J Pharm Pharmaceut Sci (www.cspscanada.org) 8(3):467-482, 2005
Pegylated Lysine Based Copolymeric Dendritic Micelles For Solubilization And Delivery Of Artemether
Dipankar Bhadra, Sulekha Bhadra, N. K. Jain
Pharmaceutics Research Laboratory, Dept. of
Pharmaceutical Sciences, Dr. H.S. Gour University, Sagar (MP),
Received June 23, 2005, Revised August 23, 2005; Accepted August 23, 2005, Published September 2 2005
Corresponding Author:
N. K. Jain, Pharmaceutics Research Laboratory,
Dept. of Pharmaceutical Sciences, Dr. H.S. Gour University, Sagar (MP), 470003,
Abbreviations:
IR: infrared; NMR: nuclear
magnetic resonance; CMC: critical micelle concentration
RES: Reticulo-endothelial system
ABSTRACT PURPOSE: A newer polymeric amphiphilic micellar
system was developed in the present study for solubilsation and controlled
delivery of an antimalarial drug, Artemether (ART). Methoxy polyethylene glycol
(MPEG) 2000 and 5000 were used as hydrophilic terminal. METHODS: The
hydrophobic di-Fluorene methoxycarbonyl-l-lysine (di-FMOC-L-lysine) was linked
initially to the single reactive end of MPEG, and to the two amino groups of
l-lysine by consecutive peptide linkages and deprotection upto 2.5 generations (G).
Half-generations are diFMOC-lysine terminated systems and full-generations are
deprotected l-lysine terminated systems. The half-generation (0.5G, 1.5 G and
2.5 G) dendritic micelles of MPEG 2000 and 5000 were used to solubilize
artemether. IR, NMR and Mass spectroscopy characterized the synthesis of these
micellar systems. The CMC of the systems was determined. Then formulations made
were characterized for solubility enhancement (i.e. drug loading) and
drug-release profile. RESULTS: There is considerable solubility
enhancement of artemether upto three to fifteen times depending on
concentration, generation and type of dendritic micelles used. The size and
shape were studied using transmission electron microscopy. The stability of the
micellar formulation was also determined by storing the micelles at various
temperatures for a definite period of time followed by its successive
dilutions. The dendritic carriers were found to form stable micelles at 10-30 mg/ml (lower
CMCs) depending on generation and type of MPEG used. The formulations increased
the stability of the drug and also prolonged the release of artemether upto 1-2
days in vitro. CONCLUSION: From all the studies performed, it can
be concluded that these micellar systems can be used for the safe and effective
delivery of insoluble bioactive.
INTRODUCTION
Copolymeric
micelles are the micelles formed through multimolecular
assembly of the block copolymers. They are comprehensively described as novel
core–shell typed colloidal carriers for controlled drug delivery and gene
targeting. Novel approaches are also used for the formation of such micelles
using functionalized poly (ethylene glycols) (PEGs) as
hydrophilic outer shell and were focused through PEG-conjugated
ligands with minimal non-specific interaction with other proteins. Surface
organization of these block copolymer micelles with
cross-linking core was also described from a standpoint of the preparation of a
new functional surface-coating with some unique macromolecular architecture
(1). These have PEG chains attached to one end in a brush configuration, which
avoid or reduce interactions with blood proteins and therefore impart RES
avoiding properties. To achieve the core shell structure described above, block
amphiphilic polymers of the PEG-R types were synthesized. R was chosen from
bioerodible polymers such as PLA (poly D, L-lactide), PLGA
(polylactideco-glycolide), PCL (poly-caprolactone), Poly (butylene terephthalate)
(PBT), poly (ortho ethers) (POE), poly-l-lysine (dendrimers) etc (2).
Earlier, we have synthesized and reported similar PEG
coated polyamidoamine (PAMAM) dendrimer based unimolecular micellar system for
the delivery of 5-fluorouracil in our laboratory (3, 4). One such similar
unimolecular dendritic micelles as solubility enhancers were obtained by
coupling polyethylene glycol (PEG) to Starburst PAMAM dendrimers of various
generations (5). There was significant change in the solubility of pyrene as
was monitored at 334 nm, its maximum absorption wavelength. A brief survey of
such host-guest interactions involving dendritic architectures was also
reported (6). The effects of ethylene glycol-based graft, star-shaped,
dendritic polymers on solubilization and controlled release of drugs like
paclitaxel and nimesulide was also studied (7, 8). The micelles from lipid
derivatives of water-soluble polymers as delivery systems for poorly soluble
drugs were also studied (9). The solubility of a poorly water-soluble drug,
Cyclosporin A, was also increased in aqueous dispersions of
dextran-grafted-polyethyleneglycol-alkyl ether (10). The drug release behaviors
of nimodipine-loaded poly (caprolactone)–poly (ethylene oxide)–polylactide
amphiphilic copolymeric nanoparticles were also studied (11).
Many newer approaches developed for effective
antimalarial chemotherapy was reviewed by Bhadra et al. (12). However, in the
present approach, poly-l-lysine based peptide dendrimers protected with
Fluorene methoxy carbonyl terminal was conjugated at methoxy-PEG-hydroxyl
terminals to form an amphiphilic peptide based AB-dendritic copolymeric
micelles for solubilization of a potent antimalarial, artemether (an
artemisinin derivative). Artemisinin derivatives are active at nanomolar
concentrations in vitro on both chloroquine-sensitive or -resistant P.
falciparum strains. ART has been included in the WHO List of Essential
Drugs for the treatment of severe multiresistant malaria (13, 14), but the
major drawback of artemisinin derivatives is their short half-life (3–5 h).
Also, the oral formulations of these drugs were rapidly but incompletely
absorbed, and their bioavailability is lower. So there was always some
necessity of administration of these derivatives by some alternative parenteral
route. The Walter Reed Institute of Research had already patented a stable,
water-soluble derivative of this family called artelinic acid (15).
However, the problem associated with such conventional
formulations is unavailability of a suitable compatible aqueous base for
sustained and controlled delivery of ART. Thus, nanoparticulate depot type
carriers were suggested for the delivery of ART compatible intravenous
carriers, as used earlier for such bioactives having short half-life (8, 16).
This approach could also increase the solubility of ART, similar to
water-soluble polymer conjugates of the anti-malarial drug, artelinic acid,
developed using water soluble and non-peptidic polymer backbones, such as
poly(ethylene glycol), mPEG, bifunctional PEG and multi-arm PEG (18). In the
present study, however, much stable MPEG-Lysine-diFMOC based dendrimeric
nanoparticulate carriers were selected for solubilization of artemether, and
prolonging its release and stability, which can enable sustained and controlled
delivery of ART in solubilized systems by i.v. route as aqueous solution.
MATERIALS AND METHODS
Materials:
The
drug ART was a generous gift sample from M/s Ipca Laboratories,
Synthesis of MPEG-lysine-diFMOC dendrimers: The MPEG-lysine-diFMOC dendrimers were
synthesized using MPEG-amine 2000D and 5000D as core and protected
diFMOC-lysine for progressive linking on terminal amino groups of prior
generations consecutively by liquid phase peptide synthesis as discussed in our
earlier work (19, 20) upto 2.5G. MPEG amine was synthesized firstly by stepwise
synthesis scheme as suggested by Zalipsky et al. (21). For the synthesis of
dendrimers, protected l-lysine was required for allowing uniform branching.
This was carried out using FMOC-Su (Fluorenyl methoxy carbonyl succinimide) to
form di-FMOC-lysine by method suggested by Lapatsanis et al (22). Finally, the
MPEG terminated lysine-di-FMOC micellar systems of various generations were
synthesized by the well-known DCC-HOBt coupling procedure in DCM: DMF (1:1)
solvent system. Deprotection of diFMOC groups from the micelles was carried out
by piperidine-based hydrolysis for synthesis of further higher generations (19,
20, 22).
The products were separated,
dried and stored in a vacuum desiccator. The protection and de-protection steps
were repeated alternately with subsequent increase in reactants (Fig. 1) for
every consecutive generations upto 2.5G. The half
generations of dendrimers of 0.5G, 1.5G and 2.5G of each MPEG2000 and 5000
types were used in the present studies for formulations and solubilization of
artemether (Table 1).
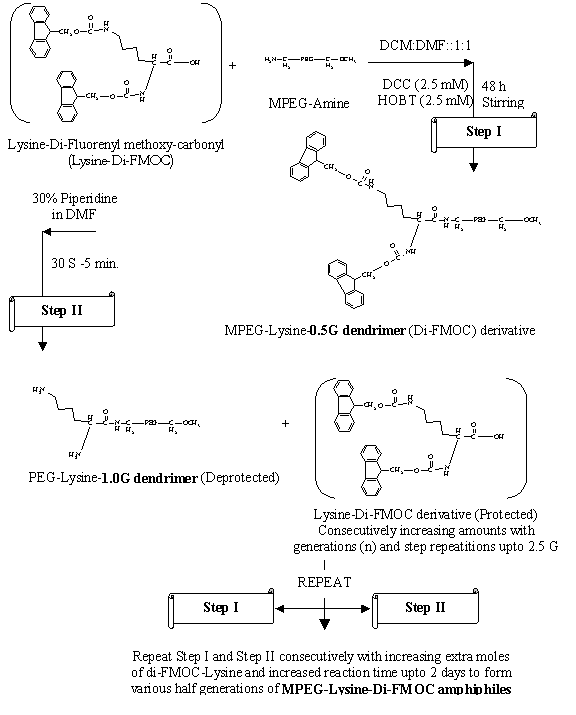
Fig. 1. Structural scheme for the synthesis of MPEG-Lysine-Di-FMOC
amphiphilic dendrimeric micellar carriers.
The dendrimeric amphiphiles
were characterized by IR, NMR, MALDI-TOF mass spectroscopy and Kaiser Test for
completion of reaction and structural elucidation as described earlier (19).
Determination of Critical Micelle Concentration: Amphiphilic block copolymers like MPEG-lysine systems of
half generation tend to form micelles at concentrations above CMC, hence determination of CMC is required for formulation
of drug-loaded carriers. Amongst the various methods used to determine CMC, log10(concentration)
vs. absorbance plot of half generation systems (0.5G, 1.5G and 2.5G systems),
at its absorbance maxima (lmax, 258 nm), was used in the present study. The half
generations of dendrimers were dissolved in water, vortexed (Superfit),
sonicated and shaken on mechanical incubator shaken at 37°C for 2 h to prepare a stock solution of 1000mg/ml. The aliquots of the
stock solution were further diluted to prepare different dilutions and kept
overnight for equilibration and formation of micelles for the study.
In the curve where there was a change in the slope of
curve at CMC. The concentration corresponding to the point of intersection of
the slopes of lower and upper curve denotes the CMC of the copolymers. This
method was based on sudden changes in absorbance due to aggregation at CMC
(23). However, the whole-generation PEG-lysine systems have no CMC, and they
actually form unimolecular nanoparticulate dendrimeric carrier systems for
loading drug by complexation or hydrotropic solubilzation within its structure
by steric hindrance and group complexation.
Phase solubilization of artemether: The studies were conducted using continuous variation method of Higuchi
and Connors (24). Briefly, an excess of the drug was added to various
concentrations of dendrimeric micellar solutions, followed by vortexing and
equilibration. Half-generations of PEGylated-lysine-diFMOC dendrimers at
concentration range 1000 mg/ml to 5000 mg/ml (i.e. above CMC values) were used for solubilization of water
insoluble ART. The final suspensions of drug and dendrimers (half-generations)
were vortexed and sonicated. The final mixture was shaken in mechanical
incubator shaker at 37°C overnight, filtered,
lyophilized and stored until further use.
Characterization of formulations: The final dendritic micellar formulations
with and without drug prepared and dialyzed were used for electron microscopic
studies. The Transmission Electron Microscopic studies were carried out using 3
mm Forman (0.5 % plastic powder in amyl acetate) coated copper grid (300 mesh)
at 60 KV using negative staining by 4% Uranyl acetate at various magnifications
on Moragagni 268D with digital TEM image analysis system of Soft Imaging
System, GmbH (Germany) at 50-60kV.
Drug loading was performed by phase
solubilization of the drug in different concentration of half-generation
dendrimeric micelles as entrapment of drug in micelles can also increase its
solubility.. For the determination of entrapment
efficiency of the systems, the drug molecules were partitioned out from 1ml
portions of aqueous formulations by shaking with 5ml portions of
dichloromethane (DCM). This led to breaking of micelles, thereby releasing the
entrapped drug. The DCM layer was dried under vacuum and methanol was used to
solubilze the residue. The methanol solubilized portion was hydrolyzed using 1
ml of 5 M HCl by heating for 15 min. The amount of drug was determined
spectrophotometrically at 254 nm after proper dilution (14, 25). The amount of
drug solubilized by various concentrations of dendrimers was used to determine
amount of drug (g) entrapped by per gram of dendrimeric micelles (w/w) and also
as molar ratio of drug, in moles of drug per moles of dendrimeric micelles.
The drug release studies were carried out by dialysis
using cellophane tubes (Pore 2.4 nm, Himedia). 100 mg of lyophilized
drug-dendrimer system was dissolved and taken in cellophane tubes and immersed
in the aqueous medium (20 ml) under magnetic stirring. Samples were withdrawn
from it at every 1 h for 8 h. After that samples were withdrawn at 24 h. and
every day thereafter. The cumulative amount of drug coming out of the micellar
carriers was plotted against time to determine the release pattern of the systems.
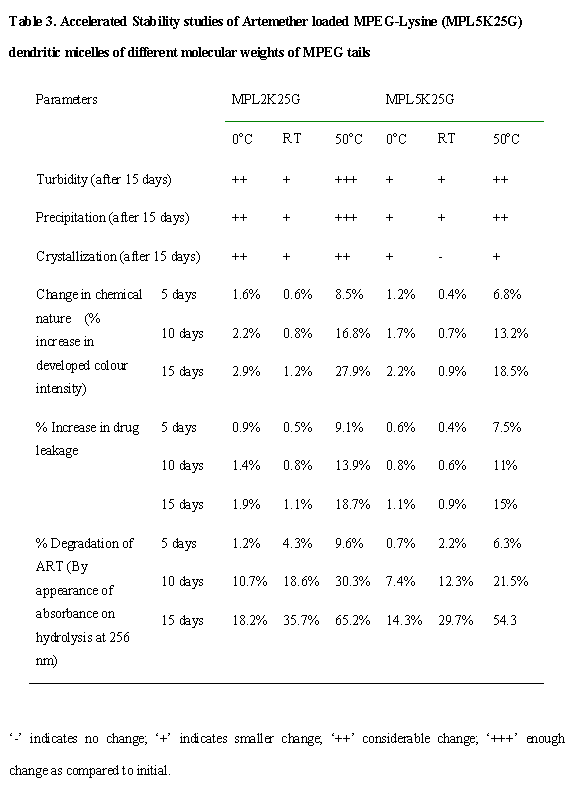
Stability studies: The dendrimer-drug micellar formulations were kept in tightly closed vials and stored at 0°C, room temperature (25°C) and 50°C (controlled oven) for a period of 15 days. The samples were analyzed initially and periodically after every week for any precipitation, turbidity, crystallization, change in colour, consistency, drug leakage and chemical nature of formulations. The data obtained was used to predict the stability, the required storage conditions, and the precautions required during storage.
Effect
on chemical nature of preparation was ascertained by comparison of the intensity of
colour developed by Kaiser Test with 1ml of formulation, spectrophotometrically
(lmax=570nm) (26). The percentage change in the
intensity of the color produced was used for the determination of free amino
groups available at the periphery of dendrimeric formulation.
The
drug leakage was determined by checking for increase in the
release rate of drug from the formulations after storage at accelerated conditions.
The formulation samples (2ml) were kept in cellulose tubing and dialyzed across
the tubing. The external medium (10ml) was analyzed for content of drug,
spectrophotometrically. The procedure was repeated every week for upto five
week. The percentage increase in drug release from the formulation was used to
analyze the effects of accelerated conditions of storage on the formulation.
The amount of residual insoluble drug (ART) present in formulations were also
analyzed for all formulations by filtration and analyzing similar as for drug
entrapment in micelles every 5 days.
Hydrolytic
attack on artemether
and degree of stability provided by the micellar formulations were determined
by mixing 0.5 g of ART-loaded micellar preparation of 2.5G of MPEG 5000D with
1.0 ml of 1 M HCl producing a stock solution of 5000mg/ml. This stock was equally divided and
stored at room temperature (25°C), for determination of effect of HCl on
encapsulated ART in separate ten 10 ml vials. Similarly, diluted methanolic
aqueous ART solution was also kept with 1M HCl, for comparison. This study was
designed to determine the protection efficacy of micelles on the encapsulated
drug.
The effect of dilution on stability of
formulations was determined by diluting 1 ml micellar formulations (5000 mg/ml) with
1-10 ml of water. These dilutions were monitored for any crystallization after
2 h. The amount of drug remaining solubilized in the formulations was
determined by filtration followed by drug estimation in the micellar solutions.
RESULTS AND DISCUSSION
In
the present study, amphiphilic poly-l-lysine based peptide dendrimers, having
PEG at hydrophilic ends and di-FMOC at other hydrophobic end was used for the
aqueous solubilization of artemether, an artemisinin derivative. Peptide
dendrimers are radial or wedge-like branched macromolecules consisting of a
peptidyl branching core and/ or covalently attached surface functional units.
The multimeric nature of these constructs, the unambiguous composition, and the
ease of production make this type of dendrimer well suited to various
biotechnological and biochemical applications e.g. diagnostic reagents, protein
mimetics, carriers for drugs, vaccines and genes. Earlier, Sadler & Tam
(27) reviewed extensively such peptide dendrimers, their synthesis and
applications. Choi et al. (28, 29) also synthesized one such barbell-like
ABA-type triblock copolymer, poly(L-lysine)
dendrimer-poly(ethylene glycol)- poly(L-lysine) dendrimer (PLLD-PEG-PLLD) by
liquid-phase peptide synthesis, similar to the present system.
Synthesis of MPEG-lysine-diFMOC dendrimers. Lysine-diFMOC was used to synthesize the proposed
peptide dendrimers by amide linkages using DCC-HOBT techniques following scheme
given in Fig 1. The RasMOL representation (Fig 2) gives the evidence of basic
linear configuration and closeness of structure as generation increases. The
reaction was allowed to complete with further addition of DCC (taken 10% molar
excess quantity) to the solution of protected lysine and HOBT (taken equimolar
to DCC). Lysine-diFMOC was taken 10-50% molar excess of stoichiometric amounts
depending on generations.
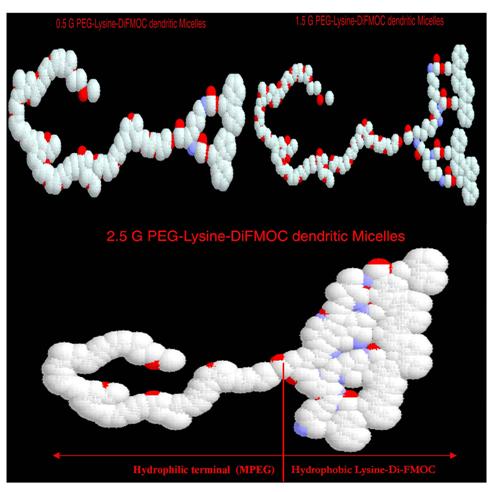
Fig. 2. Rasmol
version 2.5 representations of MPEG-Lysine-diFMOC micelles of various
dendrimeric generations, where a) 0.5G, b) 1.5G, c) 2.5G MPEG-Lysine di-FMOC
dendrimers.
The reaction of amide linkages with the protected
lysine-diFMOC took 1-5 days, depending on generations. In the case of higher
generations, the time required for completion of reactions was increased. Intermittent
checking for completion of reaction at amine termination of lysine was done by
negative Kaiser Test.
The progress of generations was confirmed
by Kaiser Tests giving Ruhemann’s purple blue chromophore absorbing at 570 nm.
This absorbance gives the number of amine groups of amino acids (Table 1). The
positive Kaiser test and absorbance of the dendrimeric generations
(full-generation) was further used for quantitative estimation of terminal
lysine on each dendrimer molecules (26). On protection by di-FMOC no such
formation occurred and only yellow colour was obtained in the reaction mixture.
The test was used to determine equivalent amino groups in each generation and
completion of reaction until there is no blue colour development in aliquots of
reaction mixtures tested for completion of reaction.
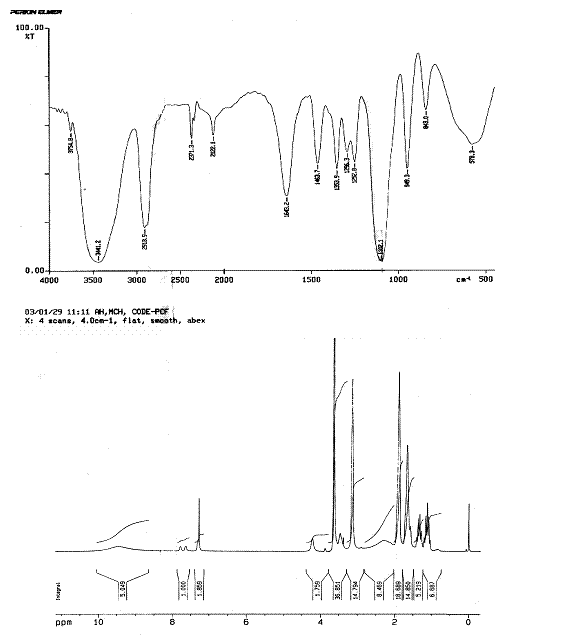
Fig. 3. IR and 1H-NMR Spectrum of MPEG-Lysine-DiFMOC type of half generation
peptide dendrimeric micelles.
Finally, IR, NMR and MALDI-TOF mass spectroscopy
confirmed the completion of synthesis. The IR spectrum (Fig. 3) showed some
distinct peaks in half-generation of dendrimers with peaks of PEG and lysine
consecutively. For example, 2931.7 & 2832.8 cm-1 for –C-H
stretch of methyl groups; 1592.2 cm-1 for N-H bends due to amine gr.
of lysine; and 1120.5 cm-1 for C-O-C str. of PEG. Peak at 1629.7 cm-1
for C=O str. and at 3043.6 cm-1 for N-H stretch confirms the
formation of amide linkage. Other important peaks were at 1360.5; 1162.5; 773.4
cm-1.
The NMR spectrum showed some distinct peaks
in half generations of dendrimers. The major shifts are at 0.9-1.1 ppm for C-H
protons of other lysine; 1.3-1.4 ppm for methylene protons of PEG; 1.5-1.8 ppm
for a-, b, and g- methylene protons of poly-lysine; 1.9-2.1
ppm for -N-H of amine of poly-l-lysine at d position; 2.2-2.6 for NH proton near COOH
gr. of lysine; 3.0-3.3 ppm for ether groups of PEGs; 3.5-3.8 ppm for ether
groups of terminal portion methoxy and residual diethyl ether left; 4.2-4.4 ppm
for amide linkage at d position; 7.1-7.4 ppm for amide linkage at
a position;
7.6-7.8 ppm for aromatic fluorene; and 8.9-10.2 ppm for carboxyl groups of
lysine. The ratios of NMR peak intensity for the ethylene protons of PEG
segment (d=3.0-3.3 ppm) and the a-, b, and g- methylene
protons of PLL dendrimers (d=1.4-1.8 ppm) were further used to determine
the ratio of lysine to PEG chains in dendrimers. The experimental ratios of the
peaks are more or less equivalent to the theoretical ratios of the peaks in the
spectrum. The MALDI-TOF-mass spectroscopy was used to determine the mass of the
dendrimers formed in each generation, protected and unprotected types. Average
mass of the systems were determined from the peaks of parent molecular ion. It
matched to a significant extent with the
actual theoretical mass (Table 1).
Determination of Critical Micelle
Concentration (CMC). The CMC values
were determined by the method of changes in turbidity and absorbance at 258nm (lmax) associated with it at concentration equal
to or more than CMC due to aggregation of unimers at that concentration leading
to an abrupt increase in absorbance (23).
The CMC values were in the range of
micromolar concentration for such polymeric micellar carriers that were found
to be decreasing with increase in generations due to in increase in the
hydrophobicity of the ends (Table 1). No general trend was observed with
increase in molecular weight of the carriers but with the increase in molecular
weight of PEGs (hydrophilic terminal), the CMC values
were significantly higher than that of the same generations made from lower
molecular weight PEGs.
This conforms to the predictions for the
CMC based on structures of the surfactants in a series of surfactants (30). The
lower CMC values indicate that the systems could be used as stable and
sustained drug delivery carriers without much effect on physicochemical
stability of formulations on dilution and at the same time, can protect the
drug molecules from degradation due to external environment (31). By the Rasmol
representation of molecular orientation and structure, it can also be ascertained
that the structures at higher generations are suitable as unimolecular
micelles. The hydrophobic end is well structured and voluminous that could well
load the hydrophobic molecules within their hydrophobic environment and at the
same time remain solubilized due to the presence of
PEG at the periphery (Fig. 4).
So it can be concluded that the higher
generations have more entrapment capacity and stabilizing potential.
Electron microscopy. The particles were not ordinarily viewable
by normal microscopy or dynamic light scattering technique as they are in
nanometric size-range. Such nanoparticulate carriers are more easily and
suitably focused by Transmission Electron Microscopy (TEM). All formulations
are in nanometric size range, spherical and uniform shaped (Fig. 5).
The micellar carriers were stained negatively by 4%
uranyl acetate, which stained the background more prominently and leave the
particles in unstained state. The drug-loaded micelles were seen as dark dots.
This might be due to the positive staining of drug-loaded carriers considerably
due to presence of drug within such carriers. The size of micelles was found to
be 5-25 nm (as evident by scale below shown by digital image analysis system
Soft imaging system version 3.1) with increase in generations from 0.5G to 2.5G
for MPEG-lysine-diFMOC carriers of 5000D.
Drug Entrapment and solubilization. The drug loading in dendrimers were carried out by
equilibrium dialysis method leading to drug loading by adsorption and physical
interaction like protein binding onto the carriers.
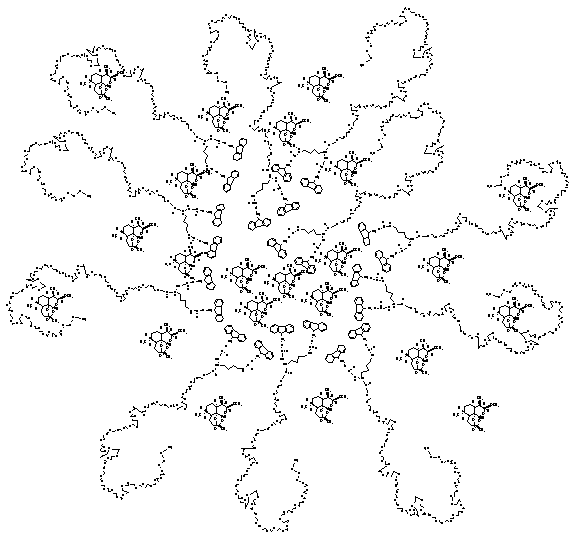
Fig. 4.
Representative
structure of insoluble artemether loaded in MPEG-Lysine-FMOC Micellar carrier
of 0.5G dendrimeric generations.
The entrapment was expressed in terms of
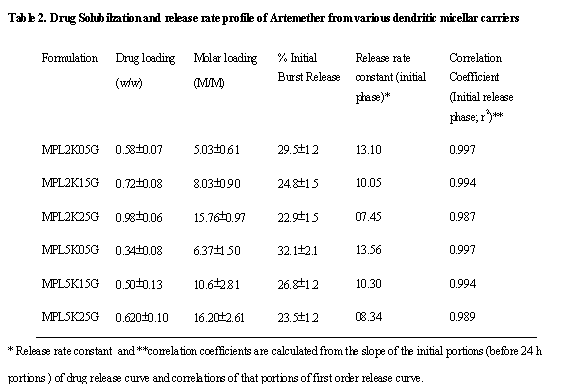
weight of drug loaded per gram of dendrimers (Table 2). This was used to
calculate stoichiometrically molecular entrapment using theoretical molecular
weights of the carriers to determine possible number of drug molecules loaded
within one molecule of dendrimer. The micellar entrapment of ART also followed
a definite trend, where increase in micellar generations causing increase in
weight-by-weight and molar drug content. This occurred because with increase in
generation of micelles, there was a distinct increase in hydrophobic tail
length and volume, which caused entrapment of drug molecules in micellar
aggregates and in tails with FMOC groups by hydrophobic interactions. Thus,
entrapment in micelles occurred both by hydrophobic and hydrotropic
complexation based interactions. The entrapment was upto 5 to 16 molecules per
molecules of unimers. These values did not undergo significant changes with
increase in molecular weight of MPEGs from 2000D to 5000D. The entrapment of
ART in weight terms undergoes significant changes with increase in molecular weights
of MPEGs (hydrophilic tails) and also with increase in generations of
dendrimeric micelles.
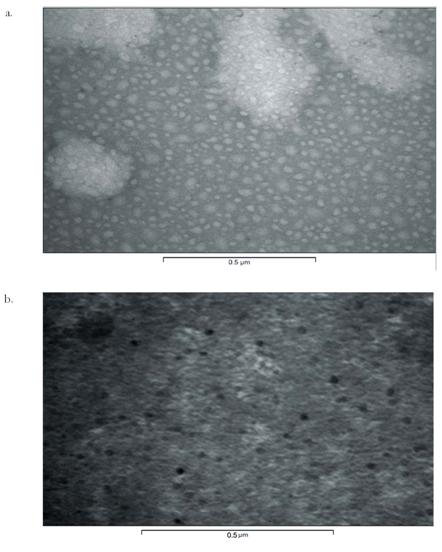
Fig. 5.
TEM
photomicrograph representations of 2.5G dendritic micelles after negative
staining with uranyl acetate where a) represents the empty micelles; b)
represents drug loaded micelles
As the CMC increases with molecular weight of MPEG,
there was an increase in the requirements of number of molecules for
micellization or aggregation for the drug loading. This led to significant
decrease in weight of drug loaded per gram of dendrimer with increase in
molecular weights of MPEGs significantly. The drug entrapment reduced
significantly from 0.98±0.06g/g for MPL2K25G to 0.620±0.10g/g for
MPL5K25G, which in molar times was however, non-significant (Table 2). However,
the weight-by-weight drug loading increased from 0.58±0.07 g/g for 0.5G generations of 2000D
micelles to 0.98±0.06 g/g for 2.5G MPEG 2000D species.
Drug Release Profile. The release of drugs from the dendrimeric formulations
was determined across dialysis cellulose tubing of 2.4nm and estimated
spectrophotometrically, after appropriate dilution. The drug release was
estimated in terms of % cumulative drug released using the average amounts of
drug loaded in the dendrimeric carriers (Table 2). The effect of mass &
generation of dendrimeric carriers on initial burst release from carriers;
release patterns and release rate constants were also analyzed.
The
micelles on dialysis get diluted and displace drug comparatively rapidly being
multi-molecular in nature. The release rate of MPL2K05G, MPL2K15G and MPL2K25G
was 13.10%, 10.05% and 7.45% per h, respectively. For higher molecular weights
of MPEGs (i.e. 5000D series), the release rate was slightly higher. It was
13.56%, 10.30% and 8.34% per h for MPL5K05G, MPL5K15G and MPL5K25G,
respectively. Similar trend was observed in case of burst release from such
carriers, which reduced with increase in generations of such carriers viz. 29.5±1.2%, 24.8±1.5% and 22.9±1.5%
respectively for 0.5G, 1.5G and 2.5G of MP2000 series and 32.1±2.1%, 26.8±1.2% and 23.5±1.2%
respectively for 0.5G, 1.5G and 2.5G of MP5000 series. This was only due to the
fact that higher CMCs of micellar carriers of 5000D made the carriers more
vulnerable for drug release (Fig. 6). With the increase in generations of
micelles, there was significant decrease in CMCs, also
there is an increase in hydrophobic fluorene groups (FMOC) in the micelles for
hydrophobic interactions leading to increase in drug loading (Table 2). With increase in generations of dendrimers, increase in groups for
complexation and additional binding caused increased steric hindrance, causing
reduction in drug leakage. The higher drug
release from the micelles of higher molecular weights of MPEG may also be
contributed to the lower wt fraction of hydrophobic core in these polymers
other than the effects of the CMC values.
Stability Studies. Micelles of high molecular weight MPEGs
are comparatively more stable as to lower molecular weight micellar carriers.
There was increase in turbidity in formulations at lower temperature more due
to lower solubility of carriers that occurs highly in lower molecular weight
PEG carriers as these carriers have lower solubility because of lesser number
PEG ethereal linkages, which additionally increases the stability of such
carriers. The decrease in solubility of such carriers at lower temperature
additionally caused crystallization of drug because of displacement of ART from
such carriers. The percentage degradation of ART as measured by appearance of
absorbance on hydrolysis at 256 nm for the formulations stored at various
conditions for testing integrity and protective nature of formulations, also
proved that higher molecular weight MPEG carriers were more stable from
degradations as compared to lower molecular weight carriers. This occurred due
to increased steric hindrance by higher molecular weight PEG that prevents
physicochemical degradation and losses of structural integrity of such
carriers.
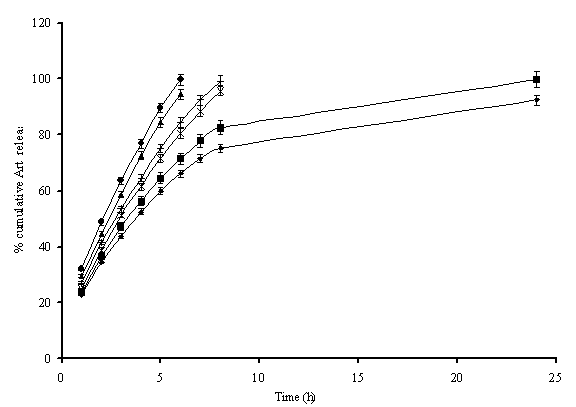
Fig. 6.
Cumulative
release pattern of artemether from the various generations of different
MPEG-Lysine peptide dendritic micelles, where ![]() represents
release from MPL2K05G; X from MPL2K15G;
represents
release from MPL2K05G; X from MPL2K15G; ![]() from
MPL2K25G;
from
MPL2K25G; ![]() from MPL5K05G; + from MPL5K15G and
from MPL5K05G; + from MPL5K15G and ![]() from MPL5K25G generations of micelles.
from MPL5K25G generations of micelles.
The
various micellar carriers were tested for hydrolytic attacks by HCl by keeping
in mild HCl at room temperature for a definite period of ten days and observed
for drug degradation pattern every day upto ten days.
For micellar carriers low molecular weight MPEG carriers (MPL2K25G) were found
less stable as compared to high molecular weight MPEG carriers. There was
appearance of ART crystallization from such carriers in 5-6 days and much heavy
crystallization was observed by 10th day.
The
higher molecular weight micellar carriers were more stable chemically even at
lower temperature and at room temperature as evident from change in chemical
nature by percentage increase in developed color intensity by Kaiser reagent (Table 3). The changes in chemical nature as
determined by percentage increase in developed colour intensity by Kaiser test
showed more colour development for lower MWt. carriers (MPL2K25G) as to
MPL5K25G, which was by 10th day only 4.7% as compared to 14.2% for
MPL2K25G. This could be attributed to molecular weight of MPEG, where increase
in number of molecular groups causes increased stability by its steric
hindrance from the attack of HCl (Fig. 7).
Encapsulation of ART in micelles also
stabilized the drug from outer environment as evidenced by HCl hydrolytic
attack for the entrapped drug. The HCl induced hydrolytic attack on
encapsulated drug after incubation showed that there was increased amount of
hydrolyzed drug in aqueous milieu from free drug solutions as compared to the
drug entrapped in micellar dendrimers (Fig. 7). The studies correlated well
with the reports on stabilizing DNA within dendrimers against nuclease attack
carried out by Rackstraw et al (31).
The various ART loaded micellar carrier
were tested for stability on dilution with water. More
drug crystallization was found from higher molecular
weight MPEG micelles MPL5K25G on dilution because of higher
CMC values so at increased dilutions they were producing drug crystals.
This could be attributed to the
intrinsic solubility of drug freed from the micelles in water, after disruption
of micelles, as the concentration of micelles falls below CMC that was earlier
preventing the drug from precipitation or crystallization from lower molecular
weight carriers on dilution. The lower CMC values and micellar aggregates
reduced drug crystallization by its release on dilution upto some extent but
after a definite dilution there is a breakdown of aggregates and release of
drug causes the drug concentration to exceed its solubility in aqueous milieu.
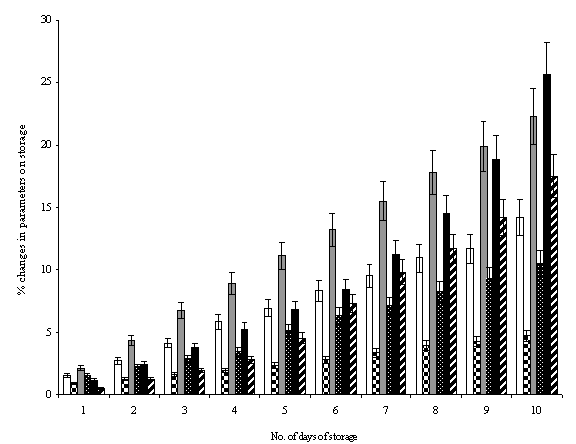
Fig. 7.
Hydrolytic stability profile of various Artemether
loaded dendrimeric micellar carriers on storage for various time intervals of
storage, where ![]() is related to percentage changes in chemical
nature of MPL2K25G and
is related to percentage changes in chemical
nature of MPL2K25G and ![]() MPL5K25G dendrimeric micelles;
MPL5K25G dendrimeric micelles; ![]() is related to percentage changes in entrapment
of artemether in MPL2K25G and
is related to percentage changes in entrapment
of artemether in MPL2K25G and ![]() in MPL5K25G; and
in MPL5K25G; and ![]() is percentage degradation of artemether with
time intervals of storage when present in MPL2K25G and
is percentage degradation of artemether with
time intervals of storage when present in MPL2K25G and ![]() when present in MPL5K25G.
when present in MPL5K25G.
It can thus be concluded that
micellar carriers of lower generations and lower molecular weight carriers can
simply be well diluted and administered intravenously as compared to higher
generations and higher molecular weight MPEG carriers. The work is only representation
of a newer and novel type of amphiphilic micellar carrier having at one end PEG
and other end is hydrophobic due to the presence of FMOC termination of
protected essential amino acid l-lysine. The systems were found very suitable
for solubilization and encapsulation of hydrophobic drugs like artemether, by
hydrophobic interactions within their FMOC terminus that is in dendrimeric form
as branched structure as shown in RasMol representations. The toxicity of such
systems might be very less due to their organization in the form of densely
clubbed hyper-branched micellar structures and possibly slow rate of
degradation, which would be further studied. All this can also be attributed to
PEG ends which can reduce toxicity of many toxic drugs, when coupled with them,
by the control of bioavailability.
ACKNOWLEDGEMENTS
The authors are pleased to acknowledge
University Grants Commission (UGC) and CSIR-New Delhi,
REFERENCES
[1]
Otsuka, H.;
[2]
Bhadra, D.; Bhadra, S.; Jain, P. and Jain, N. K., Pegnology: a review of
PEG-ylated systems. Pharmazie, 57(1): 5-29, 2002.
[3]
Bhadra, D.;
Jain, S.; Bhadra, S.; Jain, R.
and Jain, N. K., PEGylated
Dendrimers for delivery of 5-fluorouracil. Control.
Rel. Soc. Proc.,
[4]
Bhadra, D.; Bhadra, S.; Jain, S. and Jain, N.K., A
PEGylated dendritic nanoparticulate carrier of fluorouracil. Int J Pharm,
257: 111–124, 2003.
[5]
Yang, H.; Morris, J.J. and Lopina, S.T., Polyethylene glycol-polyamidoamine
dendritic micelle as solubility enhancer and the effect of the length of
polyethylene glycol arms on the solubility of pyrene in water. J. Colloid
Interface Sci, 273(1): 148-154, 2004.
[6]
Moorefield, C.N. and Newkome, G. R., Unimolecular micelles: supramolecular use
of dendritic constructs to create versatile molecular containers. Comptes Rendus Chimie, 6(8-10):
715-724, 2003.
[7]
Ooya, T.; Lee, J. and Park, K., Effects of ethylene glycol-based graft,
star-shaped, and dendritic polymers on solubilization and controlled release of
paclitaxel. J Control Rel., 93(2): 121-127, 2003.
[8]
Bhadra, S.; Bhadra, D. and Agrawal,
G.P., Amphiphilic copolymeric micelles for delivery of nimesulide: Preparation,
Optimization and Characterization.
[9]
Lukyanov, A.N. and Torchilin, V.P., Micelles from lipid derivatives of
water-soluble polymers as delivery systems for poorly soluble drugs. Adv
Drug Deliv Rev, 56(9): 1273-1289, 2004.
[10]
Francis, M. F.; Lavoie, L.; Winnik, F. M. and Leroux, J.C., Solubilization of
cyclosporin A in dextran-g-polyethyleneglycolalkyl ether polymeric micelles. Eur
J Pharm Biopharm, 56(3): 337-346, 2003.
[11]
Huo, F.; Xu, H.; Zhang, L.; Fu, Y.; Wang, Z. and Zhang, X., Hydrogen-bonding
based multilayer assemblies by self-deposition of dendrimer. Chem Commun
(Camb), 7: 874-875, 2003.
[12]
Bhadra, D.; Bhadra, S.; Jain, N. K. In
Jain, N.K. (Ed.) Progress in Controlled and Novel Drug Delivery Systems,
CBS Publishers and Distributors, New Delhi, 1st ed, pp
209-247, 2004.
[13] The use of artemisinin & its derivatives as
anti-malarial drugs world health organization. WHO,
WHO/MAL/98/1086
[14]
International Pharmacopoeia. World Health Organization, Avenue Appia,
Geneva, Switzerland, 3rd ed. Vol. 5, pp187-198, 2003.
[15]
Li, Q.; Peggins, J.O.; Fleckenstein, L.L.; Masonic, K.; Heiffer, M.H. and
Brewer, T.G., The pharmacokinetics and bioavailability of dihydroartemisinin,
arteether, artemether, artesunic acid and artelinic acid in rats. J Pharm
Pharmacol, 50: 173–182, 1998.
[16]
Kolhe, P.; Misra, E.; Kannan, R.M.; Kannan, S. and Lieh-Lai, M., Drug
complexation, in vitro release and cellular entry of dendrimers and
hyperbranched polymers. Int J Pharm, 259: 143-160, 2003.
[17]
Bhadra, D.; Bhadra, S.; Jain, S. and Jain, N.K., A PEGylated dendritic
nanoparticulate carrier of fluorouracil. Int J Pharm, 257(1-2): 111-124,
2003.
[18]
Bentley, M. D.; Zhao, X. and Clark, J. L., Water-soluble polymer
conjugates of artelinic acid. US Patent, 6,461,603.,
2002.
[19]
Bhadra, D.; Bhadra, S. and Jain, N. K., Pegylated Peptide Based
Dendritic Nanoparticulate Systems for Delivery of Artemether. STP Pharm. Sc.
Theme issue 2005 (in Press).
[20]
Bhadra, D.; Bhadra, S. and Jain, N. K., PEGylated-poly-l-lysine
dendrimers for delivery of Chloroquine phosphate. International
Conference on MEMS, NANO, and Smart Systems.
[21]
Zalipsky, S.; Gilon, C. and Zilkha, A., Attachment of drugs to polyethylene
glycols. Eur Polym J, 19(12): 1177-1183, 1983.
[22] Lapatsanis, L.; Milias, G.; Froussios, K.; Kolovos, M., Synthesis
of N-2,2,2-(Trichloroethoxycarbonyl)-l-Amino acids and
N-(9-Fluorenylmethoxycarbonyl)-l-amino acids involving succinimidoxy anion as a
leaving group in amino acid protection. Synthesis,
671-673,1983.
[23] Goni,
F. M. and Alonso, A., Spectroscopic techniques in the study of membrane
solubilization, reconstitution and permeabilization by detergents. Biochim Et Biophys Acta, 1508: 51-68, 2000.
[24] Higuchi, T.; Connors, K.A. in: Reilly, C.N.
(Ed.), Advances in analytical chemistry and instrumentation. Vol.
4, Interscience,
[25] Thomas, C.G.;
[26] Sarin, V.K.; Kent, S.B.H.; Tam, J.P. and Merrifield,
R.B., Quantitative monitoring of solid phase peptide synthesis by the Ninhydrin
reaction. Anal Biochem, 117:
147-157, 1981.
[27] Sadler, K. and TAM, J. P., Peptide dendrimers:
applications and synthesis. Rev Mol
Biotech, 90: 195-229, 2002.
[28] Choi,
J. S.; Lee, E. J.; Choi, Y. H.; Jeong, Y.J. and Park, J. S., Poly(ethylene
glycol)-block-poly(L-lysine) dendrimer: novel linear polymer/dendrimer block
copolymer forming a spherical water-soluble polyionic complex with DNA. Bioconjug
Chem, 10(1): 62-65, 1999.
[29] Choi, J S.; Joo, D. K.; Kim, C. H.; Kim, K. and Park,
J. S., Synthesis of a Barbell-like Triblock Copolymer, Poly(L-lysine)
Dendrimer-block-Poly(ethylene glycol)-block-Poly(L-lysine)
Dendrimer, and Its Self-Assembly with Plasmid DNA. J Am Chem Soc, 122: 474-480, 2000.
[30] Huibers, P. D. T.; Lobanov, V. S.; Katritzky A. R.;
Shah, D. O. and Karelsonr, M., Prediction of Critical Micelle Concentration
Using a Quantitative Structure-Property Relationship Approach. 1. Nonionic
Surfactants Langmuir, 12, 1462-1470, 1996.
[31] Rackstraw B.J.; Stolnik, S.; Davis, S.S.; Bignotti, F. and Garnett, M.C., Development of multi-component DNA delivery systems based upon poly(amidoamine)-PEG-co-polymer. Biochem et Biophys Acta, 1575, 269-286, 2002.
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
CSPS Home | JPPS Home | Search | Subscribe to JPPS