J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 5(1):24-27, 2002
In Vivo Evaluation Of 99mTc-DTPA and 99mTc-Sulphur Colloid as Tracers In Colonic Drug Delivery Systems Using Gamma Scintigraphy In Volunteers
Y.S.R. Krishnaiah1, S. Satyanarayana, Y.V. Rama Prasad
Department of Pharmaceutical Sciences, College of Engineering, Andhra University, Visakhapatnam, A.P., IndiaS. Narasimha Rao
Department of Nuclear Medicine, Andhra Medical College, King George Hospital, Visakhapatnam, A.P., India
Received September 17, 2001, Revised March 11, 2002, Accepted March 12, 2002
PDF version
Abstract
Purpose: The suitability of 99m Tc-diethylenetriaminepenta- acetic acid (DTPA) and 99m Tc-sulphur colloid ( 99m Tc-SC) as tracers in gamma Scintigraphy for the evaluation of colon-specific drug delivery systems was assessed in healthy volunteers. METHOD: Sodium chloride core tablets containing either 99m Tc-DTPA or 99m Tc-SC were prepared and compression coated with two different quantities of guar gum. The compression-coated tablets were subjected to gamma scintigraphic studies for colonic drug delivery in healthy human volunteers. RESULTS: The tablets containing 99m Tc-DTPA did not release the tracer in stomach and small intestine, and on entering the colon disintegrated completely whereas the tablets containing 99m Tc-SC remained intact in stomach, small intestine and in colon as well. The study showed that DTPA is a suitable tagging agent for 99m Tc in the evaluation of guar gum based colonic drug delivery systems containing water-soluble drugs. CONCLUSION: In the present investigation guar gum was applied externally as a compression coat over the radiolabelled core. Since the core consisted of water-soluble material (sodium chloride), it is possible that the release of the tracer from the 99m Tc-DTPA containing formulations is a combined effect of enzymatic action and diffusion of the salt. The failure of disintegration of 99m Tc-SC containing formulations might be due to its interference with the disintegration or the part diffusion observed with 99m Tc-DTPA cores. The results of the study showed that DTPA is a suitable tagging agent for 99m Tc in the evaluation of colonic drug delivery systems containing water-soluble drugs by gamma scintigraphy.
Introduction
Gamma Scintigraphy is a valuable tool in the development and evaluation of pharmaceutical dosage forms. This non-invasive technique gives information in terms of the transit times of oral dosage forms across the different regions of gastrointestinal tract (1), the time and site of disintegration of dosage forms (2) and also the effect of food, disease and size of the formulation on the in vivo performance of the dosage forms. It has become a common practice to evaluate the in vivo performance of novel drug delivery systems in healthy volunteers or patients using scintigraphic technique (3,4). Gamma scintigraphy is found to be particularly useful for the evaluation of colon-specific drug delivery systems. This technique has been used for the evaluation of in vivo behaviour of colonic delivery systems based on pH dependent polymers (5), pectins (6) and guar gum (7). Technetium-99m is the most widely used radionuclide in gamma scintigraphy because of its short half-life, low energy and ready availability. Other radionuclides used are indium-111 (8), samarium-153 (7), etc. The radionuclides are usually ligated with different tagging agents such as diethylene triamine pentaacetic acid (DTPA), sulphur colloid (SC), methylene di-phosphate and pyrophosphate depending on the organ of imaging. Of all these tagging agents, DTPA and SC are routinely used for imaging in nuclear medicine. In majority of the gamma scintigraphic studies involving the evaluation of in vivo behaviour of oral dosage forms, either technetium-99m or indium-111 are ligated with DTPA. In one study 99m Tc-sulphur colloid ( 99m Tc-SC) was used as a tracer to correlate the in vivo position of the formulation in the gastrointestinal tract with absorption of drug (9). In another study 153 Sm was used as a tracer for the pharmacoscintigraphic evaluation of guar gum formulations meant for colonic delivery of dexamethasone (7). In the present investigation, the suitability of 99m Tc-DTPA and 99m Tc-SC as tracers for the in vivo evaluation of guar gum based colonic delivery systems was studied in healthy volunteers using gamma scintigraphy.
Materials and methods
Materials
Guar gum (viscosity of 1%w/v aqueous dispersion 2300 cps at 25°C), magnesium stearate and talc were obtained from Dabur India Limited, India and were of USNF quality. Molybdenum-99 (source of 99m Tc), diethylenetriaminepentaacetic acid (DTPA) and sulphur colloid (SC) formulations were obtained from Board of Radiation and Isotope Technology, Mumbai, India. Other materials used were microcrystalline cellulose (Avicel, FMC Type pH-105) and sodium chloride [E. Merck (India) Limited].
Preparation of compression coated tablets
Each core tablet (average weight 100 mg) consisted of sodium chloride (80 mg), microcrystalline cellulose (MCC, 18 mg), talc (1 mg) and magnesium stearate (1 mg). Sodium chloride was used as a bulking agent and about 74 MBq of either 99m Tc-DTPA or 99m Tc-SC (less than 0.1% of free technetium) was adsorbed on to it. The radiolabelled sodium chloride was mixed with other excipients and compressed into tablets using 6 mm round, flat and plain punches at an applied force of 3000 Kg on a single station-tableting machine. These core tablets were compression coated with 175 mg of coating material containing either 100 mg of guar gum, 72 mg of MCC, 2 mg of talc and 1 mg of magnesium stearate (coat formulation C1) or 125 mg of guar gum, 45 mg of MCC, 3 mg of talc and 2 mg of magnesium stearate (coat formulation C2). Both 99m Tc-DTPA and 99m Tc-SC core tablets were compression coated with coat formulation C1 whereas only 99m Tc-DTPA core tablets were compression coated with coat formulation C2 at an applied force of 5000 kg using 9 mm round, flat and plain punches as described above.
In vivo gamma scintigraphic studies
Twelve healthy male volunteers of 20-23 years of age and 55-70 kg weight participated in the study as volunteers and were divided into three groups (Group-I, Group-II and Group-III) of four each. They were non-alcoholics, non-smokers and were not on any drugs. The purpose of the study was fully explained and each volunteer had given his written consent. After overnight fasting, one group (Group-I) of volunteers orally ingested 99m Tc-DTPA-containing core tablets and another group (Group-II) 99m Tc-SC containing core tablets, both compression coated with coat formulation C1 along with 200 ml of water. The third group (Group-III) had ingested 99m Tc-DTPA containing core tablets compression coated with coat formulation C2. The tablets were visualised using a gamma camera (EGC 1400, Electronic Corporation of India Limited, India) with a 36 cm field of view and fitted with a low energy parallel hole collimator. An external marker was used to allow correct alignment of volunteers during successive imaging. The volunteers were served with breakfast after 2 hours of administration of the tablets by which time the tablets were in the small intestine. The volunteers were served with lunch and dinner after 4 and 10 hours of tablet administration respectively. Anterior images of 60 seconds duration were taken with volunteers in supine position immediately after tablet administration and after 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10 and 12 hours. The procedure was in accordance with the declaration of Helsinki guidelines (1964) and approved by the institutional human experimentation committee, and informed signed consent was obtained.
Results and Discussion
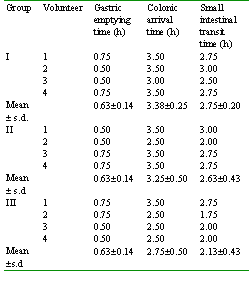
The scintigraphs showed that all the formulations were intact in the physiological environment of stomach and small intestine in all the three groups of the volunteers indicating that guar gum is capable of protecting the core from being released. The gastric emptying time, the colonic arrival time and the small intestinal transit time of the formulations in the volunteers are given in Table 1.
Table 1: The gastric emptying time, colonic arrival time and small intestinal transit time of the tablets in the volunteers
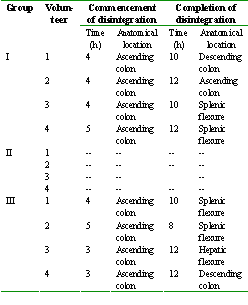
The mean gastric emptying time was found to be 0.63 ± 0.14 h in all the three groups which is almost similar to those reported for healthy volunteers under fasting conditions (1). The mean small intestinal transit time was found to vary from 2.13 ± 0.43 h to 2.75 ± 0.20 h. Thus, the mean colonic arrival time in all the volunteers was between 2.75 ± 0.50 h and 3.38 ± 0.25 h. The time and anatomical location of beginning and completion of the disintegration of the tablets in the volunteers are given in Table 2.
Table 2: The time and anatomical location of the commencement and completion of disintegration of guar gum coated tablets in the volunteers
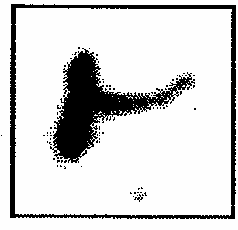
On entering the ascending colon, the 99m Tc-DTPA core tablets compression coated with coat formulation C1 began to release the tracer and by 12 hours the tablets were completely disintegrated in all the four volunteers and resulted in the distribution of the released tracer throughout the colon (Figure 1).
Figure 1: Gamma Scintigraphy showing the distribution of the released tracer, Tc-99m-DTPA, in ascending colon at hepatic flexure and transverse colon 12 h after disintegration of the coat formulation C1 in volunteer 2 (Group I).
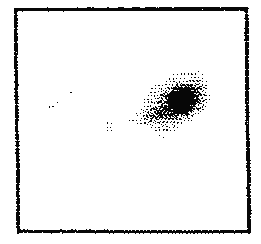
However, the formulations containing 99m Tc-SC and compression coated with C1 had not started to disintegrate even after 12 hours in the gastrointestinal tract (Figure 2).
Figure 2: Gamma Scintigraphy showing the intact Tc-99m-SC core tablet compression coated with coat formulation C1 in ascending colon at 12 h in volunteer 2 (Group II).
The degradation of guar gum by the enzymes of gut microflora was already established (10).
The biodegradability of guar gum in the form of matrix tablets (11,7) and in the form of compression coat (12) was also established. Though, there is no direct evidence for the degradation of guar gum coat by the enzymes of colonic bacteria, the absence of release of tracer either in stomach or in small intestine, and the release of the tracer soon after entering the colon (at about 3.0 h) indicate degradation of hydrated guar gum coats by colonic bacteria thereby exposing the soluble coats. Earlier studies in simulated gastric, intestinal and colonic fluids have indicated that guar gum coats would not allow the release of drug from the core, unless the coat is degraded by colonic bacterial enzymes (12). Guar gum forms a viscous gel layer around the surface of the tablets on being exposed to the GI fluids, which prevents further seeping in of fluids. Similar phenomenon of gel layer formation was observed in our earlier studies with guar gum matrix tablets as well (13). Hence, it was suggested that the degradation of hydrated gum coat is a prerequisite for the release of tracer from the water-soluble cores. However, it is possible that part of the tracer might have diffused out before the coat was completely degraded.
The studies with 99m Tc-DTPA tablets clearly indicate that the tracer material had been completely released and distributed throughout the colon. This may be due to the degradation of the gum coat by colonic bacterial enzymes and subsequent disintegration of the core tablets. In the case of 99m Tc-SC tablets coated with the same amount of guar gum, the gum coats might be degraded by the enzymes of the colonic bacteria, but the colloidal sulphur might not have allowed the disintegration of the core. In order to corroborate this hypothesis, the sodium chloride core tablets (with out guar gum coat) were prepared with pharmaceuticals DTPA and SC without 99m Tc, and were subjected to in vitro disintegration studies. The DTPA core tablets disintegrated within 3 minutes in distilled water whereas the SC core tablets took about 17 minutes to disintegrate. This might be due to the hydrophobicity of colloidal sulphur. It is possible that this hydrophobicity might have hindered the release of tracer even after degradation of the gum coat by the enzymes of colonic bacteria (14).
In order to establish the influence of DTPA, if any, on the release of tracer in the colon after coat degradation, further studies were carried out on 99m Tc-DTPA core tablets compression coated with coat formulation C2 containing more amount of guar gum (125 mg). The tablets remained intact in stomach and small intestine, and on entering the ascending colon they began to release the tracer because of degradation of the coat by enzymes of colonic bacteria. At 12 hours, the tablets were completely disintegrated in all the four volunteers and the released tracer was distributed throughout the colon (Figure 3).
Figure 3: Gamma scintigraph showing the distribution of the released tracer, Tc-99m-DTPA, throughout the colon at 12 h after disintegration of the coat formulation C2 in volunteer 2 (Group III).
The release of tracer from tablets compression coated with C2 containing more amount of guar gum indicates that increasing the coating thickness by 25 mg of guar gum did not visibly alter the release of the tracer from the core (regardless of the mechanism).
The adverse impact of hydrophobic excipients on the disintegration and dissolution of tablets is very well established (14). In the present study, the hydrophobic pharmaceutical excipient, SC, was tagged to 99m Tc, and was adsorbed on to sodium chloride. This resulted in the formation of an insoluble colloidal layer around the sodium chloride particles. On compression, SC forms a water insoluble barrier that prevents any ingression of dissolution fluids to disintegrate the tablets. As a result there is no possibility either for erosion or for disintegration of core in the colonic environment where the fluid content is very less. In the in vitro disintegration test it took longer time (17 min) for SC core tablets to disintegrate when compared to DTPA tablets (3 min) even in the presence of large volume (900 mL) of the medium. Hence it was suggested that the hydrophobic nature of 99m Tc-SC core and the limited volume of fluid available in the colon might have affected the disintegration or dissolution of core tablets containing 99m Tc-SC. It is evident from the present study that DTPA is the most suitable pharmaceutical for tagging with the radionuclide 99m Tc. Earlier workers also used DTPA as a tagging agent in the evaluation of pH dependent (5) and pectin-based (6) colonic drug delivery systems.
Conclusion
In the present investigation guar gum was applied externally as a compression coat over the radiolabelled core. Hence, the hydration and subsequent degradation of guar gum coat by colonic bacterial enzymes were unaffected by the hydrophobic core (sulphur colloid). Since the core consisted of water-soluble material (sodium chloride), it is possible that the release of the tracer from the 99m Tc-DTPA containing formulations is a combined effect of enzymatic action and diffusion of the salt. The failure of disintegration of 99m Tc-SC containing formulations might be due to its interference with the disintegration or the part diffusion observed with 99m Tc-DTPA cores. The results of the study showed that DTPA is a suitable tagging agent for 99m Tc in the evaluation of colonic drug delivery systems containing water-soluble drugs by gamma scintigraphy.
Acknowledgements
The research work was supported by Government of India, University Grants Commission, New Delhi. The authors acknowledge the financial support received from All India Council for Technical Education (AICTE), New Delhi under Thrust Area Programme in Technical Education (TAPTEC) Scheme and AICTE (MODROBS). One of the authors, Mr. Y.V. Rama Prasad is thankful to Council of Scientific and Industrial Research (CSIR), New Delhi for awarding a Senior Research Fellowship. The authors greatly acknowledge M/s. Dabur India Limited New Delhi, India for the gift sample of guar gum.
References
Khosla, R. and Davis, S.S., Gastric emptying and small and large bowel transit of non-disintegrating tablets in fasted volunteers. Int J Pharm, 52: 1-10, 1989.
Heald, D.L., Ziemiak, J.A. and Wilding, I.R., The gastrointestinal transit and systemic absorption of diltiazem HCl from a modified-release dosage form. In: Nuclear imaging in drug discovery, development and approval. Birkhauser, Boston-New York, 301-320, 1993.
Fell, J.T. and Digenis, G.A., Imaging and behaviour of solid oral dosage forms in vivo. Int J Pharm, 22: 1-15, 1984.
Wilding, I.R., Coupe, A.J. and Davis, S.S., The role of gamma scintigraphy in oral drug delivery. Adv Drug Del Rev, 7: 87-117, 1991.
Ashford, M., Fell, J.T., Attwood, D., Sharma, H. and Woodhead, P.J., An in vivo investigation into the suitability of pH dependent polymers for colon targeting. Int J Pharm, 95: 193-199, 1993.
Ashford, M., Fell, J.T., Attwood, D., Sharma, H. and Woodhead, P.J., An evaluation of pectin as a carrier for drug targeting to the colon. J Control Rel, 26: 213-220, 1993.
Kenyon, C.J., Nardi, R.V., Wong, D., Hooper, G., Wilding, I.R. and Friend, D.R., Colonic delivery of dexamethasone: a pharmacoscintigraphic evaluation. Aliment Pharmacol Ther, 11: 205-213, 1997.
Hardy, J.G., Davis, S.S., Khosla, R. and Robertson, C.S., Gastrointestinal transit of small tablets in patients with ulcerative colitis. Int J Pharm, 48: 79-82, 1998.
Wilding, I.R., Pharmacoscintigraphic evaluation of oral delivery systems: Part I. Pharm Tech Eur, 19-26, 1994.
Gibson, G.R., Macfarlane, S. and Cummings, J.H., The fermentability of polysaccharides by mixed human fecal bacteria in relation to their suitability as bulk-forming laxatives. Lett Appl Microbiol, 11: 251-254, 1990.
Krishnaiah, Y.S.R., Satyanarayana, S., Rama Prasad, Y.V. and Narasimha Rao, S., Gamma scintigraphic studies on guar gum matrix tablets for colonic drug delivery in healthy volunteers. J Control Rel, 55: 245-252, 1998.
Krishnaiah, Y.S.R., Satyanarayana, S., Rama Prasad, Y.V. and Narasimha Rao, S., Evaluation of guar gum as a compression coat for drug targeting to colon. Int J Pharm, 171, 137-146, 1998.
Rama Prasad, Y.V., Krishnaiah, Y.S.R., and Satyanarayana, S., In Vitro evaluation of guar gum as a carrier for colon-specific drug delivery. J Control Rel, 51, 281-287, 1998.
Rudnic, E.M. and Kottke, M.K., Tablet Dosage Forms, In: Modern Pharmaceutics (Banker, G.S. and Rhodes, C.T. Eds.). Marcel Dekker, Inc., New York, 333-394, 1996.
Corresponding Author: Y.S.R. Krishnaiah, Associate Professor, Department of Pharmaceutical Sciences, Andhra University, Visakhapatnam-530 003, India. krishnaysr112@rediffmail.com
JPPS Contents
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps