The anhydrous crystals are softer, weaker and less elastic. It undergoes brittle fracture much more readily and at lower stresses than the lactose monohydrate (37). The relative slow disintegration of tablets containing anhydrous lactose is the major disadvantage (38). The anhydrous lactose exhibits lesser tendency for maillard reaction and better reworkability without loss of compressibility than the spray-dried lactose (39).
Anhydrous b-Lactose
The commercial product consists of agglomerates of extremely fine crystals. It is produced by roller drying of solution of a-lactose monohydrate followed by subsequent comminution and sieving (40). It has excellent compaction properties and low lubricant sensitivity. It exhibits less brittleness than the α-lactose monohydrate (31). Due to low moisture content, anhydrous β-lactose is an ideal excipient for moisture sensitive APIs. The anhydrous b-lactose is produced by crystallization of lactose above 93°C by roller drying (41). It has relatively better reworkability than other forms of lactose. It has higher dissolution rate than a-lactose monohydrate. It has solubility up to 10 times higher than the α-lactose monohydrate. Below 55% RH, anhydrous lactose with high b-content absorbs very small amount of water and its compression properties were insignificantly affected (42).
Spray-dried lactose
Spray-dried lactose is produced by spray drying the slurry containing lactose crystals. The final product contains mixture of crystals of lactose monohydrate and spherical agglomerates of small crystals held together by glass or amorphous material. The former contributes fluidity and the latter gives the compressibility to the product. It has excellent flow properties and binding properties. It deforms plastically compared to the same sized α-lactose monohydrate particles (32). Amorphous portion of the spray-dried lactose is responsible for the better binding and plastic deformation. Compressibility is affected if it is allowed to dry below a level of 3% w/w moisture. Disintegrant is required in the formulations containing spray-dried lactose. The tablets require a lubricant, but the lubricant does not affect binding. It has poor reworkability. Spray-dried lactose discolours with certain API containing an amine group.
Guncel and Lachman were the first to describe the spray-dried lactose. They reported that the spray-dried lactose produces harder, less friable tablets, which were more susceptible to colour development following storage at elevated temperature than the tablet containing conventional lactose (43). Tablets containing spray-dried lactose exhibited increase in crushing strength with decrease in the particle size. The spherical shaped spray-dried lactose particles resulted in the strongest tablets than the angular particles (44). The disintegration time of spray-dried lactose tablets was essentially independent of compaction force (45). The spray-dried lactose undergoes fragmentation (46). At low compaction pressure, tablets containing amorphous lactose disintegrated before gel or precipitate could block the pores. At higher compaction pressure, gelling and precipitation dominated the disintegration time. The lubricant present on the granules also influenced the disintegration time (47). Spray-dried lactose exhibited strong increase in disintegration time with increase in compression force (48).
Agglomerated Lactose
It is a granulated form of a-lactose monohydrate with improved binding properties. Tablettose is an example of agglomerated α-lactose demonstrates good flowability. It has binding property better than the α-lactose monohydrate but not as good as spray-dried lactose. Bolhuis concluded that excellent compactibility of Pharmatose DCL 15 (agglomerated lactose) was due to the presence of more b-lactose, providing strong intergranular cohesion (49).
Cellulose Derivatives
Microcrystalline Cellulose
Microcrystalline cellulose (MCC) is purified partially depolymerized cellulose, prepared by treating α-cellulose with mineral acids. It is a white, crystalline powder composed of agglomerated porous microfibers (17). After purification by filtration and spray-drying, porous microcrystal are obtained. Microcrystalline cellulose occurs as a white odourless, tasteless crystalline powder composed of porous particles of an agglomerated product. Apart from its use in direct compression, microcrystalline cellulose is used as a diluent in tablets prepared by wet granulation, as filler in capsules and for the production of spheres. In the pharmaceutical market, microcrystalline cellulose is available under the brand names Avicel, Emcocel, Vivacel etc.
Reier et al. reported that MCC tablets when exposed to increased humidity (75 %, 1 week) resulted in a softening and swelling of plain microcrystalline cellulose tablets. This change disappeared on removal of humid condition (50). Microcrystalline cellulose products exhibit capping tendencies at high compression speeds, while dicalcium phosphate was highly resistant to capping. Dittgen reported no correlation between the crystallinity and tableting properties of MCC obtained from various suppliers (Hewenten 40 & 12, Vivacel 101 &102, Avicel PH 101 & 200 and Sanaq PH 101L & 102L). Authors also reported difficulty in obtaining satisfactory tablets by direct compression using Sanaq PH 101L & 102L and attributed this behavior to higher bulk volume and poor compressibility (51).
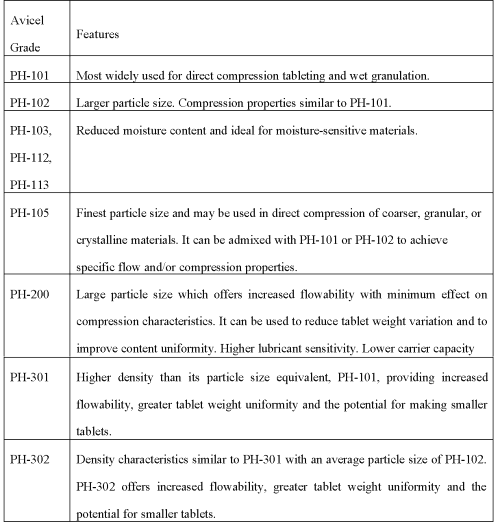
Lahdenpaa et al. demonstrated that the tablets containing higher percentage of Avicel PH101 exhibited higher crushing strength and lower disintegration time, while the tablets containing Avicel PH102 and PH 200 showed lower crushing strength, shorter disintegration time and small weight variation (52). Avicel PH 102 exhibited a much better fluidity because of its more granular form (48). Larger particles of microcrystalline cellulose (PH 102, PH 302 and SMCC 90) had better flowability and lubricity but lower compressibility. Denser particles of microcrystalline cellulose (PH 301 and PH 302) showed improved flowability, reduced lubricity and reduced compressibility (53). Obae et al. reported increase in the tensile strength of the tablets with increase in the ratio of length to diameter of particles. Celous KR 801 with more number of rod shaped particles than Avicel PH 101 yielded tablets with gave significantly higher tensile strength (54). Hardness of MCC tablets was decreased with an increase in the % of magnesium stearate while the disintegration time was unaffected by addition of lubricant (55). The physical and tableting properties of Emcocel are similar to those of Avicel (56). Paronen reported that Avicel PH-101 undergoes plastic deformation (46). Tsai and coworkers have prepared co-dried mixture of MCC and β-cyclodextrin. Authors demonstrated that the co-processed material exhibited significant improvement in flowability and compressibility than the physical blend of MCC and β-cyclodextrin (57). Garr demonstrated that incorporating up to 1% polyethylene to a mixture of 25% DCP and 75% MCC gave the intact compacts at the relatively low compaction force (58). Rues-Medina et al. reported that the UicelR 102 is more elastic than Avicel PH102 due to difference in the polymorphic form of microcrystalline cellulose present. The Uicel 102 is consists of cellulose II lattice, while Avicel PH 102 contains cellulose I polymorph (59). Levis evaluated co-processed microcrystalline cellulose - sodium lauryl sulphate prepared by an ultrasonic homogenization process followed by spray drying. The author concluded that the co-processed excipients were inferior compared with microcrystalline cellulose in a tableting for paracetamol, resulting largely from poor flow (60). Comparative properties of various grades of Avicel are depicted in Table 5.
Table 5: Comparative properties of various grades of Avicel (5, 19, 36).
Ishikawa et al. reported novel microcrystalline cellulose (PH-M Series) for preparation of rapidly disintegrating tablet using by direct compression. Study demonstrated that the acetaminophen or ascorbic acid tablets containing novel microcrystalline cellulose (PH-M Series; particle size, 7 - 32 micron) has decreased sensation of roughness and rapidly disintegrated by saliva when taken orally compared to conventional Avicel PH-102 (61). Garzo´n reported that the co-processed mixture of microcrystalline cellulose and calcium carbonate has compatibility equal or better than pure microcrystalline cellulose and tensile strength of the tablet decreased as the calcium carbonate increased (62). Kothari et al., compared the powder and mechanical properties of different batches of low crystallinity powdered cellulose (LCPC) with those of different grades of Avicel, Emcocel, Solka Floc BW-40 and Solka Floc BW-100 and concluded that the LCPC materials reported by them have powder properties that are quite different from the microcrystalline cellulose and powdered cellulose and can be recommended as a potential direct compression excipients (63). Hasegawa reported that the coarse grade microcrystalline cellulose 12 gives better results in terms if weight variation and content uniformity than the classic grade 102 (64).
Hydroxypropylcellulose
Alvarez-Lorenzo reported that the difference in flow and compaction properties, the mechanical and microstructural properties of the tablets prepared from various grades of low-substituted hydroxypropylcelluloses is attributed to difference in the specific surface (65).
Ethyl Cellulose
Crowley reported that the release rate of guaifenesin from ethyl cellulose matrix tablets prepared by direct compression was dependent on the ethyl cellulose particle size, and compaction force (66).
Sugars
Sucrose
Sucrose is widely used as filler in chewable tablets and as a binder in wet granulation (18). Bowe et al reported a co-processed sucrose based directly compressible adjuvant containing 95% sucrose and 5% sorbitol. Authors demonstrated that tablets with higher strength, which disintegrates faster can be produced using this material than tablets made with commercially available directly compressible sugars. Recently, directly compressible sugar is introduced by British sugar. It is a free flowing, directly compressible sugar comprising 95% icing sugar and 5% maltodextrin. It confirms to British pharmacopoeia monograph for compressible sugar.
Di-Pac
Di-Pac is a directly compressible, co-crystallized sugar consisting of 97% sucrose and 3% modified dextrin (5). It is a free flowing, agglomerated product consisting of hundreds of small sucrose crystals glued together by the highly modified dextrin. At high moisture level, Di-pac begins to cake and loose its fluidity. Tablets containing a high proportion of Di-pac tend to harden after compression at higher relative humidity. Its sweet taste makes it suitable for most directly compressible chewable tablets.
Rizzuto et al., demonstrated that co-crystallized sucrose and dextrin deformed readily by plastic fracture to provide much harder compacts than those obtained from sucrose crystals alone (67).
Nu-Tab
Nu-Tab is a roller compacted granulated product consisting of sucrose, invert sugar, cornstarch and magnesium stearate. It has better flowability due to relatively larger particles but has poor colour stability compared to other directly compressible sucrose and lactose. It is primarily used for preparation of chewable tablets by direct compression.
Emdex and Maltrin
Emdex is produced by hydrolysis of starch and consists of aggregates of dextrose microcrystals intermixed and cohered with a small quantity of higher molecular weight sugars. Emdex occurs as white, free flowing, porous spheres which are water soluble and non-hygroscopic. Emdex is generally used in directly compressible chewable tablets because of its sweet taste. It has good binding properties and slight lubricant sensitivity. It exhibits high moisture sensitivity, at room temperature and at 50% RH, the crushing strength of tablets decreases dramatically, whereas during storage at 85% RH tablets liquefy (68). Tablets containing theophylline prepared using Emdex exhibited higher mechanical strength, faster disintegration and rapid drug release than the tablets prepared from Maltrin M150 (69).
Mannitol
It is water soluble, non-hygroscopic and produces a semi-sweet, smooth, cool taste. It can be advantageously combined with other direct compression excipients. Sangekar et al. reported mannitol as a best sugar for chewable tablet formulation prepared by direct compression out of twenty-four formulations of placebo tablets, made from 8 excipients and 3 disintegrants (70).
Starch
Mullick et al. reported that dextrinized rice, corn, wheat and tapioca starches prepared by dextrinization exhibited very good flow, compression properties and disintegration qualities for direct compression tableting. Dextrinized tapioca starch was found to be the best (71). Preflo starch exhibited high bulk density and good flowability than starch 1500 and Star Tab as directly compressible excipients. Preflo starch containing tablets exhibited prolonged disintegration time (30 min) than the Starch 1500 (3.5 min). Preflo cornstarch formed harder tablets compared to Preflo potato starch (72). The directly compressible starch (Starch 1500) is relatively fluid, did not require a lubricating agent when compressed alone, more effective as a dry binder and gives equivalent or faster disintegration and dissolution compared to starch USP (73). Due to improved flowability and compressibility pregelatinzed starch can be used as a binder in direct compression (74).
Starch 1500
It is a directly compressible, free flowing, USP grade of partially hydrolyzed cornstarch. It is prepared by subjecting cornstarch to physical compression or shear stress in high moisture conditions causing an increase in temperature and a partial gelatinization of some of the starch granules. The product is consists of about 5% free amylose, 15% amylopectin and 80% unmodified starch (18). It provides fair to good binding properties and dilution potential, but requires high pressures to produce hard tablets. It also produces a dense tablet with good disintegration properties. Starch 1500 exhibits self-lubricating property. It has poor flowability compared to other directly compressible adjuvants and shows higher lubricant sensitivity. It is also used as filler in capsule formulation. Monedero Perales et al. demonstrated that Starch 1500 exhibited better flowability and lower binding property and plasticity than the Sepistab 200 (75). Terfenadine tablets prepared using rice starch (Era Tab) exhibited higher crushing strength and lower friability than partially pregelatinized starch, Super-Tab, Emcompress and lower than Avicel PH 101 (76).
Uni-Pure is a fully gelatinized maize starch. It gives tablets with strong binding properties and significantly faster disintegration (74). Clausen reported co-processed polymethacrylic acid-starch as a pH-sensitive directly compressible excipient for controlled delivery of model drugs amoxicillin and rifampicin (77).
Maltose
Advantose 100 is a spray-dried maltose having spherical particles with an optimal combination of fine and coarse particles that contributes superior flow. Compared to microcrystalline cellulose, spray dried maltose can tolerate significantly greater compression force without capping upon ejection from the tablet die; it has low hygroscopicity and low reactivity than microcrystalline cellulose (25).
Dicalcium Phosphate Dihydrate
Dicalcium phosphate is the most common inorganic salt used in direct compression as a filler-binder. Advantage of using dicalcium phosphate in tablets for vitamin and mineral supplement is the high calcium and phosphorous content. Dicalcium phosphate dihydrate is slightly alkaline with a pH of 7.0 to 7.4, which precludes its use with active ingredients that are sensitive to even small amount of alkali (i.e. ascorbic acid). It exhibits high fragmentation propensity. Rees et al., studied time dependent deformation of few directly compressible excipients. Authors reported that the increase in dwell time had insignificant effect on dicalcium phosphate dihydrate compacts whereas increase in dwell time increased the consolidation of other materials in the rank order sodium chloride, anhydrous lactose, microcrystalline cellulose and modified starch (78). Panaggio et al. studied the effects of varying proportions of dicalcium phosphate dihydrate and modified starch matrices in tablets prepared by direct compression and observed that at some concentrations, properties of tablets were intermediate between those of the pure components and varied linearly with small changes in relative proportions (79). Water of crystallization of dicalcium phosphate dihydrate could possible be released during processing and thus chemically interact with hydrolysable drug (80). Schüssele characterized the flowability of commonly used directly compressible adjuvants using Sotax Powder Flow Tester from good flow to poor flow in following order: Emcompress, Tablettose 80, Fujicalin, Tablettose 100, Starch and Avicel (81). Holte reported use of directly compressible alginates (Protanal LF 120 L, Protanal LF 120M, Protanal LV 120D, Protanal SF 120) in combination of dicalcium phosphate in formulation of sustained release acetyl salicylic acid directly compressible tablets (82).
Emcompress
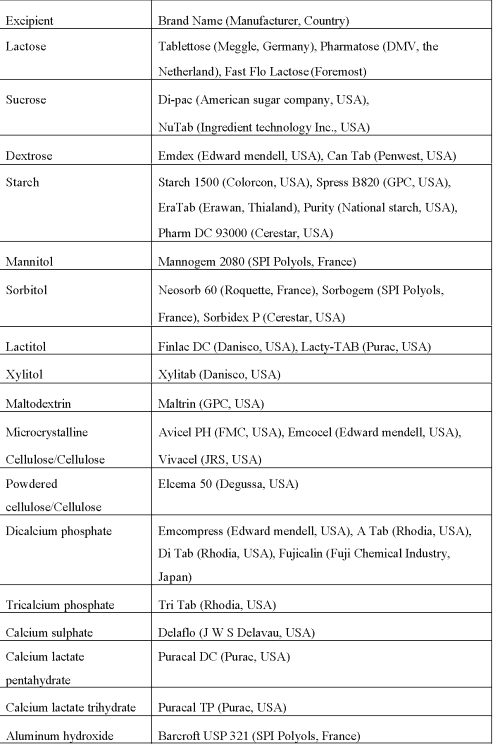
Emcompress consists of aggregates of small primary particles of dicalcium phosphate. Unlubricated Emcompress tablets are difficult to eject from dies, therefore, it requires high lubrication. Hardness of tablets containing Emcompress is insensitive to tablets machine speed and lubricant such as magnesium stearate due to the fragmentation behaviour during compression and consolidation. It can be good directly compressible adjuvant when used in combination with microcrystalline cellulose or starch (45). Dolden et al. reported that intraparticulate porosity and mean yield pressure of the dicalcium phosphate anhydrous product are higher than that of the dicalcium phosphate dihydrate (Emcompress). Authors further demonstrated that Compacts of the anhydrous product disintegrated much more rapidly in distilled water than did those of the dihydrate (83). In addition to the above commonly used directly compressible excipients various other directly compressible filler binder available in market are shown in Table 6.
Table 6: Examples of some directly compressible adjuvants.
Fujicalin
Fujicalin is a spherically granulated dicalcium phosphate anhydrous prepared by spry-drying. It has lower particle size, high porosity and high specific surface area. Fujicalin gives significantly stronger tablets than Di-Cafos (80).
Inlulin
Eissens reported effect of chain length, particle size and amount of included air in the particles of inluin on flow properties and tableting properties. Particles with larger size showed better flowability. A high lubricant sensitivity was found for amorphous inulin with a low amount of entrapped air. The disintegration/dissolution time increased with decreasing chain length of the inulin (84). Hollow inulin particles have an increased compactibility as compared with solid inulin particles and a strongly reduced lubricant sensitivity (85).
Co-Processed Diretly Compressible Adjuvants
Ludipress
Ludipress, a co-processed product, consists of 93.4% a-lactose monohydrate, 3.2% polyvinyl pyrrolidone (Kollidon 30) and 3.4% crospovidone (Kollidon CL). It consists of lactose powder coated with polyvinyl pyrrolidone and crospovidone (23). Although, Ludipress contains disintegrant, the disintegration of tablets takes longer than tablets containing α-lactose monohydrate, Tablettose and anhydrous b-lactose (27). At low compression force Ludipress gives harder tablets but the addition of glidant and disintegrant is needed. It is reported that binding capacity of Ludipress was higher than that of microcrystalline cellulose. The dilution potential was high (upto 70%) when aspirin was used a model drug (86). Baykara et al. reported that the dilution potential of LudipressR with paracetamol is lower than that of Avicel PH 101, Elcema G250 and Elcema P050 (87). The binding properties of Ludipress, both unlubricated and lubricated with 1% magnesium stearate was found to be much better than corresponding physical mixture (87). Plaizier-Vercammen et al. reported that the addition of a lubricant was necessary and its mixing time had little effect on crushing strength of Ludipress tablets. Authors also reported that Ludipress exhibits better tableting characteristics for low dose APIs, and good batch-to-batch uniformity than Cellactose (88). The compressibility of Ludipress is similar to that of Avicel PH 200. The disintegration time of Ludipress containing tablets remained unchanged at about 100 MPa compaction pressure while significant prolongation was observed with Cellactose (89, 90). Schmidt and Rubensdorfer reported that the tablets manufactured with Ludipress exhibited optimum disintegration time and compaction pressure independent dissolution of glibenclamide. While, increasing compaction pressure had a negative effect on drug dissolution from compacts containing Cellactose (90). It has been reported that among various lactose based directly compressible excipients, Ludipress exhibited a better flow rate compared to Avicel PH 101 (91). Ludipress exhibited highest flowability followed by Cellactose, Tablettose, Fast Flo lactose and anhydrous lactose as demonstrated by lower static and dynamic angles of repose than the other excipients (92). The values of compressibility could be ranked from maximum to minimum in the following order: Tablettose, Cellactose, Ludipress and Fast Flo lactose. Fragmentation propensity was from maximum to minimum in Tablettose, Cellactose, Ludipress and Fast-Flo lactose (93).
Cellactose
Cellactose is a co-processed product consisting a-lactose monohydrate (75%) and cellulose (25%). Apart from good flowability, it has good compactibility. The compactibility is attributed to a synergetic effect of consolidation by fragmentation of lactose and plastic deformation of cellulose (94). Because the lactose covers the cellulose fibers, moisture sorption is much lower than that of microcrystalline cellulose alone. Aufmuth et al reported that the Cellactose exhibited increased crushing strength of the compacts along with reduced friability and lower disintegration time than the dry blend of lactose and cellulose (20). Armstrong et al. pointed that Cellactose exhibit the dual consolidation behaviour since it contains a fragmenting component (lactose) and a substance that consolidates primarily by plastic deformation (Cellulose) (95).
Ruiz et al. and Reimerdes found that the Cellactose exhibited better compressibility compared to Ludipress, Fast Flo lactose, Tablettose, Di-pac and anhydrous lactose (11, 88). Belda and Mielck found that due to co-processing Cellactose exhibited enhanced crushing strength compared to the powder mixtures each containing 25% w/w Avicel PH-101 or Elcema P-100 and 75% w/w Tablettose or lactose (100#) (96). Casalderrey et al reported that the Cellactose tablets prepared at a compression pressure that largely eliminated macro pores had better mechanical properties but much poorer disintegration than tablets of the other blends having similar composition, particle size, and true density at the same punch pressure. Authors further reported that the tensile strength and disintegration time of Cellactose tablets decreased rapidly as the compression pressure is reduced (97). Gohel and Jogani prepared and evaluated co-processed directly compressible adjuvant containing lactose and microcrystalline cellulose using starch as a binder. The percentage fines, Carr's index of the agglomerates as well as friability and tensile strength of the tablets were affected by the ratio of lactose to microcrystalline cellulose and percentage of starch in binder solution. A product containing lactose: microcrystalline cellulose (9:1) and 1% starch paste exhibited satisfactory flow, compressibility and friability. Tablets of diltiazem hydrochloride and acetaminophen prepared using the co-processed excipients exhibited satisfactory tableting properties (98). Gohel et al. prepared and evaluated co-processed diluents containing lactose and microcrystalline cellulose using a 23 factorial design. Ratio of lactose to MCC (75: 25 and 85:15), type of binder (hydroxypropyl methylcellulose or dextrin) and binder concentration (1 or 1.5%) were studied as independent variables. The results revealed that the lactose: microcrystalline cellulose ratio 75:25 and dextrin as a binder are better than the ratio of 85:15 and hydroxypropyl methylcellulose as a binder. The tableting properties of the developed adjuvant were ascertained using diltiazem HCl as a model drug (99). Gohel and Jogani prepared co-processed directly compressible adjuvant containing lactose and microcrystalline cellulose using melt granulation technique (100). Gohel et al. demonstrated use of factorial design in development of directly compressible adjuvant of desired characteristics consisting of lactose, dicalcium phosphate and microcrystalline cellulose (101).
Pharmatose DCL 40
It is a co-processed product consisting of 95% b-lactose and 5% anhydrous lactitol. Due to spherical shape and favourable particle size, it exhibits good flowability. It has high dilution potential than other lactose based products due to better binding property. It has very low water uptake at high humidity (18).
Prosolv
It is co-processed silicified microcrystalline cellulose. It consists of 98% microcrystalline cellulose and 2% colloidal silicone dioxide. The manufacturer claim better flowability and compressibility compared to Emcocel and Avicel PH 101 or physical mixture of MCC with colloidal silicone dioxide (53, 102). Allen reported that Prosolv containing tablets were significantly robust than those produced from regular cellulose by wet granulation. In the presence of magnesium stearate (0.5 %), tablets prepared with Prosolv maintained tensile strength profiles, whereas the tensile strength of regular cellulose was significantly affected. Author further reported that Prosolv is about 20% more compactable than regular cellulose (103). Fraser et al reported that silicified microcrystalline cellulose has some improvement in flow but considerably enhanced mechanical properties (104). Lahdenpaa et al. demonstrated that Silicified microcrystalline cellulose is useful to prepare tablet containing poorly compressible ingredients by direct compression (105). The silicification affects the moisture sorption and the packing during tapping as well as the particle deformation during tableting. Prosolv showed slight increase in the tensile strength but marked increase in the disintegration time of the tablets compared to Avicel (106). Bolhuis et al. demonstrated that the co-processing of microcrystalline cellulose with colloidal silicone dioxide has no significant contribution on the tablet strength of lubricated tablets containing the physical mixture of microcrystalline cellulose and colloidal silicone dioxide (107).
StarLac
Starlac is a co-processed excipient consists of lactose monohydrate and maize starch produced by spray drying (108) The advantage of Starlac are its good flowability depending on the spray-drying process, an acceptable crushing force due to its lactose content, its rapid disintegration depending on starch (109). Gohel and Jogani demonstrated use of multiple linear regression in development of co-processed lactose and starch. Authors concluded that as the lactose/starch ratio increased Carr's index of the adjuvant and crushing strength of the tablets increased while friability decreased. Percentage of starch paste has inverse effect on the friability (110).
As discussed in this review, it is clear that no single excipient fulfils all the optimum requirements. In most instances evaluation of tableting properties of these excipients are required before selecting them as a part of formulation. Each directly compressible adjuvant has merits and demerits hence; there is still need for directly compressible adjuvant, which exhibits a satisfactory performance.
Some of the parameters and their importance are briefly outlined in the Table 7 and for further information on directly compressible adjuvants the web-address of the manufacturers of directly compressible adjuvant are given Table 8.
Table 7: Parameters useful in evaluation of directly compressible.
Table 8: List of web sites of directly compressible adjuvant manufacturers.

References
Sam, A. P., and Fokkens, J. G., Drug Delivery System: Adding Therapeutic and Economic Value to Pharmacotherapy. Part 2, Pharm. Tech. Eur., 9: 58-66, 1997.
Rasenack, N., and Muller, B. W., Crystal Habit and Tableting Behaviour, Int. J. Pharm., 244: 45-57, 2002.
Khan, K. A. and Rhodes, C. T., The Production of Tablets by Direct Compression, Can. J. Pharm. Sci., 8:1-5, 1973.
Shangraw, R. F., Compressed Tablets by Direct Compression Granulation Pharmaceutical Dosage Forms: Tablets, Vol-1, Marcel Dekker, USA, 2nd ed, 195-246, 1989.
Shangraw, R. F., Direct Compression Tableting, Encyclopedia of Pharmaceutical Technology, Vol-4, Marcel Dekker, USA, 2nd ed., 85-160, 1988.
Shangraw, R. F., and Demarest, D. A., Survey of Current Practices in the Formulation and Manufacture of Tablets and Capsules, Pharm. Technol., 17: 32-44, 1993.
Robertson, M. I., Regulatory Issues with Excipients, Int. J. Pharm., 187: 273-276, 1999.
Silverstein, I., Excipient GMP Quality Standards One is Enough, Pharm. Technol., 25: 46-52, 2002
Armstrong, N. A., Functionality Related Tests for Excipients, Int. J. Pharm., 155: 1-5, 1997.
Banker, U. V., Role of Ingredients and Excipients in Developing Pharmaceuticals, Manuf. Chem., 65: 32-34, 1994.
Reimerdes, D., The Near Future of Tablet Excipients, Manuf. Chem., 64:14-15, 1993.
Jivraj, M., Martini, L. G., and Thomson, C. M., An Overview of the Different Excipients Useful for the Direct Compression of Tablets, PSTT, 3: 58-63, 2000.
Armstrong, N. A., Selection of excipients for direct compression tablet formulation, Pharm. Technol. Eur., 9: 24-30, 1997.
Smewing, J., Powder flow analysis- the solution, Manuf. Chem., 32-33, 2002.
Rubinstein, M H., Tablets Pharmaceutics: The Science of Dosage of Form, Churchill, UK, 1st ed., 304-321, 1998.
Ibrahim, Y. K., and Olurinola, P. F., Comparative Microbial Contamination Levels in Wet Granulation and Direct Compression Methods of Tablet Production, Pharm. Acta. Helv., 66: 298-301, 1991.
Shangraw, R. F., Wallace, J. W., and Bowers, F. M., Morphology and Functionality in Tablet Excipients for Direct Compression, Pharm. Technol., 11: 136-143, 1987.
Bolhuis, G. K., and Chowhan, Z. T., Materials for Direct Compression, Pharmaceutical Powder Compaction Technology, Vol-7, Marcel Dekker, USA, 419-499, 1996
Russell, R., Synthetic Excipients Challenge All-Natural Organics —Offer advantages/ Challenges to Developers and Formulators, Pharm. Technol., 27: 38 – 50, 2004
Reimerdes, D., and Aufmuth, K. P., Tabletting with Co-processed Lactose-Cellulose Excipients, Manuf. Chem., 63: 21-24, 1992.
Puri, V., and Bansal, A., Pharmaceutical Technology: Challenges and Opportunities, Crips, 2: 2-11, 2001.
Product Information Brochure, Penwest, USA, 2000.
Product Information Brochure, SPI Polyols, France, 1999.
Joshi, V., Excipient choice in solid dosage forms, Drug. Del. Technol., 2 : http://www.drugdeliverytech.com/cgi-bin/articles.cgi?idArticle=69, 2002,
Antonio Moronim, A Novel Copovidone Binder for Dry Granulation and Direct-Compression Tableting, Pharm. Technol., 24: 8-12.
Guo, J., Lactose in Pharmaceutical Applications, Drug. Del. Technol., 4: http://www.drugdeliverytech.com/cgi-bin/issues.cgi?idIssue=26, 2004.
Whiteman., M., and Yarwood., R. J., Evaluation of Six Lactose-Based Materials as Direct Compression Tablet Excipients, Drug Dev. Ind. Pharm., 14: 1023-1040, 1988.
Lerk, C. F., Consolidation and Compaction of Lactose, Drug Dev. Ind. Pharm., 19: 2359-2398, 1993.
Molokhia, A. M., Al-Shora, H. I., and Hammad, A. A., Aging of Tablets Prepared by Direct Compression of Bases with Different Moisture Content, Drug Dev. Ind. Pharm., 13: 1933-1946, 1987.
Sebhatu, T., and Alderborn, G., Relationships Between the Effective Interparticulate Contact Area and the Tensile Strength of Tables of Amorphous and Crystalline Lactose of Varying Particle Size, Eur. J. Pharm. Sci., 8: 235-242, 1999.
Roberts, R. J., and Rowe, R. C., Effect of Punch Velocity On the Compaction of a Variety of Materials, J. Pharm. Pharmacol., 37: 377-384, 1985.
Fell, J. T., and Newton, J. M., The Tensile Strength of Tablets, J. Pharm. Sci., 20: 657-658, 1968.
Gohel, M. C., Patel, L. D., Murpani, D., and Iyer, L., Preparation of Directly Compressible Lactose and Optimization of Characteristics Affecting Direct Compression, Indian Drugs, 34: 322-326, 1997.
Gohel, M. C., Patel, L. D., Amin, A. F., Jogani, P. D., Bajaj, S. B., and Patel, G. J., Studies in Improvement of Flow and Compressional Characteristics of Lactose, Int. J. Pharm. Excip., 1: 86-92, 1999.
Michoel A, Rombaut P, Verhoye A., Comparative evaluation of co-processed lactose and microcrystalline cellulose with their physical mixtures in the formulation of folic acid tablets, Pharm Dev Technol. 7:79-87, 2002.
Gohel, M. C., and Jogani, P. D., Functionality Testing of a Multifunctional Directly compressible Adjuvant Containing Lactose, Polyvinylpyrrolidone, and Croscarmellose Sodium, Pharm. Technol., 25, 64 –82, 2002.
Wong, D. Y., Wright, P., and Aulton, M. E., Deformation of Alpha-Lactose Monohydrate and Anhydrous Alpha-Lactose Monocrystals, Drug Dev. Ind. Pharm., 14: 2109-2126, 1988.
Van Kamp, H. V., Bolhuis, G. K., Kussendrager, K. D., and Lerk, C. F., Studies on Tableting Properties of Lactose IV. Dissolution and Disintegration Properties of Different Types of Crystalline Lactose, Int. J. Pharm., 28, 229-233, 1986.
Mendell, E. J., Direct Compression Method of Producing Solid Dosage Forms. Parts 1-3, Manuf. Chem. Aero. News, 43: 47-49, 1972.
Shangraw, R. F., Wallace, J. W., and Bowers, F. M., Morphology and Functionality in Tablet Excipients for Direct Compression: Part I, Pharm. Technol., 5: 69-78, 1981.
Steven, P., and Zimmerman, K., Lactose: the Natural Excipient, Manuf. Chem., 57: 77-80, 1986.
Shukla, A. J., and Price, J. C., Effect of Moisture Content on Compression Properties of Directly Compressible High Beta-content Anhydrous Lactose, Drug Dev. Ind. Pharm., 17: 2067-2081, 1991.
Gunsel, W. C., and Lachman, L., Comparative Evaluation of Tablet Formulations Prepared from Conventionally processed and Spray-dried Lactose, J. Pharm. Sci., 52: 178-182, 1963.
Alpar, O., Hersey, J. A., and Shotton, E., The Compression Properties of Lactose, J. Pharm. Pharmacol., 22: 1S-7S, 1970.
Khan, K. A., and Rhodes, C. T., Effect of Variation in Compaction Force on Properties of Six Direct Compression Tablet Formulation, J. Pharm. Sci., 65: 1835-1837, 1976.
Paronen, P., Behavior of Some Direct Compression Adjuvants During the Tableting Process, S.T.P. Pharma Pratiques., 2: 682-688, 1986.
Vromans, H., Bolhuis, G. K., Lerk, C. F., and Kussendrager, K. D., Studies on Tableting properties of Lactose VIII. The Effect of Variations in Primary Particle Size, Percentage of Amorphous Lactose and Addition of a Disintegrant on the Disintegration of Spray-dried Lactose Tablets, Int. J. Pharm., 39: 201-206, 1987.
Bolhuis, G. K., and Lerk, C. F., Comparative Evaluation of Excipients for Direct Compression. Part 1, Pharma. Weekb, 108: 469-481, 1973.
Bolhuis, G. K., and Zuurman, K., Tableting properties of Experimental and Commercially Available Lactose Granulations For Direct Compression, Drug Dev. Ind. Pharm., 21: 2057-2071, 1995.
Reier, G. E., and Shangraw, R. F., Microcrystalline Cellulose in Tableting, J. Pharm. Sci., 55: 510-514, 1966.
Dittgen, M., Microcrystalline Cellulose in Tableting, Manuf. Chem., 64: 17-21, 1993.
Lahdenpaa, E., Niskanen, M., and Yliruusi, J., Crushing Strength, Disintegration time and Weight Variation of Tablets Compressed from Three Avicel PH Grades and their Mixtures, Eur. J. Pharm. Biopharm., 43, 315-322, 1997.
Hwang, R., and Peck, G. R., A Systematic Evaluation of the Compression and Tablets Characteristics of Various Types of Microcrystalline Cellulose, Pharm. Technol., 24: 112-132, 2001.
Obae, K., Iijima, H., and Imada, K., Morphological Effect of Microcrystalline Cellulose Particles on Tablet Tensile Strength, Int. J. Pharm., 182: 155-164, 1999.
Mitrevej, A., Sinchaipanid, N., and Faroongsarng, D., Spray-Dried Rice Starch: Comparative Evaluation of Direct Compression Fillers, Drug Dev. Ind. Pharm., 22: 587-594, 1996.
Pesonen, T., and Paronen, P, Evaluation of a New Cellulose Material as Binding Agent for Direct Compression of Tablets, Drug Dev. Ind. Pharm., 12: 2091-2111, 1986.
Tsai, T., Wu, J., Ho, H., and Sheu, M., Modification of physical characteristics of microcrystalline cellulose by codrying with b-cyclodextrin, J. Pharm. Sci, 87: 117-122, 1998.
Garr, J. S., Comparative Investigation of Novel Direct Compression Excipients, African J. Pharm. Pharma. Sci., 22: 250-256, 1992.
Reus-Medina, M., Lanz, M., Kumar, V., and Leuenberger, H., Comparative evaluation of the powder properties and compression behaviour of a new cellulose based direct compression excipient and Avicel PH 102, J. Pharm. Pharmacol., 56: , 951-956.
Levis, S.R., Deasy, P.B., Pharmaceutical applications of size reduced grades of surfactant co-processed microcrystalline cellulose, Int. J. Pharm., 230(1-2): 25-33, 2001.
Ishikawa, T., Mukai, B., Shiraishi, S., Utoguchi, N., Fujii, M., Mitsuo Matsumoto, and Watanaba, Y., Preparation of Rapidly Disintegrating Tablet Using New Types of Microcrystalline Cellulose (PH-M Series) and Low Substituted-Hydroxypropylcellulose or Spherical Sugar Granules by Direct Compression Method, Chem. Pharm. Bull,. 49: 134 –139, 2001.
Lourdes Garzo’n, M., and Villafuerte, L., Compactibility of mixtures of calcium carbonate and microcrystalline cellulose, Int. J. Pharm., 231: 33 - 41, 2002.
Kothari, S. H., umar, V., and Banker, G. S., Comparative evaluations of powder and mechanical properties of low crystallinity celluloses, microcrystalline celluloses, and powdered celluloses, Int. J. Pharm., 232: 69-80, 2002.
Hasegawa, M., Direct Compression Microcrystalline Cellulose Grade 12 versus Classic Grade 102, Pharm. Technol., 25: 50-60, 2002.
Alvarez-Lorenzo, C., Gomez-Amoza J. L., Martinez-Pacheco, R., Souto, C., and Concheiro, A., Evaluation of low-substituted hydroxypropylcelluloses as filler-binders for direct compression, Int. J. Pharm., 197(1-2): 107-116, 2000.
Crowley, M. M., Schroeder, B., Fredersdorf, A., Obara, S., Talarico, M., Kucera, S., and McGinity. J. W., Physicochemical properties and mechanism of drug release from ethyl cellulose matrix tablets prepared by direct compression and hot-melt extrusion, Int. J. Pharm., 269 (2): 509-522, 2004.
Rizzuto, A. B., Chen, A. C., and Veiga, M. E., Modification of the Sucrose Crystal Structure to Enhance Pharmaceutical Properties of Excipient and Drug Substances, Pharm. Technol., 8: 32-39, 1984.
Bolhuis, G. K., Reichman, G., Lerk, C. F., Van Kamp, H. V., and Zuuarman, K., Evaluation of Anhydrous Alpha-Lactose, a New Excipient in Direct Compression, Drug Dev. Ind. Pharm., 11: 1657-1681, 1985.
Olmo, I. G., and Ghaly, E. S., Evaluation of Two Dextrose-Based Directly Compressible Excipients, Drug. Dev. Ind. Pharm., 24, 771-778, 1998.
Sangekar, S. A., Sarli, M., and Sheth, P. R., Effect of Moisture on Physical Characteristics of Tablets Prepared from Direct Compression Excipients, J. Pharm. Sci., 61: 939-944, 1972.
Mullick, S., Sa, B., and Bandyopadhyay, A. K., Development of Some New Direct-Compression Carriers from Indigenous Starches for Tablet Compression, Indian Drugs, 29: 676-679, 1992.
Sanghvi, P. P., Collins, C. C., and Shukla, A. J., Evaluation of Preflo Modified Starches as New Direct Compression Excipients-I: Tableting Characteristics, Pharm. Res., 10: 1597-1603, 1993.
Manudhane, K. S., Contractor, A. M., Kim, H. Y., and Shangraw, R. F., Tableting Properties of a Directly Compressible Starch, J. Pharm. Sci., 58: 616-620. 1969.
Heinze, F., Starch – the natural Excipient choice, Manuf. Chem., 40 - 42, 2002.
Monedero Perales, M. C., Munoz-Ruiz, A., Velasco Antequera, M. V., Munoz Munoz, N., and Jimenez-Castellanos, M. R., Comparative Tableting and Microstructural Properties of a New Starch for Direct Compression, Drug Dev. Ind. Pharm., 22: 689-695, 1996.
Hsu, S. H., Tsai, T. R., Chuo, W. H., and Cham, T. M., Evaluation of Era-Tab as a Direct Compression Excipient, Drug Dev. Ind. Pharm., 23: 711-716, 1997.
Clausen, A. E., and Bernkop-Schnurch, A., Direct compressible polymethacrylic acid-starch compositions for site-specific drug delivery, J.Con. Rel., 75(1-2): 93-102, 2001.
Rees, J. E., and Rue, P. J., Time Dependent Deformation of Some Direct Compression Excipients, J. Pharm. Pharmacol., 30: 601-607, 1978.
Panaggio, A., Rhodes, C. T., and Schwartz, J. B., Properties of Mixtures of Two Tablet Matrices, Pharm. Acta Helv., 59: 37-39, 1984.
Schlack, H., Bauer-Brandl, A., Schuber, R., and Becker, D., Properties of Fujicalin®, a New Modified Anhydrous Dibasic Calcium Phosphate for Direct Compression: Comparison with Dicalcium Phosphate Dihydrate, Drug. Dev. Ind. Pharm., 27: 789-801, 2001.
Schüssele, A., and Bauer-Brand, A., Note on the measurement of flowability according to the European Pharmacopoeia, Int. J. Pharm., 257: 301–304, 2003.
Holte,O., Onsøyen, E., Myrvold, R., and Karlsen, K., Sustained release of water-soluble drug from directly compressed alginate tablets, Eur.J. Pharm. Sci., 20: 403–407, 2003.
Doldan, C., Souto, C., Concheiro, A., Martinez-Pacheco, R., and Gomez-Amoza, J. L., Dicalcium Phosphate Dihydrate and Anhydrous Dicalcium Phosphate for Direct Compression: Comparative study, Int. J. Pharm., 124: 69-74, 1995.
Eissens, A.C., Bolhuis, G.K., Hinrichs, W. L., and Frijlink, H.W., Inulin as filler-binder for tablets prepared by direct compaction, Eur. J. Pharm. Sci., 15: 31-38, 2002.
Eissens, A.C., Bolhuis, G.K., Adrichem, T. P., Wesselingh, J. A., and Frijlink, H.W., Hollow Filler-Binder as Excipients for Direct Compression, Pharm. Research, 20: 515-518, 2003.
Plaizier-Vercammen, J. A., and Van Den Bossche, H., Evaluation of the Tableting Properties of a New excipient for Direct Compression, Pharm. Ind., 54: 973-977, 1992.
Baykara, T., Duman, G., Ozesener, K. S., Ordu, S., and Ozates, B., Comparing the Compressibility of Ludipress with the other Direct Tabletting Agents by Using Acetaminophen as an Active Ingredient, Drug. Dev. Ind. Pharm., 17: 2359-2371, 1991.
Plaizier-Vercammen, J. A., and Van Den Bossche, H., Evaluation of the Tableting Properties of a New Excipient for Direct Compression, Drugs Made in Germany, 36: 133-137, 1993.
Schmidt, P. C., and Rubensdorfer, C. J., Evaluation of Ludipress as a Multipurpose Excipient for Direct Compression. Part 1. Powder Characteristics and Tableting Properties, Drug Dev. Ind. Pharm., 20: 2899-2925, 1994.
Schmidt, P. C., and Rubensdorfer, C. J., Evaluation of Ludipress as a Multipurpose Excipient for Direct Compression Part II: Inactive Blending and Tableting with Micronized Glibeclamide, Drug Dev. Ind. Pharm., 20: 2927-2952, 1994.
Angel Munoz-Ruiz, M., Borrero-Rubio, J. M., and Jimenez-Castellanos, M. R., Rheology of a New Excipient for Direct Compression: Ludipress, Pharm. Acta. Helv., 67: 223-226, 1992.
Angel Munoz-Ruiz, M., Perales, C. M., Antequeva, V. V., and Villar, T., Rheology and Compression Characteristics of Lactose Based Direct Compression Excipients, Int. J. Pharm., 95: 201-207, 1993.
Monedero Perales, M. C., Munoz-Ruiz, A., Velasco Antequera, M. V., and Jimenez-Castellanos M. R., Study of the Compaction Mechanisms of Lactose-Based Direct Compression Excipients Using Indentation Hardness and Heckel plot, J. Pharm. Pharmacol., 46: 177-181, 1994.
Garr, J. S., and Rubinstein, M. H., Compaction Properties of a Cellulose-Lactose Direct Compression Excipient, Pharm. Tech. Int., 3: 24-27, 1991.
Armstrong, N. A., Roscheisen, G., and Al-Aghbar, M. R., Cellactose As a Tablet Diluent, Manuf. Chem., 67: 25-26, 1996.
Belda, P. M., and Mielck, J. B, The Tabletting Behaviour of Cellactose Compared With Mixtures of Celluloses with Lactoses, Eur. J. Pharm. Biopharm., 42: 325-330, 1996.
Casalderrey, M., Souto, C., Concheiro, A., Gomea-Amoza, J. L., and Martinez-Pacheco, R., A Comparison of Cellactose with Two Ad hoc Processed Lactose-Cellulose Blends as Direct Compression Excipients, Chem. Pharm. Bull., 48: 458-463, 2000.
Gohel, M. C., and Jogani, P. D., An Investigation of the Direct Compression Characteristics of Co-processed Lactose Microcrystalline Cellulose Using Statistical Design, Pharm. Technol., 22: 54-62, 1999.
Gohel, M. C., Modi, C. J., and Jogani, P. D., Functionality Testing of a Co-processed Diluent Containing Lactose and Microcrystalline Cellulose, Pharm. Technol., 22: 40-46, 1999.
Gohel, M. C., and Jogani, P. D., Exploration of Melt Granulation Technique for the Development of Co-processed Directly Compressible Adjuvant Containing Lactose and Microcrystalline Cellulose, Pharm. Dev. Tech., 8: 175-185, 2003.
Gohel, M. C., Bariya, S., and Jogani, P. D., Investigation in Direct Compression Characteristics of Co-processed Adjuvant Containing Lactose, Microcrystalline cellulose and Dicalcium Phosphate, Pharm. Dev. Tech., 8: 143-152, 2003.
Luukkonen, P., Schaefer, T., Hellen, L., Juppo, A. M., and Yliruusi, J., Rheological Characterization of Microcrystalline Cellulose and Silicified Microcrystalline Cellulose Wet Masses Using a Mixer Torque Rheometer, Int. J. Pharm., 188: 181-192, 1999.
Allen, J. D., Improving DC with SMCC, Manuf. Chem., 67: 19-20& 23, 1996.
Fraser, S. D., Michael, T., Stephan, E., Ansong, C., Staniforth, J. N., Physicochemical and Mechanical Evaluation of a Novel high Density Grade of Silicified Microcrystalline Cellulose, Drug Dev. Ind. Pharm., 30: 103-109, 2004.
Lahdenpaa, E., Antikainen, O., and Yliruusi, J., Direct Compression with Silicified and Non-Silicified Microcrystalline Cellulose: Study of Some Properties of Powders and Tablets, S. T. P. Pharm. Sci., 11: 129-135, 2001.
Kachimanis, K., Nikolakakis, I., and Malamataris, S., J. Pharm Sci., 92: 1489-1501, 2003.
Bolhuis, G. K., Veen, B., Wu, Y. S., Zuuraman. K., and Frijlink, H. W., Compaction mechanism and tablet strength of unlubricated and lubricated silicified microcrystalline cellulose, Eur. J. Pharm. Biopharm.,59: 133-138, 2005.
Meggle AG, Technical information at www.meggle-pharma.de/en/product/uebersicht/starlac).
Hauschild, K., and Picker, K. M., Evaluation of a new co-processed compound based on lactose and maize starch for tablet formulation, http:// www.aapspharmsci.org, AAPS Pharmasci, 6: Article 16, 2004.
Gohel, M. C., and Jogani, P. D., An Investigation in Direct Compression Characteristics of Co-processed Lactose-Starch using Experimental Design, Indian J. Pharm. Sci., 65: 31-38, 2003.
Kim, H., Venkatesh, G., and Fassihi, R., Compactibility Characterization of Granular Pectin for Tableting Operation Using a Compaction Simulator, Int. J. Pharm., 161: 149-159, 1998.
Niwa, T., Takeuchi, H., Hino, T., Itoh, A., and Kawashima, Y., and Kiuchi, K., Preparation of Agglomerated Crystals for Direct Tableting and Microencapsulation by the Spherical Crystallization Technique with a Continuous System, Pharm. Research, 11: 478-484, 1994.
Deodhar, U. P., Paradkar, A. R., and Purohit, A. P., Preliminary Evaluation of Leucaena leucocephala Seed Gum as a Tablet Binder, Drug Dev, Ind. Pharm., 24: 577-582, 1998.
Kawashima, Y., Okumura, M., and Kojima, A., Direct Preparation of Spherically Agglomerated Salicylic Acid Crystal During Crystallization, J. Pharm Sci., 73: 1535-1538, 1984.
Lennartz, P., and Mielck, J. B., Minitableting: Improving the Compactibility of Paracetamol Powder Mixtures, Int. J. Pharm., 173: 75-85, 1998.
Zuuarman, K., Maarschalk, V., and Bolhuis, G. K., Effect of Magnesium Stearate on Bonding and Porosity Expansion of Tablets Produced from Materials with Different Consolidation Properties, Int. J. Pharm., 179: 107-115, 1999.
Holzer, A. W., and Sjogren, J., Evaluation of Some Lubricants by the Comparison of Friction Coefficients and Tablet Properties, Acta. Pharm. Suec, 18: 139-148, 1981.
Kuentz, M., Leurenberger, H., and Kolb, M., Fracture in Disordered Media and Tensile Strength of Microcrystalline Cellulose Tablets at Low Relative Densities, Int. J. Pharm., 182: 243-255, 1992.
Maarschalk, V., Zuurman, K., Vromans, H., Bolhuis, G. K., and Lerk, C. F., Porosity Expansion of Tablets as a Results of Bonding and Deformation of Particles Solids, Int. J. Pharm., 140: 185-193, 1996.
DeCrosta, M. T., Schwartz, J. B., Wigent, R. J., and Marshall, K., Thermodynamic Analysis of Compact Formation: Compaction, unloading and Ejection II, Int. J. Pharm., 213: 45-62,2001.
Corresponding Author: Dr. M.C. Gohel, Lallubhai Motilal College of Pharmacy, P.O. Box 4011, Navarangpura, Ahmedabad 380 009, India. mukeshgohel@hotmail.com
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.cspscanada.org