| Sustained
Release and Enteric Coated NSAIDs: Are They Really GI Safe? Manuscript received February 8th, 1999; Revised March 18th; Accepted March 19th, 1999. Neal M. Davies1 Abstract Non-steroidal anti-inflammatory drugs (NSAIDs) have commonly been associated with upper gastrointestinal (GI) tract side effects including a high incidence of gastric and duodenal ulceration. Recent reports implicate NSAID use and mucosal injury in the small and large intestine. A trend in NSAID development has been an attempt to improve therapeutic efficacy and reduce the severity of upper GI side effects through modified release dosage forms of NSAIDs such as enteric-coating (EC) or sustained release (SR) formulations. It is possible that modified release formulations may increase the exposure of active drug to the mucosa distally to the duodenal bulb, and thereby increase toxicity to distal GI regions where the effects are difficult to monitor. A systematic literature review through Medline, Embase, and Index Medicus was made to identify toxicological effects induced by modified release formulations of NSAIDs in the small and large intestine. An analysis of the identified toxicological effects of these formulations was made. SR and EC NSAID use has been associated with both small and large intestinal bleeding, anaemia, strictures, ulcerations, perforations, and death. Modified release NSAIDs may cause significant morbidity in some patients. NSAID modified release delivery systems are not guarantors of GI safety. Since SR and EC NSAIDs are widely prescribed and some EC products are available without a prescription, heightened awareness of these toxicological manifestations in more distal sites of the GI tact may reduce morbidity. Introduction NSAIDs are amongst the most commonly prescribed medications in the world attesting to their efficacy as anti-inflammatory, anti-thrombotic, anti-pyretic, and analgesic agents. During the past 30 years, there has been a substantial increase in the number of clinically available NSAIDs. However, numerous spontaneously reported adverse drug reactions, case-control, cohort, and post-marketing surveillance studies have revealed that NSAIDs are associated with extensive side effects, the most prevalent being GI disturbances (1). As awareness of the GI side effects associated with NSAIDs increases, safety becomes a primary requisite in treatment. Numerous articles examining the gastric and duodenal damage caused by NSAIDs have been published, however, only recently have the more distal intestinal disturbances induced by these drugs received close attention (2-3). There is often a poor correlation between patient reported symptoms of upper GI distress and endoscopically proven gastropathy (4-6). This may suggest afflictions of more distal parts of the intestine. The concept of selective and site-specific damage to the upper GI tract following NSAIDs has been questioned, particularly by the works of Bjarnason, who has demonstrated that patients on chronic NSAID therapy can develop small and large intestinal inflammation which may lead to anaemia, hypoalbuminemia, ucleration,‘diaphragm’ like strictures, perforation, and hemorrhage (7). A trend in NSAID development has been to improve therapeutic efficacy and reduce the severity of upper GI side effects through altering dosage forms of NSAIDs by modifying release of the formulations to optimize drug delivery. These formulations are designed to increase patient compliance through a prolonged effect and reduce adverse effects through lowered peak plasma concentrations. Indeed, enteric coated (EC) and sustained release (SR) formulations of several NSAIDs have resulted in a reduction in endoscopic findings in the stomach and duodenal bulb as these formulations are intended to release NSAIDs in the intestine (8-10). However, in other studies of NSAID gastropathy this endocsopic data has not been as convincing (11-12). For example, a comparison of a conventional formulation of ketoprofen versus the microencapsulated preparation did not show a significant reduction in gastric damage (12). The more distal intestinal toxicological manifestations of SR and EC NSAID formulations have been largely ignored; although the likelihood of its increased occurrence with more frequent use of NSAID medication has been previously predicted (13). It is possible, therefore, that the slow releasing of an NSAID at high concentrations at a more distal site in the GI tract may shift the site of damage distally. A physical association between a modified release NSAID and a distal side effect has been reported with the osmotically activated slow-release formulation of indomethacin no longer commercially available. These capsules were located at the site of perforating colonic and ileal ulcers and free in the peritoneal cavity (14). In addition, a more recent report has also suggested the location of possible diclofenac pill fragments at the site of distal intestinal ulceration and strictures (15). Finally, the introduction of a controlled release formulation of ketoprofen in the Netherlands led to an increase in the number of case reports of GI bleeding and/or perforations (16). An inherent problem in examining adverse effects of modified release NSAIDs in the distal GI tract is that diagnosis is difficult, usually requires invasive techniques, and is usually only demonstrated after one of the complications (obstruction, perforation, or hemorrhage) becomes clinically apparent. An increasing number of case reports have, however, uniformly described deleterious effects of NSAIDs in both the small and large intestine with findings ranging from asymptomatic mucosal inflammation, iron deficiency anaemia, bloody diarrhoea, strictures, perforations, and major haemorrhages (14-15,17-64). Cost containment of pharmaceuticals is important to health-care systems and the therapeutic rationale behind EC and SR formulations in terms of GI side-effects may not be clear cut. A systematic search was made through Medline, Embase, and Index Medicus to identify the possibility of distal toxicity attributable to SR and EC NSAID formulations. There is a paucity of studies directly assessing modified release preparations of NSAIDs in terms of adverse effects throughout the entire GI tract. Expectably, the therapeutic relevance of SR formulations of NSAIDs, is an important clinical and economical issue that has not been adequately dealt with. Little emphasis has been placed on formulation when reporting adverse drug reactions to medications. This review highlights and identifies an important clinical concern when NSAIDs are prescribed. Case Reports of EC and SR NSAID-induced Intestinal Toxicity 24 patients with de novo NSAID-induced damage of the small intestine attributable to EC and SR NSAID use have been described in the literature from 1970 to present (Table 1). These patients, whose ages ranged from 21 to 85 years (mean, 64.7), were taking many types of NSAIDs for inflammatory conditions such as rheumatoid arthritis and osteoarthritis. The general clinical manifestations were GI bleeding, abdominal pain, iron deficiency, anaemia, obstruction, ulceration, stricture, and diarrhoea. Of the 23 in whom gender was reported, 15 were female, and 8 were male. Of the 19 patients in whom duration of NSAID use was reported, 12 had been taking NSAIDs for less than 1 year and 6 patients were described with NSAID-induced enteropathy after use of medication for less than 3 weeks. Of the patients whom drug and formulation were reported, 13 had been taking SR indomethacin, and 8 diclofenac preparations. The majority of literature case reports describe a temporal relationship between NSAID use and occurrence of small intestinal side effects. In 3 cases NSAID-use contributed to and resulted in mortality.
NR, not reported The cessation of NSAID therapy generally results in rapid improvement, in most cases without additional therapy (21, 32, 35). The use of sulphasalazine, metronidazole or a prostaglandin analogue may help resolve the toxicity. Case Reports of EC and SR NA\SAID-induced Large Intestine Toxicity 86 patients with large intestinal side effects attributable to NSAID use have been described in the literature from 1978 to present (Table 2). The main clinical manifestations were GI bleeding, iron deficiency anaemia, ulceration and stricture formation. Of the 50 in whom gender was reported, 36 were female, and 14 were male. Of the patients in whom duration of NSAID use was reported, 15 had been taking NSAIDs for less than 1 year and 6 patients were described with NSAID-induced colonopathy after use of medication for less than 2 weeks. In several cases rechallenge with NSAID gives a more convincing relationship (37-42). However, ethics of rechallenge due to severity of the presumed cause prohibited this in many patients.
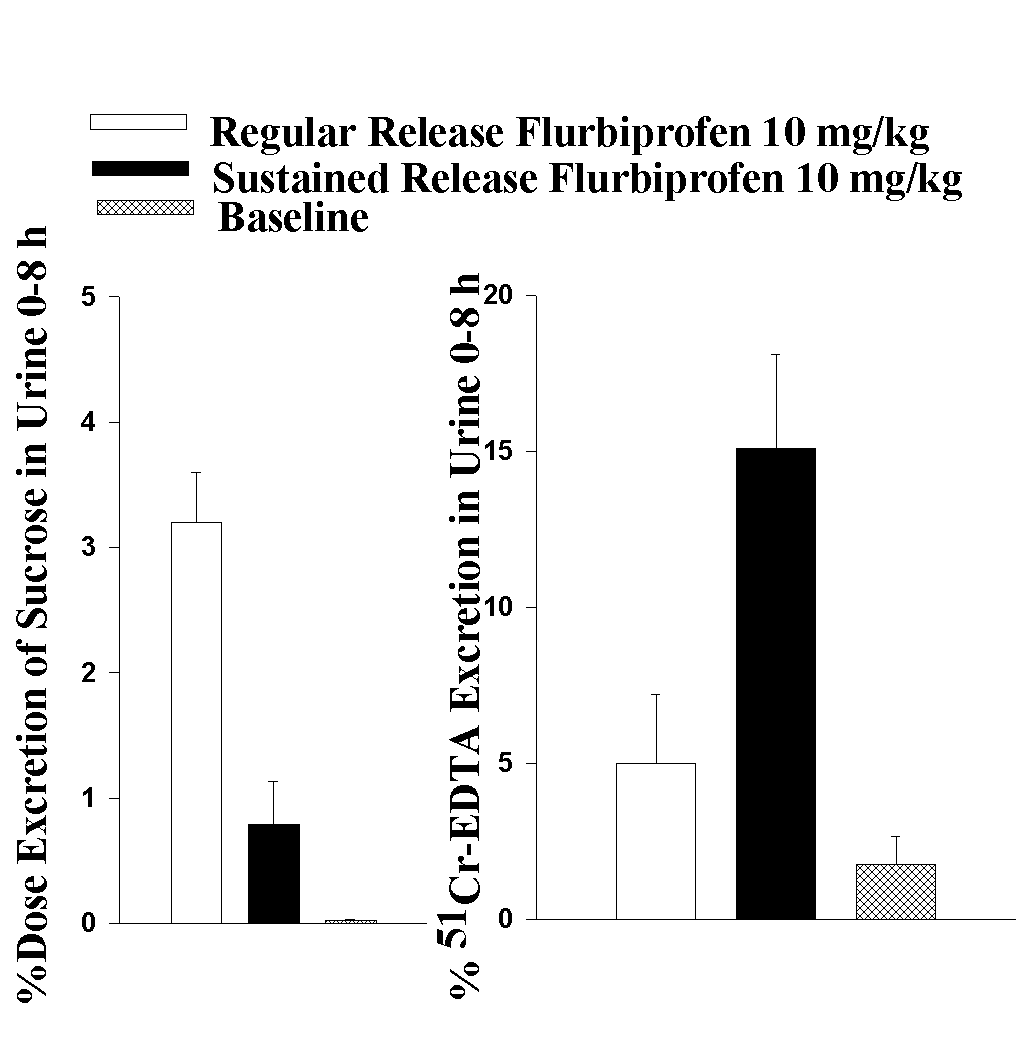
NR, not reported The use of sulphasalazine, metronidazole 5-acetylsalicylic acid, or prednisone is often sufficient to induce remission (37-55). Although, immediate surgery with resection of affected bowel segments may be required depending on the severity of toxicity. Discussion Toxicological effects of modified release NSAID formulations on the distal intestine have been demonstrated in the literature. However, the inability to ascertain from many other case reports the use of other drugs obtained without prescription and to definitively identify weather the NSAID was a modified release formulation is often evident. In addition, more than 200 brands of analgesic medications are available over the counter, patients may not know or recall that a non-prescription medication used was EC acetylsalicylic acid; they may consider the information irrelevant or may not accurately recall all medications used. It is imperative that literature reports of adverse drug reactions report the formulation as well as the drug in order to ascertain whether there are formulation-dependent side effects. The magnitude of this problem can not be ascertained from a review of sporadic case reports. Indeed, there may be problems in extrapolating epidemiological trends from case reports. Numbers of cases of intestinal side effects of SR and EC NSAIDs almost certainly under represent the extent of the actual problem. A preferred way in which to design a study to quantify such a problem is to use large hospital or insurer provider databases of complications associated with these NSAID formulations. Such methodology could provide a more reliable estimate of the true impact (numbers and variety) of this problem since the method would not rely on clinicians taking the initiative to offer a formal published report, and unfortunately this information does not yet exist. Although regular release NSAIDs are designed for gastric or proximal intestinal absorption, they too have some small intestinal and colonic side effects that are reported in the literature. After over thirty years of wide spread clinical use the ability to accurately rank individual NSAIDs adverse effects to the GI tract are still not possible. Many post-market surveillance and epidemiological studies of NSAID toxicity also differ from one another. The discrepancy between the epidemiological safety scores of NSAIDs may be attributed to three important but often overlooked reasons: A) Most studies generally only reflect the toxicity in the upper GI (gastroduodenum), B) Channeling the use of EC and SR products to high risk patients. C) Rarely the type of the formulation used (e.g., conventional, SR or EC) are considered in the interpretation of data. In addition to adverse effects on the gastrointestinal mucosa, side-effects of NSAID use manifests as iron deficiency anaemia, bloody diarrhoea, inflammation, and in severe cases hemorrhage (Tables 1-2). Blood loss from the small and large intestine may result in significant morbidity and contribute to the anaemia of patients with arthritis taking NSAIDs. While the clinical presentation of NSAID-induced gastropathy may be more dramatic than small and large intestinal damage, the pathogenesis of short or long-term importance of this adverse effect of NSAIDs on the distal GI is unknown. Several case histories stress the importance of distal intestinal toxicity attributable to modified release NSAIDs (Table 1-2). These lesions are often difficult to diagnose on enteroscopy, colonoscopy and barium scans as radiological findings can be subtle and easily missed. The literature demonstrates that distal GI damage induced by modified release NSAIDs is rare but this may also reflect the lack of clinical recognition and reporting of such adverse effects. The prescribing of SR NSAID formulations to patients with pre-existing bowel disease (i.e., diverticulitis, inflammatory bowel disease) and physiological conditions that affect drug-release or lead to delayed transit (stenosis, anticholinergic drugs, diverticuli) may represent relative or absolute contraindications to such formulations and require special clinical considerations. Increased awareness of this potential avoidable condition should allow for earlier clinical recognition and appropriate patient management. In cases of extensive inflammation, temporary medication with metronidazole or sulfasalazine may be advisable. Another problem in evaluating adverse effects brought about by the use of novel delivery systems is that as these formulations claim to cause fewer adverse effects than the conventional formulation they could be prescribed for patients who are most prone to develop such adverse effects. This phenomena has been termed "channeling" and may complicate any interpretation of the epidemiology of adverse effects (16). Several case histories stress the importance of small and large intestinal toxicity induced by NSAIDs and it is clear that NSAID ingestion should be considered as a factor in the differential diagnosis of undiagnosed blood loss, inflammation, strictures and relapse of inflammatory bowel disease (Table 1-2). However, other causes for the formation of strictures in the distal GI cannot be ruled out in some of the aforementioned cases. Nevertheless, several case reports have uniformly suggested the possible association between EC and SR NSAID use and small and large intestinal damage with remission demonstrated upon discontinuation of NSAID therapy. These studies have reported that amongst NSAID users with GI complications, there was no correlation of GI blood loss and endoscopic findings, whereas intestinal inflammation and blood loss correlated well with one another but not with the macroscopic appearance of the gastroduodenal mucosa (65-66). This supports the assertion that the small intestine is a main site of NSAID-induced chronic blood loss. In the assumption that mucosal lesions are partly due to a local toxic effect of NSAID, modified release formulations have been developed to reduce upper GI toxicity by allowing the drug to bypass the gastroduodenal mucosa before dissolving, delaying exposure of the active drug until the drug has reached the small bowel. The identified case reports uniformly suggest that by modifying dosage forms of NSAIDs to reduce ulcerogenicity in the upper gastroduodenum has not alleviated adverse-effects in the more distal parts of the GI tract. These regions of the GI tract are less available for controlled evaluation, and such an effect may provide more serious sequelae of complications. Modified release NSAIDs are expected to be released mainly in the intestine and these changes in the release rate and its effect of prolonged intestinal presence may affect the overall GI integrity. The effect of modified release formulations on NSAID-induced distal intestinal damage has not been adequately addressed in clinical studies. One of the few clinical studies to directly compare a conventional release and a SR product demonstrated 44% of the SR group versus 35% in the conventional group experienced adverse events to flurbiprofen without a significant differences in upper or lower GI tract (67). In a Latin-square crossover study, naproxen 500 mg twice daily for 7 days as plain tablets, EC tablets, or EC granules in capsules was administered to healthy male volunteers. All drugs induced a significant increase in intestinal permeability but no statistical differences between these formulations were detected. A considerable inter-individual variation was seen, however, it did appear that the median urinary excretion values for the enterogranulate capsules and the EC tablets were higher than after plain tablets (68). These data are consistent with a more recent study that demonstrated increased intestinal permeability with a SR formulation of diclofenac but not with conventional release diclofenac (69). The results of the intestinal permeability of a SR product appear to be more variable than the conventional release product, which is postulated to be due to the sustained release of drug and its longer presence within the intestinal tract, which may induce more local distal intestinal damage in addition to the systemic intestinal effects (32). A study comparing GI blood loss of plain and EC acetylsalicylic acid after acute and chronic ingestion demonstrated that plain acetylsalicylic acid significantly increased GI blood loss as compared with EC acetylsalicylic acid. However, EC acetylsalicylic acid also significantly increased GI blood loss as compared with control (70). A recent study has suggested that there may be a subset of elderly patients who are unable to absorb EC acetyl salicylic acid which may lead to high local concentrations in the ileum and colon and to increased distal GI damage in this age group (71). Other studies have reported a high incidence of intake of NSAIDs especially acetylsalicylic acid with non-ulcer GI bleeding including bleeding in the colon. The odds ratio of lower GI versus upper GI bleeding in NSAID users was 1.71, indicating no predilection for site. However, a higher prevalence of lower GI bleeding than upper GI bleeding for acetylsalicylic acid intake was evident although no information of the type of formulations administered was provided and no predilection for site was apparent (72). Salsalate (salicylsalicylic acid, DisalcidÒ ) which was developed as a newer non-acetylated form of salicylates designed to be insoluble in the acidic gastric fluids and to dissolve and be absorbed in the small bowel. Several studies have shown that salasalate may produces less gastric mucosal toxicity than EC acetylsalicylic acid and EC naproxen (73, 74). However, there was no difference in the duodenal mucosal damage induced by these drugs and a higher incidence in adverse experiences with salsalalate (74). Furthermore, the possibility of the more distal intestinal side effect were ignored in these studies although it had been previously reported that salsalate can induce multiple ulcerations of the small bowel (75). The establishment of an animal model for toxicological studies of the entire GI tract has enabled experimental quantitative assessment in both the upper and lower GI and determination of the relative safety profile of conventional and SR formulations of NSAIDs. It appears that SR NSAIDs (e.g. tiaprofenic acid, and flurbiprofen) although safer on the upper GI tract may cause greater intestinal toxicity on the distal intestine than a conventional release formulation (76, 77) (Figure 1). The pathogenesis of NSAID-induced mucosal damage in the distal GI tract remains poorly understood. Some NSAIDs undergo enterohepatic recirculation and it is generally believed that biliary excretion is important in the pathogenesis in NSAID enteropathy (78). However, other investigators have argued through pharmacokinetic/pharmacodynamic analysis that there is a major systemic component involved in NSAID enteropathy (80). Nevertheless, as a regular release formulation and a sustained release formulation undergo equivalent amounts of enterohepatic re-circulation, basic and clinical studies which suggest greater toxicity for SR formulations are consistent with the formulations altering the toxicity profile through a local effect.
Several other basic and clinical findings also question the validity of the importance of enterohepatic recirculation such as the clinical observation that acetylsalicylic acid which does not undergo any enterohepatic recirculation can cause both intestinal and colonic damage (Table1-2). Considering the fact that NSAIDs are the most frequently prescribed class of therapeutic agents, their side effects present a relatively major problem for the public. To overcome this problem, various approaches have been taken, including development of EC and SR NSAIDs. After over 20 years of clinical use it is evident that novel drug delivery systems have not solved the problem of NSAID-induced GI side effects and may have extended local toxicity distally. There is no convincing clinical evidence in the literature suggesting safer profiles for NSAID modified release formulations. Indeed for lack of this information, clinicians and scientists are debating the therapeutic value of modified release formulations and third party insurance agencies are considering delisting modified release formulations of NSAIDs from their health care benefits lists due to their higher costs (79). Conclusions Whereas, NSAIDs side effects are not limited to the upper GI tract, it is necessary to monitor the entire GI tract from mouth to anus when evaluating NSAID-induced GI toxicity. There are many confounders in determining the actual incidence of NSAID modified release adverse effects in the distal intestine, however, this review emphasizes that there is substantial basic and clinical scientific evidence that these delivery systems are not guarantors of GI safety. Adverse drug reaction data, therefore, should consider the type of formulation administered as well as the drug in NSAID toxicological evaluations. The main goal still remains to solve the problem of NSAID-induced GI toxicity with its resultant morbidity and mortality and to obtain optimal therapeutic effect with least possible side effects. There are emerging alternatives to traditional NSAIDs (i.e. cyclooxygenase-2 inhibitors) that hold such promise. However, in view of all the difficulties that have bedevilled this area of clinical investigation over the years, this must be tempered with an additional caution that any new therapeutic agent, no matter what the rationale behind its introduction, must be examined throughout the GI tract and assumed to induce GI toxicity until clinically proven otherwise. References
Corresponding author: Neal M. Davies, University of Sydney, College of Health Sciences, School of Pharmacy, Department of Pharmacy, Sydney, New South Wales, Australia 2006. Email: ndavies@pharm.usyd.edu.au Key Words: NSAID, sustained-release, enterocoated, toxicity Published by the Canadian Society for Pharmaceutical Sciences. Copyright © 1999 by the Canadian Society for Pharmaceutical Sciences. |