J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 7(1):65-69, 2004
Antinociceptive effects of Peganum harmala L. alkaloid extract on mouse formalin test.
Hamid Reza Monsef, Ali Ghobadi, Mehrdad Iranshahi
Department of Pharmacognosy, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, IranMohammad Abdollahi1
Department of Toxicology & Pharmacology, Faculty of Pharmacy, and Laboratory of Toxicology, Pharmaceutical Sciences Research Centre, Tehran University of Medical Sciences, Tehran, IranReceived 15 December 2003, Revised 13 February 2004, Accepted 13 February 2004, Published 19 February 2004
PDF Version
Abstract
PURPOSE: To evaluate the effect of Peganum harmala (Syrian rue) a wild-growing flowering plant belonging to the family Zygophylaceae and found abundantly in Iran on formalin-induced pain response in mice. METHODS. Total alkaloid extract was prepared from dry seeds of Peganum harmala. All doses of extract were dissolved in normal saline and administered intraperitoneally 30 minutes before formalin injection to the mouse paw. Nociception was recorded 0-5 (early phase, A) and 15-40 (late phase, B) minutes after formalin injection. The alkaloid extract was subjected to silica gel column chromatography using a linear gradient with a CHCl3 -MeOH system and different fractions collected. The effective fraction in formalin test were further purified and isolated by preparative thin layer chromatography (TLC) and identified on the basis of nuclear magnetic resonance (NMR) and mass spectrometry (MS) analysis. RESULTS. Alkaloid extract in doses (mg/kg) used induced significant reduction in pain response when compared to control as follow: 16 (28.63%), 20 (59.15%), 24 (80.75%), 28 (90.14%) and 30 (100%) in the early phase and 20 (24.67%), 24 (59.93%), 28 (78.52%) and 30 (100%) in late phase. Observed responses in both phases of A and B were dose-dependent with r2 of 0.93 and 0.99 respectively. ED50 for phases of A and B were 27.87 and 24.63 mg/kg respectively (p<0.001 for all groups). CONCLUSION. Harmaline, the last step of extraction is the main effective antinociceptive agent of the Peganum harmala alkaloid extract.
Introduction
Medicinal plants are believed to be an important source of new chemical substances with potential therapeutic effects. Thus, study of plant species that traditionally have been used as pain killers should still be seen as a logical search strategy, in research for new analgesic drugs [1-4]. The Peganum harmala L. (Syrian rue) is a wild-growing flowering plant belonging to the Zygophylaceae family and is found abundantly in Middle East and North Africa [5]. From ancient times, it has been claimed to be an important medicinal plant. Its seeds are known to possess hypothermic, and hallucinogenic properties [6,7]. It has been used traditionally as an emmenagogue and an abortifacient agent in the Middle East and North Africa [8]. There are several reports in the literature indicating a great variety of pharmacological activities for Peganum harmala L such as anti-bacterial, antifungal and MAO-inhibition [9]. It has also been known to interact with a2-Adrenoceptor subtypes [10] and have hallucination potency and be effective in the treatment of dermatosis [11], hypothermic [12] and cancer [13]. Among the several models of persistent nociception, formalin has been well established as a valid model to study central sensitization events at the spinal level after peripheral inflammatory states. In this test, two types of nociception were postulated; a short-lasting nociception caused by a direct effect on nociceptors followed by a long-lasting nociception due to inflammation. Since the formalin test measures the response to a long-lasting nociceptive stimulus, it has a closer resemblance to clinical pain. [14-16].
The objective of this study was firstly to determine the Peganum harmala analgesic and anti inflammatory properties and secondly to find the active antinociceptive ingredient(s) of the extract.
Materials and Methods
Plant material
The seeds of Syrian rue, collected from local market of Tehran province, in May 1999, were used in this investigation.
Extract preparation
The dry seeds of Syrian rue (100 g) were grinded and then were extracted with 80% ethanol for 24 hr in a continuous extraction (soxhlet) apparatus (Iranian Scientific and Industrial Research Center, Tehran). The extract was filtered, and ethanol was evaporated on a rotatory evaporator under vacuum at a temperature of 45°C to a small volume. Then a small amount of NH3 (25%) was added to make pH of 9. Subsequently, 100 ml of chloroform was added and slowly shaked for 10 minutes until alkaloids separated from water and enter to the chloroform phase. This was repeated for three times and then total chloroform phase was evaporated, yielding a total alkaloid extract.
Animals
Male albino N-MRI mice from Institute de Pasteur of Tehran weighing 25-32 g were used in the experiments. All experiments were performed according to "The Animal Welfare Act" (Act P.L. 99-198) considering all ethical circumstances. The animals were housed in standard stainless steel cages in a temperature controlled room (22±2°C) with a 12-12 hr light-dark cycle. The mice were randomly distributed into groups of seven as control and test subjects. All animals had access to food and water throughout the experiments. For antinociception recording, mice were allowed to acclimatize for 30 minutes before any injection.
Preparation of doses
The doses of 12, 16, 20, 24, 28 and 30 mg/kg of the alkaloid extract were used. Doses were selected on the basis of extract dry weight and being at the range of 0.1 of extract's LD 50 [17]. Sodium chloride 0.9% was used as solvent. All doses were administered intraperitoneally 30 min before formalin injection to animals.
Formalin test
Each mouse received 25 ml of formalin (0.5%) subcutaneously into the dorsal surface of the right hind paw using a microsyringe with a 26-gauge needle. Immediately after formalin injection, animals were placed individually in a glass cylinder (20 cm wide, 25 cm long) on a flat glass floor and a mirror was arranged at an angle of 45°C under the cylinder to allow clear observation of the paws of the animals. Only licking or biting of the injected paw was defined as a nociceptive response. The total time of the response was measured during periods of 0-5 min (early phase) and 15-40 min (late phase) [16].
Isolation and identification of the effective alkaloids
The alkaloid extract (5 g) was chomatographed on a silica gel column (3.5∞90 cm), using a linear gradient with a CHCl3-MeOH system, and collected in 5 fractions (9.5-0.5, 9-1, 8.5-1.5, 8-2, 7.5-2.5). All fractions was concentrated in room temperature, then examined for antinpcipetive activity using the formalin test. The effective fraction of alkaloid extract in terms of antinociception in formalin test was purified again and isolated with precoated silica gel plates used for TLC. The solvent system for the TLC was CHCl3 -MeOH-NH3 (50:50:3). 13C NMR and 1H NMR were further used to determine the effetice component of the alkaloid [18].
Statistical analysis
Comparison between groups was made by one-way analysis of variance (ANOVA) followed by Newman-Keul's test. Differences with P<0.05 between experimental groups were considered statistically significant. Microsoft Excel software was used to examine the dose dependency and ED50 of data presented in figure 1.
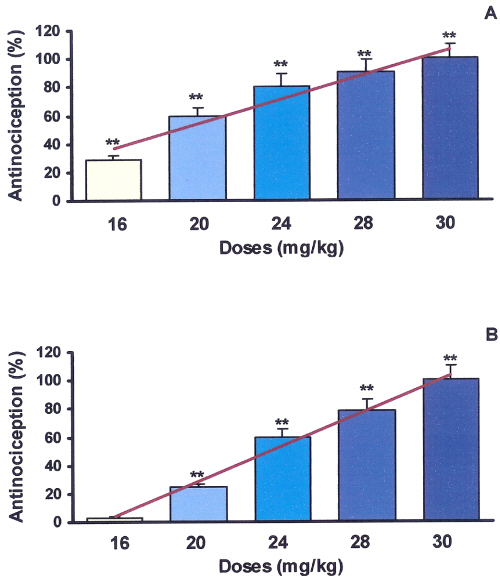
Figure 1: Antinociceptive effects of Peganum harmala extract on mouse formalin test.
All doses of extract were dissolved in normal saline and administered intraperitoneally 30 minutes before formalin injection to the mouse paw. Nociception was recorded 0-5 (early phase, A) and 15-40 (late phase, B) minutes after formalin injection. Each point is the mean±SE of seven animals that represent percent of inhibition of nociception response in respect to control. Mean±SE of control values for phase A and B were respectively (215±19.2 and 310±29.6). **Difference between control & treated groups is significant at P<0.001. Antinociception in both phases of A (r2 =0.93) & B (r2 =0.99) are dose-dependent. ED50 in phases of A & B are 27.87 & 24.63 mg/kg respectively.
Results
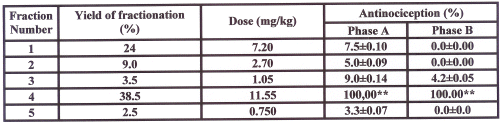
Antinociceptive induced pharmacological activity with different doses of alkaloid extract on the formalin-test in mice are shown in figure 1. Alkaloid extract in all doses (mg/kg) used induced significant reduction in pain response (P<0.01) in comparison to control as follow: 16 (28.63%), 20 (59.15%), 24 (80.75%), 28 (90.14%), and 30 (100%), in the early phase and 20 (24.67%), 24 (59.93%), 28 (78.52%) and 30 (100%), in the late phase. Observed responses in both phases of A and B were dose-dependent with r2 of 0.93 and 0.99 respectively. ED50 for phase of A and B were 27.87 and 24.63 mg/kg respectively. In comparison to saline, no significant differences were observed in animals treated by a dose of 12 mg/kg of extract (P>0.05). Table 1 shows that among the 5 fractions obtained from the alkaloid extract, the fourth fraction demonstrated greatest antinociceptive (100% inhibition of pain response in both phases of formalin test, P<0.001).
Table 1: Effect of five isolated fractions from total alkaloid extract of Peganum harmala on mouse formalin test.
The alkaloid extract (5 g) was chomatographed on a silica gel column (3.5∞90 cm), using a linear gradient with a CHCl3 -MeOH system, and collected in 5 fractions (9.5-0.5, 9-1, 8.5-1.5, 8-2, 7.5-2.5). All fractions was concentrated in room temperature. Doses were selected based on the ratio of weight percentage for each fractions considering 30 mg/kg as the most effective dose of total alkaloid extract. All doses of extract were dissolved in normal saline and administered intraperitoneally 30 minutes before formalin injection to the mouse paw. Nociception was recorded 0-5 (early phase, A) and 15-40 (late phase, B) minutes after formalin injection. Each point is the mean±SE of seven animals that represent percent of inhibition of nociception response in respect to control. Mean±SE of control values for phase A and B were respectively (215±19.2 and 310±29.6). **Difference between control and treated groups is significant at P<0.001.
TLC separation of the fraction 4 demonstrated two bands with Rf of 0.33 & 0.63. The compound with Rf of 0.33 induced 96% (P<0.001) and 87% (P<0.001) inhibition of pain response in acute and chronic phases of formalin test respectively (Table 2). The active component of fraction 4 was identified as harmalin by 13C NMR and 1H NMR as follows: mp= 229°c, 1H NMR (CDCl3 ): δ 8.05 (bs, 1H, H-1), 7.47 (d, J= 8.8 Hz, 1H, H-9), 6.85 (d, J = 2.4 Hz, 1H, H-12), 6.82 (dd, J= 8.8 Hz, J= 2.4 Hz, 1H, H-10), 3.86 (s, 3H, OCH3), 3.8 (t, J= 8.4 Hz, 2H, H-5), 2.82 (t, J= 8.4 Hz, 2H, H-6), 2.34 (s, 3H, CH3). 13C NMR (CDCl3): 158.3 (C-3), 157 (C-11), 137 (C-13), 128.5 (C-2), 128 (C-8), 120.5 (C-9), 116.5 (C-7), 110.5 (C-10), 92 (C-12), 54.5 (OCH3), 48 (C-5), 21.9 (CH3), 19.4 (C-6).
Table 2: Effects of isolated fraction 4 from total alkaloid extract of Peganum harmala on mouse formalin test.
The effective fraction number 4 (see table 1) was purified and isolated with precoated silica gel plates and then examined by preparative TLC and also identified on the basis of NMR and MS spectroscopic analysis. The solvent system were CHCl3-MeOH-NH3 (50:50:3). Two bands with Rf of 0.33 and 0.63 were isolated and based on formalin test result (table 1), the effective alkaloid was in band with Rf of 0.33. Doses were selected based on the ratio of weight percentage for each fractions considering 30 mg/kg as the most effective dose of total alkaloid extract. All doses of extract were dissolved in normal saline and administered intraperitoneally 30 minutes before formalin injection to the mouse paw. Nociception was recorded 0-5 (early phase, A) and 15-40 (late phase, B) minutes after formalin injection. Each point is the mean±SE of seven animals that represents percent of inhibition of nociception response in respect to control. Mean±SE of control values for phase A and B were respectively (215±19.2 and 310±29.6). **Difference between control and treated groups is significant at P<0.001.
Discussion
The results of the present experiments demonstrates the significant antinociceptive effects of an alkaloid extract in both phases of the formalin test at doses of 16, 20, 24 and 28 mg/kg. In the formalin test, the initial pain (early phase) is explained as a direct stimulation of nociceptors and the late phase is thought to be secondary to the inflammatory reactions [14-16]. We further isolated the effective alkaloid fraction using column chromatography and TLC. The effective fraction was purified by preparative TLC and then analyzed by NMR and GC-MS indicating that suggests that the effective alkaloid is harmalin. In the formalin test, several mediators such as histamine, kinin, serotonine and prostaglandins are released from damaged cells which take part in the inflammatory response and are able to stimulate nociceptors and induction of pain [19]. To our knowledge based on a search of the literature no studies have been conducted on the interactive effects of Syrian rue with these mediators. Among several b-carbolines (harmine, harmaline, harmalol, harman, vasicine and vasicinon) derived from Peganum harmala extract, harmaline has been found to be the major active alkaloid. Harmaline (harmidine, C13H14N2O) in moderate doses has been reported to cause tremors and clonic convulsions without increasing spinal reflex excitability [20]. Harmaline acts as a reversible MAOI [9] and in common with other beta-carbolines bind to 5-HT receptors [12]. Harmaline has also been reported to induce spasmolytic effects on guinea-pig isolated trachea with interaction to muscarinic, histaminic and b-Adreno-receptors [21]. Harmaline also inhibits MK-801 (noncompetitive NMDA channel blocker) binding to the NMDA receptor in rabbit brain [22]. In addition, b-carbolines generally have an affinity for the opioid delta and mu receptors [23]. All of these pharmacological properties of harmaline may conceivably be responsible for antinociceptive effects of Peganum harmala extract. In conclusion, this study demonstrates analgesic activity of an extract of Syrian rue which parallels the traditional use of this extract as an analgesic and antinflammatory medicine. The mechanisms) of action of harmaline remain to be elucidated by further studies.
acknowledgments
The authors are grateful for the financial support from Deputy of Research, Tehran University of Medical Sciences.
References
Bisset NG. Herbal drugs and phytopharmacuticals, 2nd ed. CRC Press, New York, 2001; pp 342-344.
Blumenthal M. 2000 Integrative Medicine Communications. Herbal medicines, Austin, 2000; pp 419-423.
Farnsworth NR. Screening plants for new medicines. In: Wilson EO, editor, Biodiversity, Port II. National Academy Press, Washington, 1989; pp 83-97.
Mattison N, Trimple AG, Lasagna I. 1988. New drug development in the United States, 1963 through 1984. Clin Pharmacol Ther 1988; 43:290-301.
Zargari A. Medicinal Plants. Vol. 1, Tehran University Press, Tehran, 1989; pp 637-639.
Lamchouri F, Settaf A, Cherrah Y. Antitumour principles from peganum harmala seeds. Therapie 1999; 54: 753-758.
Kuhn MA, Winston D. Herbal therapy and supplements, a scientific and traditional approach. Lippincott, New York, 2000; pp 347-350.
Fleming JB. Beta-Carbolines as Potetiating Agents. http://diseyes. Lycaeum. Org/dmt/alche.txt. p1-3.
Abdel-Fattah AFM, Matsumoto K, Murakami Y. Central serotonin level-dependent changes in body temperature following administration of tryptophan to pargyline- and harmaline- pretreated rats. Gen Pharmacol 1997; 28: 405-409.
Saleem A, Engstrom M, Wurster S. Intraction of folk medicinal plant with human α2-adrenoceptor subtypes. Med Plant Pakistan 2001; 57: 332-338.
Saad EL, Rifaie M. 1980. Peganum harmala: its use in certain dermatoses. Int J Dermatol 1980; 19: 221-222.
Abdel-Fattah AFM, Matsumoto K, Gammaz HAK, Watanabe H. Hypothermic effect of harmala alkaloid in rats: involvement of serotonergic mechanism. Pharmacol Biochem Behav 1995; 52: 421-426.
Adams SM. The antineoplasticeffects of prunusarmeniaca and peganum harmala; Diss Abstr Int (Sci) 1983; 44: 1052-1055.
Hunskaar S, Fasmer OB, Hole K. Formalin test in mice, a useful technique for evaluating mild analgesics. J Neurosci Methods 1985; 14:69-76.
Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effect of morphine, meperidine and brain stem stimulation in rats and cats. Pain 1977; 4:74-161.
Shibata M, Ohkubo T, Takashi H, Inoki R. Modified formalin test, characteristic biphasic pain response. Pain 1989; 38:347-52.
El-Bahri L, Chemli R. Peganum harmala L: A poisonous plant of North Africa. Vet Hum Toxicol 1991; 33: 276-277.
Claude A, Coune Luc J. ¹³C NMR des alcaloides des strychnos. Phytochemistry 1980; 19: 2009-2011.
Rang HP, Dale MM, Ritter JM. Pharmacology. Churchill Livingston, New York, 1998; pp 614-616.
Budavari S, O’Neil MJ. The merk index. 12th ed. CRC Press, New Jersey, 1996; pp 4644-4645
Shi CC, Liao JF, Chen CF. Spasmolytic effect of three harmala alkaloids on guinea-pig isolated trachea. Pharmacol Toxicol 2001; 89: 259-264.
Du W, Aloyo VJ, Harvey JA. Harmaline competitively inhibits [(3)H]MK-801 binding to the NMDA receptor in rabbit brain. Brain Res 1997; 770: 26-29.
Airaksinen MM, Saano V, Steidel E, Juvonen H, Huhtikangas A, Gynther J. Binding of beta-carbolines and tetrahydroisoquinolines by opiate receptors of the delta-type. Acta Pharmacol Toxicol (Copenh) 1984; 55: 380-385.
Corresponding Author: Mohammad Abdollahi, Associate Professor of Pharmacology and Toxicology, Department of Toxicology & Pharmacology, Faculty of Pharmacy, and Laboratory of Toxicology, Pharmaceutical Sciences Research Centre, Tehran University of Medical Sciences, Tehran, PO Box 14155-6451, Iran mohammad.abdollahi@utoronto.ca
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps