J Pharm Pharmaceut Sci (www.cspscanada.org) 8(3):516-527, 2005
Functional comparison of single- and double-stranded mdr1 antisense oligodeoxynucleotides in
human ovarian cancer cell lines.
Veronika Jekerle1,2,
1 Pharmaceutical
Institute,
2
Department of
Pharmaceutical Sciences,
3
Department of
Nuclear Medicine, University Health Network,
Received August 17, 2005; Revised September 21 2005; Accepted September 22, 2005; Published September 23, 2005
Corresponding
Author:
Micheline Piquette-Miller, Department of
Pharmaceutical Sciences,
Abbreviations:
ODNs, Oligodeoxynucleotides
PS, Phosphorothioate
ss, single-stranded
ds, double-stranded
mdr1 , multidrug resistance gene
Pgp , P-glycoprotein
A2780/Adr, Adriamycin resistant A2780
A, antisense
S, sense
R, random
PBS, phosphate-buffered saline
FACS, fluorescence assorted cell sorting
DTPA, diethylenetriaminepentaacetic acid
FITC, fluorescein isothiocyanate
DAPI, 4,6-diamidino-2-phenylindole
DNR, Daunorubicin
Abstract
PURPOSE. P-glycoprotein mediated multidrug resistance
presents a major obstacle in the successful therapeutic treatment of solid
tumors such as ovarian cancer. Among the more promising techniques used to
overcome multidrug resistance in ovarian cancer, is the transcriptional
suppression of P-glycoprotein by antisense oligodeoxynucleotides (ODNs). To
design more potent antisense ODNs, we explored the concept that double-stranded
antisense ODNs may offer advantages in stability and potency over
single-stranded in analogy to double-stranded siRNA. METHOD. Single-stranded phosphorothioate antisense ODNs against the
human mdr1 gene were compared to the
duplex of the active antisense and sense sequence of the same length. Concentration
dependant effects on P-glycoprotein (Pgp) expression and functionality were
quantitatively compared in the Pgp overexpressing ovarian cancer cell line
A2780/Adr and its parental cell line A2780. Antisense ODNs were 111Indium-
and fluorescein isothiocyanate-conjugated for stability, cellular uptake and
nuclear localization studies. Duplex formation significantly enhanced
transcriptional inhibition of Pgp surface expression and functionality. Cellular
uptake and distribution to the nucleus was improved when utilized as
double-stranded DNA. CONCLUSION. Novel
findings from this study suggest that double-stranded antisense ODNs more
effectively inhibit target protein expression and consequently enhance
chemoresponsiveness through improvements in cellular uptake and distribution to
the nucleus.
Introduction
Antisense oligodeoxynucleotides (ODNs) are powerful tools for the
selective, sequence-specific regulation of gene expression (1). Though
extensively investigated and convincing in theory, practical applications have
proven to be rather challenging (2). To date, only one antisense-based drug has
been released to the market (3). Whereas some successes have been described
for target genes that are present at relatively low levels (4, 5), the
application of antisense technology for target genes with high expression has proven to be difficult due to
excessive toxicity, insufficient
in vivo stability and
cellular uptake (4, 6). Since their introduction, numerous chemical
modifications of ODNs have been described in efforts to increase their
stability and specificity. In the first generation of
ODNs, a single oxygen atom at each phospate group was replaced with a sulfur
atom producing phosphorothioate (PS) ODNs, rendering them relatively nuclease
resistant. More efforts have been placed to develop second and third generation
antisense ODNs including the 2’-O-alkyl RNA derivatives (7) and particularly
the phosphorodiamidated morpholino oligomers (8). While those newer antisense
technologies offer advantages such as reduced non-specific effects and more
efficient target protein arrest, the conventional PS ODNs are still the best
known and most widely used in research and therapy (6, 9-11).
Interest in the antisense field has
recently exploded subsequent to the discovery that short double-stranded siRNA
molecules could be used to specifically suppress gene expression within cells. The
double-stranded siRNA molecules form 3’-overhangs that specifically inhibit
gene expression. Recently, functional effects of single-stranded (ss) and
double-stranded (ds) siRNA have been compared and it has
been demonstrated that double -stranded siRNA possess unexpectedly high
efficacy and stability both in cell culture (12) and in animal models
(13). While much research has been
directed toward examining these double-stranded RNA molecules, little effort
has been placed toward examining potential benefits in the stability and
activity of double-stranded DNA based antisense ODNs. Recently, an enhanced cellular uptake of a
multidrug resistance gene (mdr1) ODN
duplex consisting of a phosphorothioate antisense sequence combined with a
shorter non-phosphorothioate sense sequence was reported, suggesting that
double-stranded ODN delivery may provide an effective means for increasing
cellular uptake of antisense oligonucleotides (14).
Antisense ODNs
directed against the mdr1 gene have
been well described and are reported to effectively inhibit expression of mdr1 and the protein it encodes for,
P-glycoprotein (Pgp) (15, 16).
Overexpression of Pgp, a member of the ATP-binding cassette transporter
family, is a common cause of multidrug resistance in many types of tumors
including ovarian and breast cancer.
This membrane bound efflux pump expels chemotherapeutic agents from
cells, resulting in decreased intracellular drug concentrations and loss of efficacy
(17-19). ODNs, designed mostly against the region of the initiation codon of mdr1, have been demonstrated to inhibit
the Pgp mediated multidrug resistant phenotype in vitro in cell culture (10) and in vivo in human tumour xenografts (20). Due to relatively high Pgp
levels present in multidrug resistant tumor cells advances in the efficacy,
stability and cellular uptake of antisense ODNs are needed to successfully
apply this technique for therapeutic use.
Using a human mdr1 antisense sequence that has previously been used by others to inhibit Pgp expression (10, 21); we investigated the potential advantages in stability, cellular uptake, toxicity and efficacy of double-stranded versus single-stranded ODNs. Phosphorothioate antisense and sense ODN of the same length were utilized and compared in the resistant A2780/Adr human ovarian cancer cell line which highly expresses Pgp, as well as its sensitive parent A2780 cell line. Novel findings from this study are the first to demonstrate that efficacy of mdr1 ODNs is increased when used as double-stranded phosphorothioate ODNs versus single-stranded. Enhanced suppression of Pgp expression was confirmed by functional analysis. Based on fluorescence microscopy analysis of tagged ODNs, increased cellular retention and nuclear localization of the ds ODN is the likely mechanism involved.
Materials and Methods
Cell culture. Adriamycin resistant (A2780/Adr) and parent
A2780 ovarian cancer cell lines were purchased from ECACC, UK. Cells were
cultivated in RPMI 1640 medium supplemented with 10% fetal bovine serum, 50 mg/ml penicillin and streptomycin and incubated
in a 5% CO2 atmosphere at 37°C. A2780/Adr were incubated with 10mM Adriamycin every 10 passages.
Pgp overexpression in A2780/Adr cells was confirmed by
RT-PCR (Fig. 1) using previously described methods (22). Briefly, total RNA was
isolated from A2780/Adr and A2780 cells using the TRIzol extraction
kit (GIBCO-BRL; Life
Technologies,
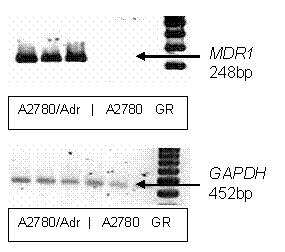
Figure 1.
RT-PCR
analysis of mdr1 expression. A
representative RT-PCR gel depicting relative mdr1 gene expression in A2780/Adr and A2780. RT-PCR was performed
and PCR products were separated and visualized as reported in Method. GAPDH was
used as housekeeping gene. Lanes 1-3 contain A2780/Adr, lanes 4-6 A2780 samples
and lane 7 is the DNA ladder (100 bp Gene Ruler- GR).
Product sizes were verified by a 100 bp gene ruler (Invitrogen,
Transfection with
oligodeoxynucleotides. Phosphorothioate
ODNs with aminohexyl modifications at the 5’-end were purchased from
Invitrogen, (Carlsbad, CA). Sequences used were; Antisense (A): Aminohexyl-5’-CCA TCC CGA CCT CGC GCT CC-3’,
Sense (S): 3’-GGT AGG GCT GGA GCG CGA GG- 5’-Aminohexyl and Random (R): Aminohexyl
-5’-GCT CCC CCA CGC GCC TCC AT-3’. Double-stranded AS-S duplexes were formed by
combining 100 mM stock-solutions of ss AS and S and
incubating at room temperature for 10 min at concentrations between 25-250 nM. Formation
of AS-S duplexes was routinely verified on 4% polyacrylamide gels. A2780 cells
were plated onto 6 well plates at a concentration of 33,000 cells per well and
incubated for 24 hour prior to treatments with increasing concentrations of AS
and double-stranded AS-S ODNs; single-stranded random ODNs were used as
controls. SuperFect (Qiagen,
The stability of
double-stranded AS-S and single-stranded AS mdr1 ODNs was examined by incubation in PBS (PBS) containing 5%
fetal bovine serum (Sigma, Kanata, ON) at 37°C. Samples were removed at various
time intervals (3-24h) and enzyme reactions terminated by heating at 95°C for
10 min (24). All samples were subsequently loaded onto a 4% polyacrylamide gel,
run at 150 V and visualized using the SYBR gold nucleic acid stain. Freshly
prepared ODNs served as controls.
Effect of ODN on Cell Viability
and Proliferation. Cell
number as a measure of cell viability was assessed at 72 hours after final ODN
treatments. Plated cells were trypsinized, centrifuged at 1000 g, 4°C for 4
min, redissolved in medium and aliquots analyzed on a Casy 1 electronic particle counter (Schaerfe Systems GmBH,
Cell proliferation was further assessed using the MTT
assay. This assay measures mitochondrial enzyme succinyldehydrogenase activity
in viable cells. Cells from each treatment group were plated onto 96 multiwell
plates (100,000 cells/well) for 3 hours (5% CO2, 37°C), 20 µl of MTT
solution (5 mg/ml) added and incubated for 1 hr. Cells were then solubilized
with isopropanol-HCl (1:300) and absorption of solubilized formazan was measured
using a BMG Fluostar (BMG LABTECH GmbH, Offenburg, Germany). Formazan
absorbance was calculated by measuring absorbance maximum at 595nm and
subtraction of background absorbance at 690nm. Cell proliferation was
calculated as a percentage of controls.
P-Glycoprotein surface
expression. After final ODN
treatments, cells were washed several times with PBS and attached cells were then
trypsinized, counted and prepared for antibody staining. 106 cells were
washed with 1 ml of buffer (PBS w/ 0.5% BSA) and dissolved in 1 ml of staining
buffer (PBS, 0.5% BSA, 0.1% NaN3) containing 20 µl FITC labeled
monoclonal anti human Pgp antibody (BD Biosciences, USA). Tubes were protected
from light and incubated on ice for 40 min, cells were then washed with 1 ml of
ice-cold staining buffer to remove unbound antibody and resuspended in buffer. Binding
of FITC-labeled Pgp-antibody was analysed on a Becton flow cytometer
(FACSCalibur, Becton Dickinson, Heidelberg, Germany) with excitation at 488 nm
and emission collected through a 530/15 band pass filter for FITC (FL1-H).
10000 events were collected while gating on physical parameters to exclude cell
debris. The number of events within the gate for intact cells remained
consistently well above 75% for all ODN treatments of 25-200 nM and were
consistent with cell viability studies. A lower cell intact fraction of 53% was
seen only in the 250 nM ds AS-S treated cells. Data was quantified using the
Cell Quest Pro and Win MDI software. Autofluorescence of A2780/Adr and A2780 cells
were tested to be identical. As both cell lines do not express BCRP,
non-specific binding to the BCRP binding antibody BXP-21 (Abcam, Cambridge, MA)
and anti-mouse Ig fluorescein-linked whole antibody (Amersham Biosciences,
Piscataway, NJ) was used to evaluate unspecific binding. Minimal non-specific
binding was detected and differences in fluorescent intensities were not seen
between the two cell lines (data not shown).
Daunorubicin chemosensitivity. Cells treated for 72 hr with 50 nM or 100 nM
ODNs and untreated controls were plated in media at 50,000 cells/well into 96
multiwell plates and incubated with increasing concentrations of Daunorubicin
(100 nM - 1mM in 10 µl) for 72h under standard conditions (5% CO2 at
37°C). Cell proliferation and viability was then assessed using the MTT-assay. Concentrations
of Daunorubicin required to decrease cell proliferation by 50% (IC50
values) were determined for each ODN treatment group from the
concentration-response curves of the MTT assay. The concentration-response
curves and IC50 values were obtained by nonlinear regression
assuming a sigmoidal dose response curve with variable hill slope, generated
with GraphPad Prism 3.0 Software.
Intracellular Daunorubicin
uptake. Using previously
described methods (25, 26); cellular uptake of the fluorescent drug
Daunorubicin was analyzed by FACS. Briefly, 106 ODN treated cells
were immediately diluted in 2 ml phenol red-free RPMI medium and incubated for
1h at 37°C. Daunorubicin (10mM) was added
and cellular daunorubicin uptake was measured every 30 min for 3 h on a Becton
flow cytometer (FACSCalibur, Becton Dickinson, Heidelberg, Germany) with
excitation at 488 nm and emission collected through a 585/22 band pass filter
for yellow daunorubicin (FL2-H). 5000 event were collected while gating on
physical parameters to exclude cell debris and sample analysis was repeated
three times. Data was analyzed by Cell Quest Pro software and accumulation
plateaus were generated using GraphPad Prism 3.0 software.
Cellular uptake of radiolabeled
ODNs. Antisense ODNs
containing an aminohexyl group at the 5’-end were combined with a 100-fold
molar excess of DTPA anhydrate (Sigma, Kanata, ON) at room temperature for 30
min as previously described (27). DTPA-derivatized ODNs were purified using
size-exclusion chromatography on a P-2 mini-column (Bio-Rad,
Intracellular distribution of
fluorescent-labeled ODNs. Intracellular
distribution of fluorescent-labeled ODNs was visualized in attached viable
cells using fluorescence microscopy. For FITC labeling of AS ODNs, a 1mg/mL
FITC-isothiocyanate solution was prepared in 100mM sodium bicarbonate buffer
(pH 9). Aminohexyl-modified AS ODNs were incubated with 300mM of FITC-isothiocyanate-solution (1:100 ratio) for 30
min at room temperature in the dark. FITC-labeled ODNs were purified by
size-exclusion chromatography on a P-2 column. A2780 and A2780/Adr cells were
grown on glass supports. At 60% confluency, cells were treated with 200nM of
double-stranded AS-S and single-stranded AS FITC-labeled mdr1 ODNs for 3 hr. For nuclear morphology analysis, cells were
incubated with a 1 mg/ml DAPI in PBS for 5
min. Cells were washed and examined under a 100x 1.30 oil immersion objective
using a Nikon Eclipse E400 microscope (Nikon,
Data analysis. Statistical analysis was performed using the unpaired
Student’s t-Test (Excel; Microsoft,
Results and Discussion
Inhibition of cell surface expression of P-glycoprotein. The effects of double-stranded AS-S and single-stranded AS mdr1 ODNs on Pgp expression were compared using flow cytometry. A conventional single-stranded random control ODN was used to evaluate nonspecific effects. Preliminary studies examined dose and time-dependency of Pgp expression with the ODN treatments. Taking toxicity into account, we found that significant reductions in Pgp surface expression were seen after 48 h with the most pronounced suppression seen at 72 h (data not shown). As the half life of Pgp is 14 h, this corresponds to treatment periods of 3-5 Pgp half lives (28). We observed significant, dose-dependent reductions in Pgp surface expression in cells treated with single-stranded AS and double-stranded AS-S preparations (Fig. 2). The double-stranded AS-S ODN was significantly more effective than single-stranded AS at all tested concentrations with the largest difference in suppression seen at the lowest concentration (23 % increase in Pgp suppression at 25nM). On the other hand, the strongest suppression of Pgp surface levels was seen in cells treated with 250 nM of double-stranded AS-S. Representative FACS histograms, demonstrating reduced Pgp surface expression in treated cells, are depicted in Figure 3. Histograms indicate homogeneity of the ODN treated cell population, since the peaks tail slightly but do not divide into a second population.
Daunorubicin chemo sensitivity and intracellular accumulation.
Changes in Pgp
surface expression were confirmed by functional studies examining
chemosensitivity and intracellular accumulation of the Pgp substrate
daunorubicin (DNR). DNR accumulation and chemosensitivity have been shown to
directly correlate with Pgp expression (29, 30). Chemosensitivity was
determined after treatment regimens of 50 nM or 100 nM using the MTT assay and
are reported as IC50 values in
Table 1. As compared to
single-stranded AS, IC50 values for double-stranded AS-S were
significantly decreased. This indicates a higher chemosensitivity and lower
resistance in cells treated with double-stranded AS-S ODN regimens.
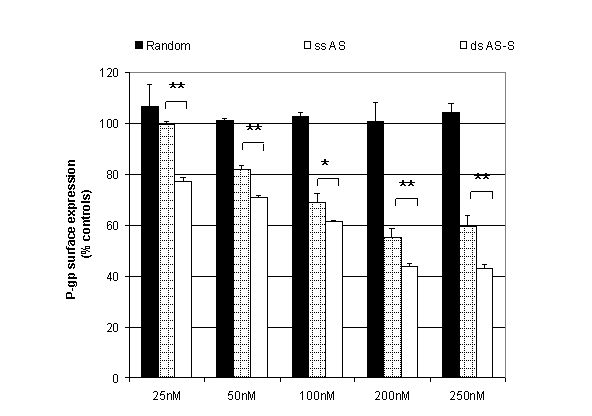
Figure 2. Effect of single-stranded
antisense (ss AS) and double-stranded (ds AS-S) mdr1 ODNs on P-glycoprotein surface expression. A2780/Adr cells
were transfected with different concentrations of ODNs as described in methods.
A conventional single-stranded random sequence was included to evaluate
non-specific effects on protein expression. For detection, a FITC-labeled
monoclonal human P-glycoprotein antibody was used with flow cytometry detection.
Cells were gated for the intact population and
the geometric mean of 10000 events was recorded. Results are expressed as a percentage
of untreated A2780/Adr and represent the average of three individual
experiments.
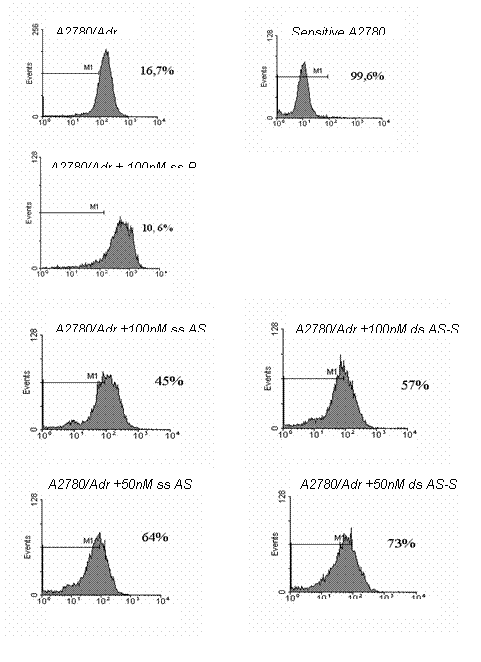
Figure 3. Representative
histograms of Pgp surface expression analysis. The effects of treatments with 50 and 100 nM
double-stranded (ds) and single-stranded (ss) AS mdr1 ODNs on Pgp surface expression in A2780 are presented. Random
control, untreated resistant A2780/Adr and sensitive A2780 were used as
negative and positive controls. Cells were prepared by incubation with a
FITC-labeled monoclonal anti-P-glycoprotein-antibody and Pgp expression was
detected by flow cytometry analysis. Acquisition was set to 10000 events and
results depict the viable/ intact cell population as routinely gated in the
forward and side scatter plot. The fluorescent intensity of the bound FITC
antibody was detected in the FL1-H channel. In the histograms, the gate M1 is
placed to include sensitive A2780 cells (99.6%) and excluding Pgp expressing
A2780/Adr cells (16.7%). Percentages in each panel represent the proportions of
cells (=events) that do not express Pgp (A2780).
The increased chemosensitivity of
DNR in ODN treated cells was associated with enhanced intracellular
accumulation of DNR as detected by FACS analysis (Fig. 4). Differences in Pgp
expression were clearly illustrated by the 3 fold differences in DNR
accumulation between A2780/Adr and A2780 cells (Fig. 4A). Whereas ODN treatments
did not significantly alter DNR
accumulation in A2780 cells, DNR levels were appreciably increased in
A2780/Adr cells. In A2780/Adr cells, we detected significantly higher
accumulation of DNR in cells treated with double-stranded AS-S ODN (Fig. 4B). Whereas
treatment of cells with 50 nM of the AS-S duplex resulted in a 30% increase in
DNR accumulation, single-stranded AS did not significantly impact DNR levels. DNR
accumulation was further increased by more than 2 fold in cells treated with
100nM of double-stranded AS-S ODN. By comparison, DNR accumulation was
increased to a maximum of 145% in cells treated with 50- 250 nM of the single-stranded
AS preparation.
Table 1. Daunorubicin
chemosensitivity.
Daunorubicin
chemosensitivity was determined in A2780/Adr cells treated for 72 hr with
single-stranded AS, double-stranded AS-S
mdr1 ODNs or random controls. Cells
were incubated with Daunorubicin or PBS and cell vitality was determined with
the MTT-assay. The concentration that produces 50% inhibition of cell vitality
(IC50) was calculated. Results were generated from means of six data
points. two separate experiments. *
indicates a p < 0.05 between ss AS and ds AS-S ODN
treatment.
|
Treatment |
IC50
± SEM /
mM |
||
|
|
ss AS |
ds AS |
PBS |
|
Control |
|
|
37.9
± 0.8 |
|
50 nM |
19.1
± 0.8 |
8.3
± 0.8 * |
|
|
100 nM |
9.7
± 0.9 |
2.8± 0.8 * |
|
Cellular toxicity of ODN
transfection.
Cellular
toxicity has been associated with the exposure to PS ODNs (4) and remains a
major challenge in antisense application. We examined whether double- and
single-stranded preparations of PS ODNs differ in toxicity. Cell viability
status and cell number counts as analyzed on a Casy 1® cell counter,
did not detect any significant differences between the single- and
double-stranded ODN preparations (Fig. 5A). Decreased cell viability was seen
in cells treated with 250 nM of ss-AS or ds-AS-S ODN that was consistent with
cell gating in the FACS studies. On the other hand, cell proliferation was
significantly lower in cells treated with single-stranded AS as compared to the
double-stranded AS-S preparations (Fig. 5B).
Intracellular ODN stability, accumulation
and localization.
To investigate the cellular mechanism underlying the enhanced effectiveness of ODNs to suppress Pgp expression and function when presented as a double-stranded duplex as compared to a single-strand, we examined the intracellular accumulation, cellular localization and stability of these preparations. ODN stability, as routinely examined in bovine serum albumin (25), did not detect differences between the stability of the single- or double-stranded AS ODN preparations over a 24 h time period. All samples remained stable under these conditions and no degradation products were detected. As fresh ODN are replaced in the media every 24h during our treatment periods, this implies that differences in AS activity are not due to changes in stability.
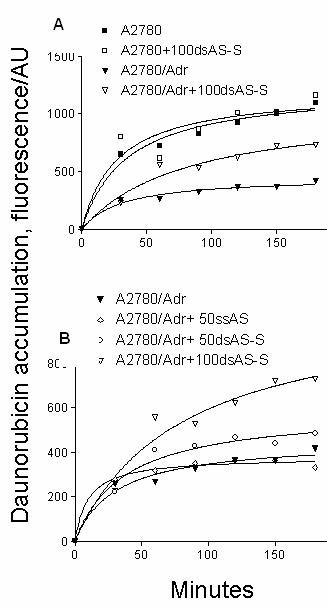
Figure 4. Effect of Antisense Treatments on Daunorubicin accumulation. Daunorubicin accumulation is shown in A. resistant A2780/Adr and A2780 cells transfected with 100 nM of double-stranded AS-S mdr1 ODNs. B. in A2780/Adr cells transfected with 50 and 100 nM of double-stranded and single-stranded AS mdr1 ODNs. Cells were incubated with 10 mM of Daunorubicin or PBS in a single cell suspension. In order to evaluate ds AS-S ODN effects on non-Pgp expressing cells, A2780 cells incubated with 100nM of ds AS-S ODNs were used as controls. After different time points an aliquot was removed and flow cytometry analysis was performed. Acquisition was set to 5000 events and gated for viable/ intact cell population. Data represent the geometric mean and similar results were obtained in two separate experiments.
To determine possible differences in
cellular uptake of the AS preparations, AS ODNs were radiolabeled with
111Indium.
A2780/Adr and A2780 cells were incubated with different concentrations of [111In]-DTPA-labeled
AS or AS-S ODNs. As compared to the single-stranded AS, we observed a
significant increase in the intracellular accumulation of
111Indium
in cells treated with the double-stranded AS-S ODN (Fig. 6). This was
significant at all concentrations above 100nM and detectable in both A2780/Adr
(Fig. 6A) and A2780 (Fig. 6B) cells. While a substantially higher accumulation
of the radiolabeled double-stranded AS-S duplex was seen in A2780/Adr cells as
compared to the A2780 cells, cell line associated differences in the
accumulation of the single-stranded AS were not seen. Of note, we observed a
higher variability in cellular uptake when using 250 nM of ODN. This may be due
to either saturation of active transport mechanisms or toxic effects.
Identification and stability of [111In]-DTPA-labeled ODNs were
confirmed by thin layer chromatography which
verified detection of
ODN-associated rather than free 111Indium. Thin layer chromatography
analysis of cell lysates demonstrated that more than 75% of radioactivity was
associated with the ODN bound 111Indium for both preparations.
Cellular uptake and distribution were
further examined by fluorescence microscopy. As shown in
Figure 7, the cellular
uptake and retention of FITC-labeled AS was dramatically greater in A2780/Adr,
as compared to the non-Pgp expressing A2780 cells (Fig. 7C, D).
Increased fluorescence was observed in
cells treated with the double-stranded AS-S preparation (Fig. 7A) compared to
the single-stranded AS preparation (Fig. 7B). The nuclear localization of
FITC-labeled ODNs was confirmed through overlapping DAPI (Fig. 8B) and FITC
(Fig. 8C) stains. Likewise, a stronger fluorescent signal was detected in the
nuclei of cells treated with the double-stranded AS-S preparation (Fig. 8C) as
compared to cells treated with the single-stranded AS (Fig. 8D).
Conclusions
In this study, we report an increased activity of double-stranded versus single-stranded mdr1 AS ODNs. Suppression of protein levels and functionality was increased along with chemoresponsiveness in the Pgp overexpressing A2780/Adr human ovarian cancer cell line. Effects of the double-stranded mdr1 AS were dose-dependant and directly associated with changes in Pgp functionality, as measured by both increased accumulation and chemosensitivity towards the Pgp substrate, daunorubicin. While double-stranded RNA molecules have been extensively studied and successfully applied in the past, double-stranded DNA have received very little attention. Only recently, an increased activity of a double-stranded DNA preparation, consisting of a duplex between an active antisense strand and an easily degradable shorter complementary sense strand, was reported. This study demonstrated higher activity towards the suppression of cell surface protein but did not examine further effects on protein functionality and efficacy (14). Using fully active, phosphorothioate AS and S ODNs in single- and double-stranded preparations we were able to directly compare treatment efficiencies for the preparations. Interestingly, double-stranded ODNs were more effective but did not increase toxicity.
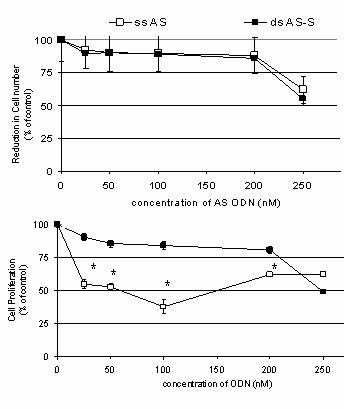
Figure 5. Effect of Antisense Treatments on (A) Cell Viability and (B) Cell Proliferation. Results depict effects of transfection (72h) with different concentrations of single-stranded AS and double-stranded AS-S mdr1 ODN on A2780/Adr cells. To evaluate direct ODN related toxicity, untreated A2780/Adr cells were used as controls. Viability was determined by cell counts in Casy1® and are expressed as the reduction in cell number as a % of untreated controls. Cell proliferation was determined using the MTT assay. Data is expressed as the mean ± SD of three data points. Similar results were obtained in three individual experiments. No significant differences were detected.
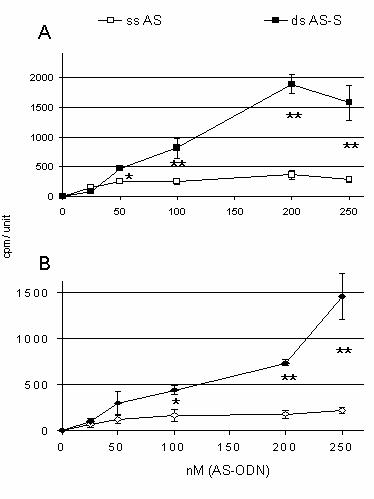
Figure 6.
Intracellular uptake of
111In-DTPA-labeled
ODNs. Cellular bound recovery of
111In-DTPA-labeled AS in the
presence and absence of equimolar amounts of unlabeled sense ODNs in
A.
resistant A2780/Adr and B. sensitive A2780 cells is presented. Cells
were transfected with different concentrations of single-stranded and
double-stranded mdr1 ODNs for 6 h. Results are expressed as amounts of
intracellular bound 111Indium in cpm per 126.6mm2
confluent cells (area of 1 well = 1 unit) and represent the average of three
individual experiments.
The increased activity of double-stranded ODNs was likely due to an increase in cellular accumulation and localization to the nucleus. This was confirmed in studies using either 111In-DTPA-labeled or FITC-labeled ODNs that demonstrated increased intracellular levels. The results indicate that double-stranded ODN preparations strongly increase the delivery into cells and most likely into the nucleus, as visualized by fluorescence microscopy. Cellular uptake of DNA is generally thought to occur through receptor-mediated endocytosis. The fact that we observed increased nuclear and cellular uptake of the double-stranded DNA as compared to single-stranded DNA suggests that there may be a difference in the affinity to active transporters or additional uptake processes for double-stranded DNA. This seems plausible, as many receptor-mediated endocytosis pathways exist (DNA receptor protein, nucleic acid binding receptor-1, heparin binding protein, nucleoli-like proteins, and porin-like proteins) (31).
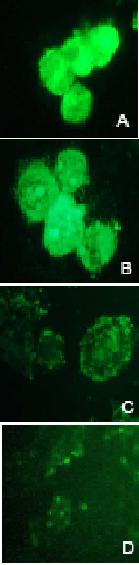
Figure 7.
FITC-Fluorescent microscopy
analysis of intracellular ODN in A2780/Adr (A, B) and A2780 (C, D) cells.
Cells were transfected with double-stranded AS-S
mdr1ODNs (A, C) and
single-stranded AS mdr1 ODNs (B, D) for 3 h.
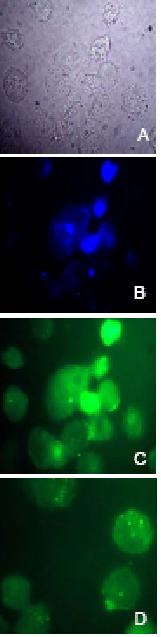
Figure 8.
Fluorescent microscopy of
nuclear ODN delivery. Nuclear localization analysis of oligonucleotides. Phase
contrast (A, grey); DAPI nuclear stain (B, blue) and FITC-fluorescence (C,
green) microscopy images were taken of A2780/Adr cells. Overlapping stains in B
and C indicate nuclear localization of FITC-labeled ODNs. Furthermore,
florescent images of cells transfected with double-stranded (C) FITC-labeled AS-S
and single-stranded (D) AS mdr1 ODNs are compared.
Chemically, the single-stranded DNA molecule is a highly polar electrolyte with a high negative charge density and a s urrounding cloud of counter ions in its vicinity (32). In the duplex conformation, even though the charge density is increased, the double-strand can more effectively neutralize this charge than the single-strand. Due to this more effective charge neutralization, the transport of the duplex through the non-polar environment of the membrane is facilitated and therefore increased in comparison to the transport of the single-strand. Furthermore, conformational changes of the AS sequence when presented as double-stranded DNA might potentially increase binding to target RNA and activation of RNase H, such as that reported for double-stranded siRNA (33). On the other hand, it is possible that both antisense and sense strands, once delivered to the nucleus could each interact with nuclear targets that alter gene expression. These hypotheses however, need to be confirmed with further experimentation.
It could also be argued that alterations in intracellular stability could be responsible for the enhanced cellular retention of the double-stranded ODNs. However, no differences in stability could be detected when analyzed using radiolabeled ODNs with thin layer chromatography analysis or via enzymatic incubation with gel electrophoretic separation and detection.
From these findings, we conclude that the formulation of double-stranded complementary phosphorothioate ODNs may have significant advantages over the use of single-stranded AS ODNs. Through advantages in cellular uptake and localization and potential contribution of the sense strand, double-stranded mdr1 ODNs may be more effective in the suppression of Pgp without increasing toxicity. Ultimately, this could increase chemoresponsiveness to antineoplastic agents and hence provide improvements in overcoming Pgp-mediated multidrug resistance. Whether this approach holds true for differently modified oligonucleotides and in vivo applications is a question that will be of further interest in the antisense field. For future antisense applications of phosphorothioate oligonucleotides in the multidrug-resistance field, double-stranded ODN delivery may be a suitable approach.
ACKNOWLEDGMENTS AND SOURCE OF FUNDING
This research was supported by the Deutsche Forschungsgemeinschaft (Graduiertenkolleg 804) and the Government of Canada Award. The authors wish to thank Kerstin Breitbach for kind and excellent support with flow cytometry; and Judy Wang, Jing-Hung Wang and Deborah A. Scollard for excellent technical assistance with 111Indium-labeling and fluorescent microscopy studies.
References
[1] Benett, C.F.; Cowsert, L.M. Antisense Oligonucleotides as a tool
for gene functionalization and target validation.
Biochim. Biophys. Acta. 1489:19-30; 1999.
[2] Lebedeva, I.;Stein, C.A. Antisense oligonucleotides: promise and
reality. Ann. Rev.
Pharmacol. Toxicol.
41:403-419; 2001.
[3] Roehr, B. Fomivirsen approved for CMV retinitis.
J Int Assoc Physicians AIDS Care 4. 14-6; 1998
[4] Kurreck, J. Antisense technologies: Improvement through novel
chemical modifications.
Eur. J.
Biochem. 270: 1628-1644; 2003.
[5] Bennett, M.R.; Schwartz, S.M. Antisense therapy for angioplasty
restenosis. Some critical considerations.
Circulation. 92(7):1981-93; 1995.
[6] Scherer, L.J.; Rossi, J.J. Approaches for the sequence-specific
knockdown of mRNA.
Nat Biotechnol.
12:1457-65; 2003.
[7] Johansson, H.E.; Belsham, G.J.; Sproat, B.S.; Hentze M.W. Target
specific arrest of mRNA translation by antisense
2’-O-alkyloligoribonucleotides.
Nucleic
Acid Res. 11;22:4591-8; 1995.
[8] Iversen, PL. Phosphorodiamidate morpholino oligomers: favorable
properties for sequence specific gene inactivation.
Curr Opin Mol Ther. 3(3):235-8; 2001.
[9] Kurreck, J.; Wyszko, E.; Gillen, C.; Erdmann, V.A. Design of
antisense oligonucleotides stabilized by locked nucleic acids.
Nucleic Acids Res. 30:1911-1918;
2002.
[10] Alahari, S.K.; Delong, R. M.; Fisher, H.; Dean, N.M.; Viliet, P.;
Juliano, R.L. Novel chemically modified oligonucleotides provide potent
inhibition of P-glycoprotein expression.
JPET.
286:419-428; 1998.
[11] Eckstein, F. Phosphorothioate oligonucleotides: What is their origin
and what is unique about them?
Antisense
Nucleic Acids Drug Dev. 10:117-121; 2000.
[12] Xu, Y.; Linde, A.; Larsson, O.; Thormeyer, D.; Elmen, J.;
Wahlestedt, C.; Liang, Z. Functional comparison of single- and double-stranded
siRNAs in mammalian cells.
BBRC.
316: 680-687;2004.
[13] Aharinejad, S.; Paulus, P.; Sioud, M.; Hofmann, M.; Zins, K.;
Schafer, R.; Stanley, E.R.; Abraham, D. Colony-stimulating factor-1 blockage by
antisense oligonucleotides and small interference RNAs suppresses growth of
human mammary tumor xenografts in mice,
Cancer
Research. 64(15):5378-84; 2004.
[14] Asriab-Fisher, A.; Fisher, M.; Juliano, R.; Herdewijn, P. Increased
uptake of antisense oligonucleotides by delivery as double stranded complexes.
Biochemical Pharmacology.
68:403-407; 2004.
[15] Alahari, S.K.; Dean, N.M; Fisher, M.H.; DeLong, R.; Manoharan, M.;
Tivel Robinson, K.L. Inhibition of expression of the multidrug
resistance-associated P-glycoprotein by phosphorothioate and 5’
cholesterol-conjugated phosphorothioate antisense oligonucleotides.
Mol Pharmacol. 50:808-19;
1996.
[16] Dassow, H. Modulation of multidrug resistance gene (hmdr1) with
antisense oligodeoxynucleotides.
Int J
Clin Pharm Ther. 38:209-16; 2000.
[17] Dalton, W.S. Is P-glycoprotein a potential target for reversing
clinical drug resistance?
Cur Opin
Oncol. 6:595-600; 1994.
[18] Leyland-Jones, B. Reversal of multidrug resistance to cancer
chemotherapy.
Cancer. 72:3484-3488; 1993.
[19] Ling, V. P-glycoprotein and resistance to anticancer drugs.
Cancer. 69:2603-9; 1992.
[20] Ramachandran, C.; Wellham, L.L. Effect of MDR1 phosphorothioate
antisense oligodeoxynucleotides in multidrug-resistant human tumor cell lines
and xenografts.
Anticancer Res.
23(3B):2681-90; 2003.
[21] Brigui, I.; Djavanbakht-Samani, T.; Jolles, B.; Piagaglio, S.;
Laigle, A. Minimally modified phosphodiesther antisense
oligodeoxyribonucleotide directed against the multidrug resistance gene mdr1.
Biochemical Pharmacology.
65:747-754; 2003.
[22] Lee, G.; Piquette-Miller, M. Influence of IL-6 on MDR and
MRP-mediated multidrug resistance in human hepatoma cells.
Can J Physiol Pharmacol. 79:876-884;
2001.
[23] Hartmann G, Cheung AK, Piquette-Miller M.
Inflammatory cytokines, but not bile acids,
regulate expression of murine hepatic anion transporters in endotoxemia.
JPET
2002; 303(1): 273-281
[24] Takei, Y.; Kadomatsu, K.; Itoh, H.; Sato, W.; Nakazawa, K.; Kubota,
S.; Muramatsu, T. 5”-,3’-Inverted
Thymidine-modified Antisense Oligodeoxynucleotide Targeting Midkine.
JBC. 26:23800-23806; 2002.
[25] Koizumi, S. Flow cytometric functional analysis of multidrug
resistance by Fluo-3: a comparison with rhodamine-123. Eur J Cancer. 31A(10):1682-1688; 1996
[26] Twentyman, S. Comparison of rhodamine 123 accumulation and efflux in
cells with P-glycoprotein mediated and MRP-associated multidrug resistant
phenotype. Eur J Cancer.
30:1360-1369; 1994.
[27] Dewanjee, M.K., Chafouripour, A.K.; Kapadvanjwala, M.; Dewanjee, S.;
Serafini, A.N.; Lopez, D.M.; Sfakianakis, G.N.
Noninvasive Imaging of c-myc
Oncogene Messenger RNA with
Indium-111-Antisense Probes in a Mammary Tumor-Bearing Mouse Model.
J Nucl Med. 35:1054-1063; 1994.
[28] Aleman, C.; Annereau, J.P.; Liang, X.J.; Cardelli, C.O.; Taylor, B.;
Yin, J.J.;
Aszalos, A.; Gottesman, M.
M.
P-Glycoprotein, Expressed in
Multidrug Resistant Cells, Is Not Responsible for Alterations in Membrane
Fluidity or Membrane Potential.
Cancer
Research.
63:3084-3091;
2003.
[29] Marks, D.C.; Belov, L.; Davey, M.W.; Davey, R.A.; Kidman, A.D. The
MTT cell viability assay for cytotoxicity testing in multidrug-resistant human
leukemic cells.
Leuk Res.
16(12):1165-73; 1992.
[30] Coley, H.M.; Sargent, J.M.; Williamson, C.J.; Titley, J.; Scherper,
R.J.; Gregson, S.E.;
Elgie, A.W.;
Lewandowicz, G.M.; Taylor, C.G. Assessment of the classical MDR phenotype in
epithelial ovarian carcinoma using primary cultures: a feasibility study.
Anticancer Res. 22(1A):69-75; 2002.
[31] Mukherjee, R.N.; Ghosh,
F.R. Maxfield Endocytosis.
Physiol Rev.
77(3): 759-803; 1997.
[32] Tikhomirova A.;
Chalikian T.V.
Probing
hydration of monovalent cations condensed around polymeric nucleic acids.
J Mol Biol. 341:551-563;
2004.
[33] Baker, B.F.; Monia, B.P. Novel mechanisms for antisense-mediated regulation of gene expression Biochim Biophys Acta. 1489:3-18; 1999.
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
CSPS Home | JPPS Home | Search | Subscribe to JPPS