J Pharm Pharmaceut Sci (www.cspscanada.org) 8(2):322-325, 2005
Stability Of Levamisole Oral Solutions Prepared From Tablets And Powder
Fouad Chiadmi1, Abdel Iyer1,
Salvatore Cisternino1, Audrey Toledano1, Joël Schlatter1,
Robert Ratiney2, Jean-Eudes Fontan1
1Department of Pharmacy and Toxicology, University Hospital of Jean Verdier, avenue du 14 juillet, 95140 Bondy, France.
2Department of Pharmacy, University Hospital of Rene Muret – Bigottini, Avenue du Docteur Schaeffner, 93270 Sevran, France.
Received April 8 2005, Revised May 24, 2005, Accepted June 4 2005, Published 12 August 2005
Corresponding author: Joël Schlatter, Department of Pharmacy and Toxicology, University Hospital of Jean Verdier, Avenue du 14 Juillet, 93140 Bondy, France, Tel: 33 1 48 02 66 03, Fax: 33 1 48 02 66 23, Email: joel.schlatter@jvr.ap-hop-paris.fr
ABSTRACT. Purpose: To study the
stability of levamisole oral solutions (25 mg/mL) prepared from powder and
tablets stored at 4 ±
Introduction
Treatment of recurrent idiopathic nephrotic syndrome in children is often complicated by the toxicity of the therapeutic regimen with corticosteroids, alkylating substances or cyclosporine. An alternative is the treatment with levamisole, a potent anthelmintic compound with immuno-modulating properties (1-3). In children, levamisole is given orally as 2 - 5 mg/kg daily or every other day depending on the patient response (4-6). Levamisole is commercially available only in 50-mg tablets under the trade name Ergamisol (Janssen Pharmaceutica). Levamisole is labelled by the United States Food and Drug Administration (FDA) as adjuvant treatment with fluorouracil but not labelled for the treatment of nephrotic syndrome. No liquid dosage form of levamisole is available. An oral solution would be highly desirable for children who are unable to swallow tablets and allows the dose to be easily adjusted. Thus, an oral solution could be helpful for levamisole administration. Few data exist on the levamisole stability in liquid form. The purpose of this study was to determine the chemical stability of levamisole in solution prepared from tablets and powder and stored at two temperatures (4 and 23°C) in glass prescription bottles over 90 days period.
Methods
Formulation of levamisole solutions
One hundred 50-mg levamisole tabletsa were
crushed to a fine powder in a glass mortar. Twenty mL of sterile water for
irrigationb were added and levigated into a paste. After 30 min, 100
mL of sterile water for irrigation were added and allowed to stand for 30 min.
Then, the milky suspension was filtered through a 7-µm filter of paperc
to exclude the insoluble excipients. Sterile waterb was added to the
clear solution in a volumetric flask to obtain a theoretical levamisole
concentration at 25 mg/mL. The recovery of levamisole (n = 5)
obtained from compressed tablets was measured. Another solution of levamisole
25 mg/mL was prepared with levamisole pure powderd. The 5-g powder
was weighed and mixed with waterb in a 200 mL volumetric flask. Ten
millilitres from each levamisole solutions were transferred in 16 amber glass
prescription bottlese.
Storage of solutions
Six bottles of each formulation were stored at 4 ± 3°C and six
other bottles were stored at room temperature (23 ± 2°C) protected from light. Four bottles
were used to determine the stability study at 60 ± 5°C.
Sampling
From each
bottle, a sample (100 µL) was taken and mixed with 900 µL of waterb.
Then, 100 µL of this dilution was mixed with 300 µL of internal standard (1.5
mg/mL) and 600 µL of water in order to obtain a theoretical levamisole
concentration at 250 µg/mL. The diluted sample was assayed in duplicate by
high-performance liquid chromatography (HPLC), immediately after preparation
(day 0) and after 1, 3, 4, 7, 15, 30, 60 and 90 days. The apparent pH of each
solution was measured using a digital pH meterf at the beginning and
the end of the study. The appearance and color of the samples were assessed by
observing samples against black and white backgrounds, and any changes of odor
were noted at each time. The stability-indicating method was proven to ensure
that potential degradation products did not interfere with levamisole and
internal standard peaks.
HPLC Analysis
The HPLC method
was adapted from Vandamme et al (7).
The assay instrumentation required an isocratic pumpg, a manual
injectorh with a 20 µL loop, an ultraviolet light detectori
set at 235 nm, a C18 columnj set at room temperature and a recording
integratork. The mobile phase consisted of potassium hydrogen
phosphatel
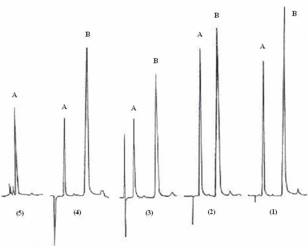
Figure 1: Chromatograms of a levamisole standard solution (1), an oral solution
prepared from tablets stored at 25°C (2), solutions with acid (3) and base (4)
and a solution with oxygen peroxide (5). A = levamisole; B = Quinine (internal
standard).
Data analysis
The initial concentration of levamisole was defined as 100%, and sample concentrations were expressed as a percentage of the initial concentration remaining. The solution was defined as being stable if the drug concentrations were not <90% of the initial drug concentration. The significance of any difference between initial and final pH values was evaluated by a Student’s t-test (α = 0.05).
Results
The
recovery of levamisole from tablets was 100 ± 2.1%. In the oral solution prepared from
powder, the mean concentrations of levamisole were >99% of the initial
concentration at 4°C and >96% of the initial concentration at 25°C for the 90-days
study period (Table 1). The mean concentrations of levamisole were >94% of
the initial concentration at 4°C and <64% of the initial concentration at
25°C over the study period for solution prepared from tablets (table 1). No
change of appearance, color or odor was noted with any of the solutions. The
apparent initial pH was significantly different between solutions prepared from
tablets and those prepared from the powder (Table 1). The final pH values of
solutions prepared from tablets stored at 4°C and 25°C increased significantly
(p <0.001) by 1.07 and 1.57 units, respectively, as compared with the
initial pH values. The final pH values of solutions prepared from powder stored
at 4°C and 25°C increased significantly (p <0.001) by 1.48 and 1.68 units,
respectively, as compared with the initial pH values.
Table
1. Stability of levamisole
solutions prepared from tablets and powder at 4 and 25°C.
% Initial Concentration Remaininga
4°C 25°C
|
Day |
Tabletsb |
|
Powderc |
Tabletsd |
|
|
Powdere |
|
|
|
|
0 |
100 ± 1.85 |
100 ± 1.64 |
100 ± 0.53 |
100 ± 2.60 |
|
1 |
99.82 ± 3.19 |
99.98 ± 0.63 |
99.81 ± 1.36 |
99.86 ± 2.66 |
|
2 |
99.51 ± 3.00 |
99.95 ± 1.73 |
99.51 ± 0.96 |
99.80 ± 3.61 |
|
3 |
99.80 ± 0.96 |
99.94 ± 2.31 |
98.85 ± 2.28 |
99.78 ± 2.44 |
|
4 |
99.75 ± 2.56 |
99.96 ± 2.35 |
98.95 ± 1.27 |
99.92 ± 3.36 |
|
7 |
99.55 ± 2.85 |
99.88 ± 1.36 |
97.33 ± 2.21 |
99.79 ± 2.28 |
|
15 |
99.63 ± 0.79 |
99.86 ± 2.28 |
94.26 ± 1.59 |
99.54 ± 1.58 |
|
30 |
98.11 ± 1.52 |
99.81 ± 1.37 |
88.57 ± 2.33 |
99.10 ± 3.21 |
|
60 |
95.62 ± 3.50 |
99.26 ± 1.85 |
77.82 ± 1.96 |
98.11 ± 2.18 |
|
90 |
94.41 ± 3.22 |
99.23 ± 2.81 |
63.56 ± 3.32 |
96.77 ± 1.75 |
aMean ± SD of 12 samples
bAdded mean concentration was 25.52 ± 1.23 mg/mL, initial pH was 5.30 ± 0.03
cAdded mean concentration was 24.89 ± 1.03 mg/mL, initial pH was 4.57 ± 0.03
dAdded mean concentration was 25.35 ± 0.96 mg/mL, initial pH was 5.28 ± 0.02
eAdded mean
concentration was 25.22 ± 1.22 mg/mL, initial pH was 4.55 ± 0.01
Discussion
The complete recovery of levamisole hydrochloride from tablets was related to its high water solubility reported to be 210 g/L (8). Levamisole solutions prepared from tablets and stored at 23°C appeared to be less stable than those prepared from powder. Refrigeration storage was shown to permit a better stability of levamisole solutions. Levamisole 25 mg/mL oral solutions prepared from powder and tablets stored at 4°C were shown stable at least 90 days and could be then used in clinical practice. This period of storage was conditioned by a possible microbiological contamination. With lack of preservatives in solutions, a period of 30 days stability seems more reasonable. Like temperature of storage, pH had an impact on the chemical stability of levamisole. It was shown that the rate of decomposition of levamisole rapidly increased between pH 5 and 7 and at pH 8 it was about seventy times faster than at pH 2 (9-11). Excipients and pH of solutions prepared from tablets could probably explain the difference in stability. Thus, the use of the solution prepared from powder would be preferable in practice. The taste of solutions was bitter and would be masked by an additive.
Conclusion
An oral liquid solution of levamisole at 25 mg/mL prepared from tablets or pure powder in sterile water was shown chemically stable 90 days under refrigeration. These oral solutions appear to be widely used as an alternative to the administration of dry levamisole forms for the pediatric patients.
References
(1) Arvind Bagga, Amita Sharma, R. N. Srivastava. Levamisole therapy in corticosteroid-dependent
nephrotic syndrome. Pediatr Nephrol, 11:415-417, 1997.
(2) Tenbrock
K, Muller-Berghaus J, Fuchshuber A, Michalk D, Querfeld U. Levamisole treatment
in steroid-sensitive and steroid-resistant nephrotic syndrome. Pediatr Nephrol,
12:459-462, 1998.
(3) Donia
AF, Amer GM, Ahmed HA, Gazareen SH, Moustafa FE, Shoeib AA, Ismail AM, Khamis S,
Sobh MA. Levamisole: adjunctive therapy in steroid dependent minimal change
nephrotic children Pediatr Nephrol, 17:355-358, 2002.
(4) MJ
Kemper, O Amon, K Timmermann, H Altrogge, DE Muller-Wiefel. The treatment with
levamisole of frequently recurring steroid-sensitive idiopathic nephrotic
syndrome in children. Dtsch Med Wochenschr, 123:239-243, 1998.
(5) L. Fu,
CS Chi. Levamisole in steroid-sensitive nephrotic syndrome children with
steroid-dependency and/or frequent relapses, Acta Paediatr Taiwan, 41:80-84,
2000.
(6) U.
Dayal, A.K. Dayal, J.C. Shastry, P. Raghupathy, Use of levamisole in
maintaining remission in steroid-sensitive nephrotic syndrome in children,
Nephron , 66:408-412, 1994.
(7) TF
Vandamme, M Demoustier, B Rollmann. Quantitation of levamisole in plasma using
high performance liquid chromatography. Eur J Drug Metab Pharmacokinet, 20:145-149,
1995.
(8) Levamisole
Hydrochloride. The Merck Index on CD-ROM version 12.3. Merck and Co Inc,
(9) NA
Dickinson, HE Hudson, PJ Taylor. Levamisole: its stability in aqueous solutions
at elevated temperatures. I. Isolation and identification of decomposition
products formed in aqueous solutions of levamisole stored under nitrogen and
oxygen at 100 degrees C. Analyst, 96:235-243, 1971.
(10) NA
Dickinson, HE Hudson, PJ Taylor. Levamisole: its stability in aqueous solutions
at elevated temperatures. II. An assay specific for levamisole and applicable
to stability studies. Analyst, 96:244-247, 1971.
(11) NA Dickinson, HE Hudson, PJ Taylor. Levamisole: its stability in aqueous solutions at elevated temperatures. 3. A chromatographic and polarimetric study of the kinetics of degradation, Analyst, 96:248-253, 1971.
Footnotes
aLevamisole 50-mg tablets, ERGAMISOL®, Janssen Pharmaceutica, Beerse, Belgium, lot 02FB831. Tablets contained 59 mg of levamisole hydrochloride (= 50 mg levamisole base) cellulose, lactose, oil vegetable and silica.
bSterile water for irrigation, VERSOL®, Aguettant, Lyon, France, lot F3133.
cFilter paper N7,
Cooperation Pharmaceutique Française (COOPER), Melun, France.
dLevamisole powder, Synopharm Laboratory, Barsbüttel, Germany, lot 0307A108.
eGlass prescription bottles reference 2506230, 125 mL, Cooperation Pharmaceutique Française (COOPER), Melun France.
fModel pH302, Hanna Instruments, Tanneries, France.
gModel LC-6A, Shimadzu Europe, Duisburg, Germany.
hModel 7125, Rheodyne Europe GmbH,
Bensheim, Germany.
iModel SPD-6A, Shimadzu Europe, Duisburg,
Germany.
jNucleosil®
(4.6-mm, 25 cm), Agilent Technologies, Massy, France.
kModel CR-6A, Shimadzu Europe, Duisburg,
Germany.
lPotassium hydrogen
phosphate, Merck KGaA, Darmstadt, Germany.
mAnalytical grade acetonitrile, CHROMASOLV®, Sigma-Aldrich Chimie, Lyon, France.
nEthanol 90% v/v, COOPER, Melun, France.
oQuinine hydrochloride
solution 30 mg/mL, AGEPS, Paris, France, lot T02081.
pHydrochloride acid
37%, NORMAPUR®, VWR International SAS, Prolabo, Fontenay-sous-bois, France.
qSodium hydroxide
1.0M, NORMADOSE®, VWR International SAS, Prolabo, Fontenay-sous-bois, France.
rHydrogen peroxide 10%, COOPER, Melun, France.
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.