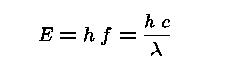An Electromagnetic Wave
is an alternating oscillations of the Electric (E) and Magnetic (B)
fields
- The EM wave travels in vacuum at a constant speed
V = c = 3 x 108m/s
- If the EM wave has wavelenth in the visibile range
400 nm < &lambda < 700 nm
our eyes can detect the EM wave. nm = nanometer = 10-9m)
- Our eyes interpret EM waves with different wavelengths
in the visible range as different colours.
-
In order of long wavelength to short wavelength:
red, orange, yellow, green, blue, indigo, violet (ROYGBIV)
|
 |