Racemic Drugs: Racemic Mixture, Racemic Compound, or Pseudoracemate? Alan G. Mitchell, Faculty of Pharmaceutical Sciences, University of British Columbia, Vancouver, BC, Canada V6T 1Z3 "When I use a word", Humpty Dumpty said in a rather scornful tone, "it means just what I choose it to mean - neither more nor less". Through the Looking-Glass by Lewis Carroll 1871. In science, words should be used to elucidate. This requires a general acceptance of scientific conventions and rules for nomenclature. Stereochemistry can be said to have begun in 1848 when Pasteur observed that crystals of sodium ammonium tartrate obtained by slow recrystallisation at room temperature were of two mirror image crystallographic forms. He was able to pick out these two forms by hand and show that solutions of equal concentration caused an equal and opposite rotation of plane polarised light. The rotation disappeared when the two crystal modifications were mixed in equal amounts before making a solution. This experiment provided the basis for the IUPAC rules for the nomenclature of organic stereoisomers, but also sowed the seeds for some of the current confusion regarding the correct description of racemic drugs in the solid state. The IUPAC rules of 1970 (1) and 1979 (2) state that "molecules that are mirror images of one another are termed enantiomers and may be said to be enantiomeric", and, "when equal amounts of enantiomeric molecules are present together, the product is termed racemic independently of whether it is crystalline, liquid or gaseous". Thus in the IUPAC rules the word "racemic" (adjective) is applied to an optically inactive product in any state of matter and "racemic mixture" would appear to be the correct terminology for a 1:1 mixture of enantiomers in any physical state. However, in the solid state, the rules specifically refer to a racemic mixture as "a mixture of equimolar amounts of enantiomeric molecules present as separate solid phases". Perhaps in recognition of the historical significance of Pasteur’s observations, sodium ammonium tartrate was the material chosen in the IUPAC rules to distinguish between a racemic mixture as defined above and a racemic compound. The IUPAC rules define a racemic compound as "any homogeneous solid composed of equimolar amounts of enantiomeric molecules". Sodium ammonium tartrate when crystallized below 27.8° C from an aqueous solution gives equal amounts of dextrorotatory and laevorotatory mirror-image crystal forms, i.e. a racemic mixture. The crystals separating out above 27.8° C constitute a homogeneous solid phase in which each symmetrical crystal contains an equal amount of the two salts, i.e. a racemic compound. If the temperature of Pasteur’s solution had been above 27.8° C, he would have been unable to recrystallise the racemic mixture and conduct the classic first resolution by hand separation into two distinct phases. The choice of sodium ammonium tartrate in the rules to distinguish between a racemic mixture and a racemic compound was probably unfortunate since it may give the impression, firstly, that any given material can be recrystallised either as a racemic mixture or as a compound simply by changing the experimental conditions and secondly, that in a racemic mixture the two mirror-image crystallographic phases can be identified by visual inspection and possibly separated by hand. This combination of properties is very rare and is probably unique to sodium ammonium tartrate. For most racemic materials the solid recrystallises either as a racemic mixture or as a racemic compound of which the latter is by far the most common. For those materials which do recrystallise as a racemic mixture, the individual crystal phases usually occur in conglomerates and not as distinct separate crystals. Jacques et al (3) prefer the term racemic conglomerate to racemic mixture since this avoids ambiguities in the definition of racemic mixture and more accurately describes the physical appearance of the solid. Although the term racemic compound is free from ambiguity the term "racemate" is commonly used to describe racemic compounds. This is in agreement with the IUPAC 1970 definition (1): "Any homogeneous solid containing equimolar amounts of enantiomeric molecules is termed a racemate". In the 1979 revision, however, the phrase "any homogeneous solid" was changed to "any homogeneous phase". This had the effect of expanding the definition of racemate to include an equimolar mixture of enantiomers in any physical state. Thus in the gaseous and in the liquid (melt or solution) states the terms "racemic mixture" and "racemate" can be used interchangeably. In the gaseous and liquid states, both types of racemic modification will behave as ideal or nearly ideal mixtures with physical properties (e.g. boiling point, refractive index, density) which are indistinguishable from those of the pure enantiomers. However both the 1970 and 1979 rules agree that in the solid state, the term "racemic mixture" refers to a heterogeneous mixture of two separate solid phases (e.g. sodium ammonium tartrate recrystallised below 27.8° C) while the term "racemate" refers to a homogeneous single phase (e.g. sodium ammonium tartrate recrystallised above 27.8° C). A survey of recent literature reveals a tendency to use "racemic mixture" interchangeably with "racemate". For solids this is incorrect. Figure 1 summarizes nomenclature for the solid state which is consistent both with the IUPAC rules and most texts.
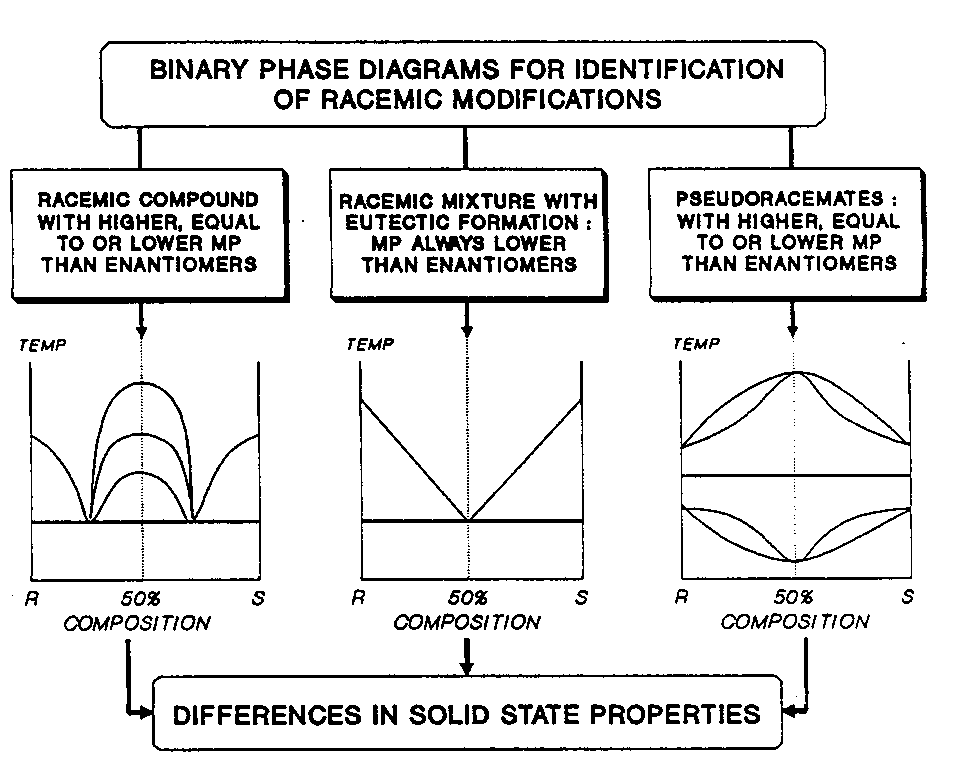
Until advances in technology made possible the large-scale synthesis and analysis of single enantiomers, the question of using an enantiomeric drug rather than its racemic modification was largely academic. Synthesis of a chiral drug from achiral precursors always leads to a racemic modification (4). Now that the preparation of pure enantiomers is a commercial reality the decision whether to market a pure enantiomer or the racemic drug depends on many factors of which clinical efficacy and safety are of overriding importance. Pure enantiomers often show different pharmacodynamic and pharmacokinetic behavior. Very often one isomer possesses the desired pharmacological properties whilst the other isomer (or isomeric pair where there is more than one asymmetric carbon atom) may be inert, completely different in its pharmacological activity or even toxic (5, 6). Another problem which is becoming acute with the increasing number of chiral drugs is that the approved drug names and accompanying monographs very often fail to give any indication of enantiomeric composition. As pointed out by Lee and Williams (7), Martindale’s Extra Pharmacopoeia (8) lists 39 beta-adrenergic blockers, only 14 of which are identified as being either the racemic drug or the pure enantiomer. Of the drugs not identified, at least two have two chiral centers (i.e. four isomers) and one has three chiral centers (i.e. eight isomers). Gal (9) suggested there is an urgent need for the adoption of an explicit system for naming stereoisomeric drugs such as that proposed by Simonyl (10). Where clinical efficacy and safety are not compromised, a racemic drug may still be preferred to an enantiomer on the basis of considerations such as lower cost and more desirable physical properties. If racemic drugs were always administered in gaseous or solution form, the discussion summarized in Figure 1 would be of semantic interest only. In practice drugs are more often administered as solids whether in tablet, suspension or inhalation dosage forms. The solid state properties of racemic mixtures and racemic compounds are likely to be very different from their corresponding enantiomers. Such differences in physical properties must be considered both for new chemical entities as well as for established drugs where the racemic modification is in current use, but where stereoselective synthesis provides an opportunity to use a pure enantiomer. In addition to the racemic mixtures and racemic compounds described above, one other racemic modification is encountered in the solid state, namely the pseudoracemate. These three racemic modifications originate from differences in the packing forces in the crystal lattice. In a racemic mixture, each enantiomer has a greater affinity for molecules of its own kind than for those of the other enantiomer and the two enantiomers crystallize in separate phases. In a racemic compound, each enantiomer has a greater affinity for molecules of the opposite type than for its own kind. The unit cell of the crystal thus contains an equal number of molecules of each enantiomer and the product is a true addition compound. In cases where there is little difference in the affinity between enantiomers of like or opposite configuration, the two enantiomers exist in an unordered manner in the crystal., i.e. the racemic modification shows nearly ideal mixing and forms a racemic solid solution (pseudoracemate or mixed crystal). Characterization of Racemic Modifications Racemic mixtures, racemic compounds and pseudoracemates can be differentiated from one another on the basis of their melting point behavior. Provided either both enantiomers or the racemic drug and at least one pure enantiomer are available, a two component phase diagram is readily constructed using differential scanning calorimetry (DSC) (11-17). A racemic mixture, Figure 2, corresponds to a simple eutectic in which the melting point always occurs at the 50:50 enantiomeric composition. Like any other physical mixture the melting point is always lower than that of the pure components. The solubility of a racemic mixture is always higher than that of the pure enantiomers, and where the two phases behave independently of one another, (i.e. ideal behavior) the solubility will be exactly double. Other solid state properties such as density, powder x-ray diffraction pattern and solid state infra-red spectra are identical with those of the pure enantiomers.
The melting point diagram of a racemic modification with racemic compound formation shows two eutectic points, Figure 2. The melting point of the racemic compound is always greater than the eutectic but may be higher or lower than the melting point of the pure enantiomers. Unlike a racemic mixture the solubility of a racemic compound may be greater or less than that of the individual enantiomers. It has been shown that the racemic compounds ICRF 159 and dexclamol hydrochloride are about five times less soluble in water than their respective enantiomers (18, 19). Maxima in the solubility-composition profiles will occur at the compositions corresponding to the eutectic of the racemic compound with each of the pure enantiomers. For example, the solubility at the eutectic points of racemic ibuprofen with either the R or S enantiomers is about twice that of the racemic compound (14). The powder X-ray diffraction patterns and solid state infra-red spectra of a racemic compound are quite distinct from those of the parent enantiomers. Melting point diagrams of pseudoracemates, i.e. enantiomers forming solid solutions at all concentrations, fall into three types, Figure 2. In Type I, mixtures of (R) and (S) enantiomers in all compositions melt at the same temperature as the pure enantiomers. In Type II, the melting point diagram shows a maximum at the 1:1 composition while Type III shows a minimum melting point at this composition. For all three types, the solid solution at the 1:1 composition can be correctly described as a racemate since it is a homogeneous one phase solid containing equimolar amounts of the enantiomeric molecules. However unlike a racemic compound, the powder X-ray diffraction patterns and solid state infra-red spectra will be identical with that of the pure enantiomers. Although pharmaceutical pseudoracemates appear to be uncommon, the enantiomers of pindolol free base form a series of solid solutions with a maximum at the 1:1 composition, (i.e. racemic pindolol is a Type II pseudoracemate) (15). Much more likely than pseudoracemates are racemic mixtures and racemates with partial solid solution formation. With both racemic mixtures and racemates, solid solution formation can occur in the regions of the pure enantiomer, while for racemates there is the additional possibility of solid solution formation in the region of the racemic compound. Thus the phase diagram of bevantolol free base shows solid solution formation in the region of the racemic compound (i.e. the 1:1 enantiomeric composition) (15). Complete binary diagrams are not usually necessary to distinguish between the various racemic modifications. Reference to Figure 2 shows that admixture of either enantiomer with a racemic drug will increase the melting point of a racemic mixture, decrease the melting point of a racemic compound and have little effect on the melting point of a Type I pseudoracemate. As discussed above, the solubility of the enantiomers is almost always different from that of the racemic modification. In addition to recognizing this difference in solubility, a decision to select a single enantiomer as a drug should consider the effect of incomplete resolution on its solubility. Ternary phase diagrams are the most convenient means of expressing the solubility composition relationship between two enantiomers and a solvent. such diagrams will show the effect of traces of the second enantiomer on the solubility of the selected enantiomer. Alternatively, phase solubility diagrams were used by Liu and Hurwitz (19) to show the reduction in aqueous solubility of dexclamol hydrochloride (the biologically active isomer) in the presence of the inactive enantiomer and the racemic compound. Polymorphism and Hydrate Formation in Racemic Modifications Polymorphism (the ability of a given compound to crystallize in more than one form) further complicates an understanding of the solid state properties of racemic drugs . Jacques and others (3) have given a detailed analysis of the various types of polymorphism which are theoretically possible with enantiomers but relatively few examples have been found. Dwivedi and Mitchell (unpublished data), using DSC and hot-stage microscopy, found that racemic tocainamide hydrochloride melts at 210° C and immediately recrystallises to give a new phase melting at 246° C. Powder X-ray diffraction confirmed that the diffraction pattern of the form melting at 246° C is distinct from that of the lower melting point form. The R and S enantiomers melt at 270° C and have a powder X-ray diffraction pattern distinct from both the low and high melting point forms. It was concluded that racemic tocainamide hydrochloride is a racemic compound with at least two polymorphic forms. Racemic modifications are frequently hydrated and transformations from one racemic modification to another with increases in temperature can be associated with a reduction in the degree of hydration. For example, below 27.8° C, sodium ammonium tartrate (‘Pasteur’s salt’) recrystallises as a racemic mixture with four waters of crystallization, but the racemic compound which recrystallises above 27.8° C, contains only one molecule of water of crystallization. Conversely, histidine hydrochloride recrystallises as a dihydrate racemic compound below about 45° C but, at temperatures of 45° C and above, recrystallises as an anhydrous racemic mixture. It is apparent from these examples of polymorphism and solvation that, depending on the temperature of crystallization, it is possible for a racemic drug to crystallize in more than one racemic modification and vary in its extent of hydration. Only molecules with chiral centers have been discussed in this report. Other elements of chirality (i.e. axis, plane and helix) in the molecule have been excluded. Furthermore, only molecules with one asymmetric carbon atom have been considered. However, drugs may have two or more chiral centers. Thus, labetalol has two asymmetric centers and therefore has four stereoisomers, i.e. two racemic pairs RR and SS; SR and RS. The RR and SS isomers are enantiomers of each other as are the SR and RS isomers. other combinations of pairs of isomers are not mirror images (i.e. they are diastereoisomers). A melting point phase diagram has not been reported but, on the basis that racemic compound formation is the most common racemic modification, it may be speculated that labetalol is a racemic mixture of two racemic compounds i.e. a simple eutectic mixture containing two solid phases namely the racemic pair, RR and SS, and the racemic pair SR and RS. However, the possibility of other racemic modifications cannot be excluded. Labetalol is now clinically available as the RR isomer and it is apparent that the solid state properties of the RR isomer will be different from those of the racemic drug. Since the number of stereoisomers increases geometrically with the number of chiral centers, the complexities possible in the solid state become readily apparent particularly when the possibilities of polymorphism and hydrate formation are included. This brief introduction has stressed the importance of using correct terminology and the need for careful solid-state characterization of racemic drugs. Solid-state characterization is particularly important where the type of racemic modification obtained depends on the crystallization conditions or where a decision is made to replace a racemic drug in a solid dosage form by a pure enantiomer. Acknowledgments The author wishes to thank Dr. S.K. Dwivedi for helpful discussions and for the preparation of the figures. References
Published by the Canadian Society for Pharmaceutical Sciences. Copyright © 1997 by the Canadian Society for Pharmaceutical Sciences. |