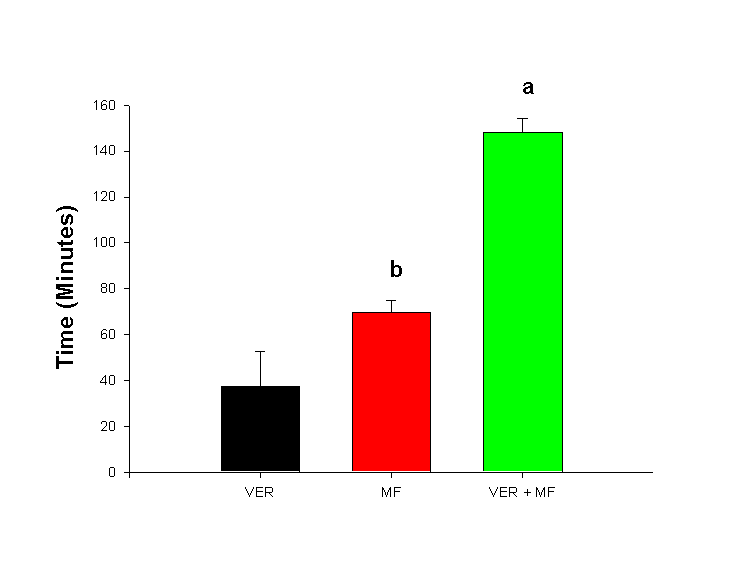
Methoxyflurane Anesthesia Augments The Chronotropic And Dromotropic Effects of Verapamil. Manuscript received March 15, 1999; Revised April 30, 1999, Accepted April 30, 1999. Patrick R. Mayo, Fakhreddin Jamali1 Abstract Introduction: Inhalation anesthetics have been shown to have electrical suppressant effects on excitable membranes such as the cardiac conduction system. Therefore, the anesthetized patient or laboratory animal may respond differently to cardiac drugs when compared with their conscious counterparts. The purpose of this study was to assess the effects of anesthesia with methoxyflurane (MF) on the dromotropic and chronotropic effects of verapamil (VER) in the rat. Methods: A lead I ECG was measured using subcutaneous electrodes placed both axilli and over the xyphoid process in male Sprague-Dawley rats. Dromotropic effect was measured using the PR-interval which indicated the electrical spread across the atria to the AV-node and chronotropic effects were determined using RR-interval. A total of six animals were randomized to receive 10 mg/kg s.c. of verapamil in the presence or absence of general anesthesia containing methoxyflurane. In addition, PR-interval and RR-intervals were determined in the presence of only methoxyflurane and at rest without any drug exposure. The time for the ECG to normalize after exposure to methoxyflurane and/or verapamil was also determined. Results: Exposure to verapamil alone resulted in a 5% prolongation in PR-interval and 6% prolongation in RR-interval. Methoxyflurane alone had a larger effect than verapamil demonstrating a 14.5% prolongation in PR-interval and a 12.3% in RR-interval which was statistically significant. The combination of MF + VER resulted in a synergistic prolongation in PR-interval to 28.7% while the effect on RR-interval was additive with an increase to 17.6%. The time for the ECG to normalize after exposure to VER, MF and VER + MF was 37.5 ± 15.1 min, 69.8 ± 5.3 min, and 148.5 ± 6.6 min respectively. Conclusion: General anesthesia with MF enhances the dromotropic and chronotropic effect of VER. This should be considered when MF-anesthesia is used in experimental procedure. Introduction Inhalation anesthetics have been shown to have anti-arrhythmic or arrhythmogenic potential in both animals and man (1, 2). For example, halothane and enflurane prolong refractory periods that may suppress re-entrant ventricular arrhythmia in the post-myocardial infarction (MI) patient (3), whereas methoxyflurane (MF) has been shown to increase the automaticity of the atrial conducting system (4). It has also been noted that a majority of patients experience some form of arrhythmia during surgical anesthesia due to multiple causes ranging from hypoventilation to the type of anesthesia (5). In addition, the anesthetized patient is significantly different from the conscious patient. For example, the addition of antiarrhythmic drugs for ventricular ectopy may actually increase the incidence of fatal ventricular arrhythmia (6). This results in two major problems. First, the unconscious patient or the unconscious laboratory animal differs physiologically from their conscious counterparts. Most notably, autonomic tone is altered by general anesthesia (6). Thus, the reaction to drugs may differ from the conscious to the unconscious state. For example, it has been shown with verapamil (VER) that the minimum effective antiarrhythmic dose is 0.5 mg/kg iv in anesthetized animals and 2 mg/kg iv in conscious animals (7). The second problem involves the possibility of a drug interaction between the anesthetic agent and the test drug. A good example is the synergistic interaction between class I antiarrhythmics such as lidocaine and the inhalation anesthetic halothane (8). An unknown drug interaction could confound the results for the basic researcher and have fatal results for the patient. Therefore it is important to conduct research in both anesthetized and unanesthetized animals to determine if differences and/or drug interactions exist. Verapamil (VER) is a phenlyalklyamine L-type Ca2+ channel blocker. It decreases electrical conduction across the atria and slows conduction through the AV-node. Therefore, it is widely used for supraventricular tachyarrthymias, and has a well-described concentration effect relationship (9, 10). Methoxyflurane (MF) is a volatile inhalation anesthetic that is no longer used in humans due to renal toxicity, but is commonly used for surgical anesthesia in animals. Several structurally similar inhalation anesthetics such as halothane, isoflurane and enflurane have been shown to inhibit the Na+/Ca2+ exchange and Ca2+ channels in the heart (11). It is therefore possible that MF could interact with VER increasing the possibility of AV-block or supraventricular arrhythmia. In addition, pharmacokinetic-pharmacodynamic animal studies require the surgical placement of cannulae for serial blood sampling. Therefore, it is important to understand the combined effects anesthesia and test drug on Pharmacodynamics. The objective of this study was to determine the effect of MF anesthesia alone or in combination with VER on the ECG of the rat. In addition, the time required to return to baseline values after treatment with VER, MF and MF + VER was determined. Methods and Materials Verapamil hydrochloride was a gift from G.D. Searle (Skokie, Ill), manufactured by Knoll Pharmaceuticals (Stuttgart, Germany). Methoxyflurane (Metofane) was purchased from Janssen pharmaceuticals, veterinary division (North York, Canada). The braided stainless steel, Teflon coated electrodes were purchased from Cooner Wire Co (Chatsworth, California). The study followed the University of Alberta guidelines established for ethical handling of live animals. Adult, male, Sprague-Dawley rats (Charles River Colony, n = 6, 311 ± 23 g) were used in the study. The animals were acclimated to a 12-hour day-night cycle, housed in rodent cages, and fed standard rodent chow. On the morning of the experiment ECG electrodes were placed under light anesthesia with MF. The placement of the ECG electrodes required no more than 10 minutes in all animals. The animals were placed in a Plexiglas restraining cage and allowed to recover for a two-hour period. All animals had their ECG's recorded every 10 mins during this 2-h recovery period to ensure that the animal was free of any residual effects of the anesthesia. The ECG was then recorded for an additional 2-h period to obtain baseline values. The animals were then randomized to each of three treatments: Methoxyflurane (MF) animals had surgical anesthesia induced with 0.15% MF and maintained for 2 hours while recording their ECG. The MF was then discontinued and the animal allowed to regain consciousness while their ECG was monitored until baseline values were re-established. Surgical anesthesia was defined as an absence of foot withdrawal to painful stimuli; absence of ear movement to ear prick and lack of eyelid response to brushing accompanied with deep rhythmic breathing. VER group received VER 10 mg/kg s.c. and had their ECG's recorded over a two hour period. The VER + MF group was placed under surgical anesthesia with 0.15% MF then VER 10 mg/kg s.c was administered. The anesthesia was maintained for a 2-h period then discontinued. ECG’s were recorded until the baseline values were restored. Animals were then crossed over to the remaining treatments such that each animal served as its own control and at the end of a four-day period an animal had received all four treatments. Each treatment effect was determined by comparing the ECG measurements recorded over a two hour period and calculating the percent change from baseline. The study is summarized in Figure 1.
Statistical Analysis Data are expressed as the mean ± SEM. The ANOVA with Fishers protected least significant difference post-hoc test was used to test for differences amongst the treatment groups. Statistical significance was set at a = 0.05. Results The MF and VER both demonstrated significant dromotropic effects as measured by PR-interval (Figure 4). VER alone produced a significant 5% prolongation in PR-interval.
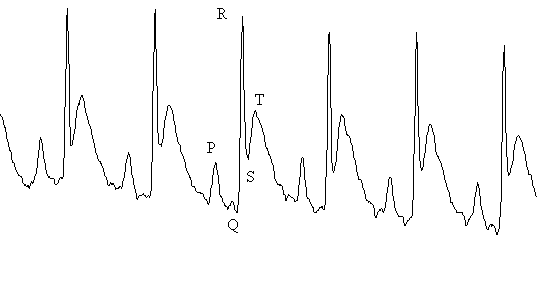
Discussion The time to re-establish baseline was 37.5 ± 15.1 minutes for VER, 69.8 ± 5.3 minutes for VER and 148.5 ± 6.6 minutes for MF + VER. Methoxyflurane and verapamil appeared to have the greatest effect on dromotropism and chronotropism as measured by PR-interval and RR-interval (Figure 4). It is generally assumed that VER has a much greater effect on the AV-node and is therefore most commonly indicated for supraventricular tachyarrythmias (12). In our study, VER alone produced a 5% prolongation in PR-interval, which was statistically significant and reflects the dose of 10 mg/kg s.c. that was administered to the rats. A similar chronotropic effect was observed with a 6% prolongation in RR-interval. This agrees well with clinical data, since VER is know to slow resting heart rate (12). It is clear that dose of MF required for surgical anesthesia, is sufficient to impair electrical conduction across the atria and through the AV-node as demonstrated by a 14.5% prolongation in PR-interval (Figure 4). However, VER + MF clearly demonstrated synergistic dromotropic effects with a 28.7% prolongation in PR-interval. MF has been shown to increase the automaticity of parts of the atrial conducting system other than the sinus either through a direct mechanism or through increased adrenergic sensitivity (4). VER is known to decrease the incidence of epinephrine-induced supraventricular tachyarrythmia in halothane anesthetized dogs (13). However, greater prolongation in PR-interval and direct depression of myocardial contractility was also observed. This appears similar to our observation of increased PR-interval prolongation with MF + VER. It is important to note that VER demonstrates "use-dependent" block of L-Type Ca2+ channels (14). This means that inhibition of the channel accumulates with repetitive stimuli. Furthermore, VER acts only after binding to the intracellular side of the membrane (15). Thus, VER must wait for L-type Ca2+ channels to open in order to reach its receptor. After the channel shuts, the drug remains trapped intracellularly and must slowly diffuse off the receptor at rest (16). This implies that VER will have a greater effect if conditions exist which increase the rate or duration of Ca2+ channel opening. While little has been written on MF and Ca2+ channels, much work has been done on the structurally related anesthetics halothane, enflurane and isoflurane. All three volatile anesthetics have been shown to have inhibitory effects on cardiac L-type Ca2+ channels (17). Greater effects have been observed on low-voltage-activated (LVA) calcium currents than on the high-voltage-activate L and N-types of Ca2+, however halothane showed no preferential effect for LVA, L or N-type channels (18). Furthermore, halothane has been shown to increase channel open time constants in sarcoplasmic reticulum calcium channels. This suggests halothane can bind to both open and closed configurations of the channel (19). Thus, the negative inotropic effect of halothane and isoflurane is attributed to Ca2+ influx inhibition in the rat heart. This suggests that the observed synergism between MF and VER may be due to cardiac L-type Ca2+ channel inhibition similar to halothane and isoflurane. Therefore, a VER-MF drug interaction exists presumably at the level of the L-type Ca2+ channel or through indirect effects on Ca2+ flux through other channel types. MF and VER appeared to have less effect on other parts of the heart. The R-wave is produced during contraction of the ventricles and the entire QRS complex reflects ventricular depolarization and the T-wave, ventricular repolarization (20). The RR-interval is used to calculate heart rate and is indicative of chronotropic effects. VER is a potent negative chronotrope (12), and the small dose used in this study did result in a 6% prolongation in RR-interval. MF prolongs RR-interval even more than VER alone and the combined effects of both VER + MF resulted in a 17.6% prolongation in RR-interval. This translates into a substantial slowing of heart rate suggesting that the combination can significantly depress the normal pacemaker areas of the heart. Effects on ventricular function as measured by the QRS-interval were subtle. Both VER and MF caused a 5% increase in QRS-interval (Figure 4). The combination of VER + MF caused a slight increase to 6.8% which was different from VER alone, but not MF. These data suggest that VER and MF possess little Class IC antiarrhythmic activity. However, the rat ECG lacks a distinct S-T segment and an isoelectric line. Thus differences in QRS-intervals may be difficult to determine and it may be best to be avoided (21) unless the signal can be digitized and integrated reliably. The time to re-establish baseline (Figure 5) was 37.5 ± 15.1 for VER, 69.8 ± 5.3 minutes for MF and 148.5 ± 6.6 minutes for MF + VER. This is an important measure, since any study on drug effect must include a sufficient wash-out period to ensure no residual anesthetic effects alter the pharmacodynamics of the test drug. In addition, general anesthesia has also been shown to alter the pharmacokinetics of drugs. For example, thiopental, ketamine and propofol have been shown to increase the AUC of VER with a decrease in Vd (22). It is not known if MF has a similar effect, however the augmented effect on PR-interval by VER + MF could result from elevated concentrations of VER. Anesthetic use is not contraindicated in pharmacokinetic-pharmacodynamic studies, but its possible effects on the results should be understood. In addition, data collected in studies performed on an anesthetized animal should not be extrapolated to conscious animals since this could result in erroneous conclusions. If anesthesia is to be used for a procedure on the day of the study, a sufficient wash-out period should be allowed to minimize any residual effects. However the use of a control group treated in an identical manner will allow for a valid comparison. In conclusion, a significant interaction was observed between MF and VER with an increase in dromotropic and chronotropic effects. This suggests that MF could augment the effects of VER in an unconscious animal. If the effects of VER alone are to be studied a washout period of at least 3 to 4 hours is required. This work further suggests that drug effects measured in anesthetized animals may differ significantly from studies in conscious animals. While it does not invalidate either form of experimentation, it does suggest that the two types of experiments must be interpreted cautiously. Clinically, patients may respond differently to the same drug given while under anesthesia than when awake. This suggests that in addition to changes in pharmacokinetics due to anesthesia and surgery, that pharmacodynamics may also be altered. References
Corresponding author: Fakhreddin Jamali, Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta, Edmonton, Alberta, Canada. Email: fjamali@pharmacy.ualberta.ca Published by the Canadian Society for Pharmaceutical Sciences. Copyright © 1999 by the Canadian Society for Pharmaceutical Sciences. |
|||||||||||||||