|
Objectives of Bioprocessing Research:
-
Develop novel biocatalysts for transformation of hydrophobic compounds,
such as crude oil components, alkanes and polycyclic aromatic hydrocarbons.
-
To understand the transport mechanisms for the uptake and conversion
of these compounds in bacteria, in order to improve the efficiency of
bacteria as biocatalysts.
-
To develop novel bioreactors for use with hydrophobic compounds.
Objectives of Bioremediation Research:
- Apply principles from biology and engineering to the science and technology
of bioremediation.
- Understand the underlying mechanisms of microbial treatment of contaminated
soil and water.
- Focus on hydrocarbons and related compounds.
Research Program Areas
Development of Novel Biocatalysts
Work with JM Foght and H Dettman is focussed on developing novel
biocatalysts for transforming oil fractions. Specific projects include:
-
Viscosity reduction of heavy oils be selectively cleaving the sulfur
bridges that hold the large asphaltenic molecules together. PhD student
Kathlyn Kirkwood is developing novel biocatalysts for this type of reaction.
-
Biological opening of saturated rings is being studied by MSc student
Paul Chernik. Bacterial enzymes can promote reactions that are very
unfavorable with conventional catalysts, such as selective oxidation
reactions.
-
Biological conversion of napthenic acids to remove these unwanted contaminates from petroleum.
Uptake and Efflux of Hydrocarbons
-
Most microbial degradation occurs within bacterial cells.
-
Hydrophobic compounds partition into the cell membranes at high concentration
.
-
Some bacteria have the ability to pump hydrophobic compounds out of
cells. This type of pumping has an important impact on intracellular
concentrations.
-
Current research is focused on the selectivity of these pumps, their
biochemistry and genetics and their ability to alter intracellular concentrations.
In order for bacteria to transform hydrocarbons into more desirable compounds,
the hydrocarbons need to cross the cell wall to contact intracellular
enzymes. Bacteria have the capability of using energy to pump compounds
out of the cell interior, to control toxic effects of solvents, and to
use energy to take up low solubility compounds like n-alkanes. The objective
of our research is to investigate these transport mechanisms in bacteria,
in order to develop more effective biocatalysts.
-
Pseudomonas fluorescens LP6a can selectively transport three-ring
aromatic compounds across the cell membranes, but not one- or two-ring
aromatics. PhD student Beth Hearn has identified the gene for this
transport process, and is determining its sequence and activity.
-
Many bacteria that degrade alkanes accumulate hydrocarbons in intracelullular
inclusions, presumably as a method of storing energy. Post-doctoral
fellow In Seon Kim demonstrated that Rhodococcus erthyropolis
can selectively transport n-alkanes and leave branched alkanes
behind. Current work is investigating the mechanism for this energy-dependent
selection and transport process.
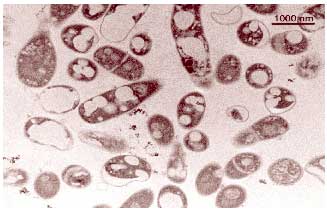
Transmission electron micrograph of a cross section if cells of Rhodococcus
erythropolis grown on n-hexadecane. The spots inside the cells are
inclusions rich in n-hexadecane.
Cell Attachment to Oil/Water Interfaces
A second factor that controls the ability of cells to attack hydrocarbons
is the proximity of the cells to the insoluble oil components. Many bacteria
that degrade hydrocarbons attach directly to the oil-water interface,
to maximize the flux of hydrocarbons into the cells.
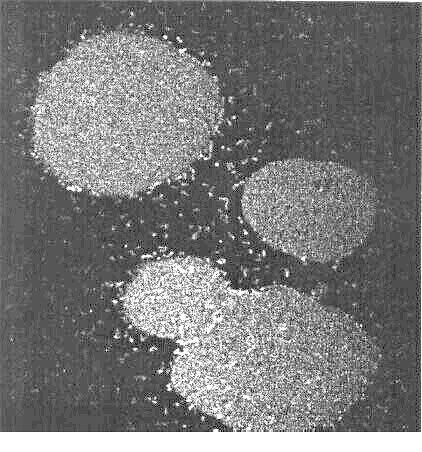
This confocal micrograph shows droplets of a oil-in-water emulsion formed
by Rhodococcus erythropolis 20S-E1-c. The fluorescent bacteria
surround the hexadecane droplets, and prevent them from coalescing.
The stability of the droplets is illustrated in this movie (above), showing two
oil droplets that are coated with bacteria being pushed together.
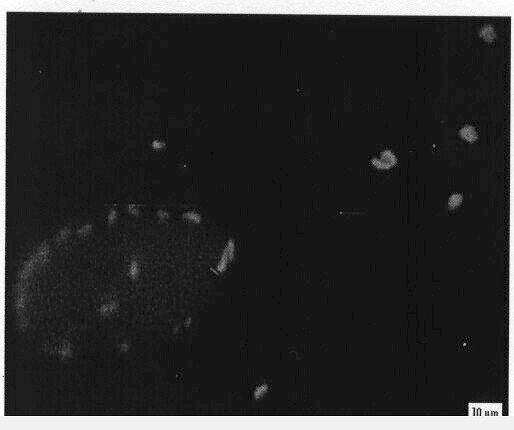
The confocal micrograph shows droplets of a water-in-oil emulsion formed
by Rhodococcus erythropolis 20S-E1-c. Clusters of bacteria surrounded
by a thin film of water can be seen at the right. Graduate student Loredana
Dorobantu demonstrated that the ability of bacteria to form such emulsions
is controlled by their surface hydrophobicity. Hydrophilic bacteria do
not attach to the interface, and no oil emulsion forms, while highly hydrophobic
bacteria can pass into the oil phase, as shown above. The largest volume
of emulsion was formed by cells of intermediate hydrophobicity, because
all of the cells were localized at the oil-water interface.
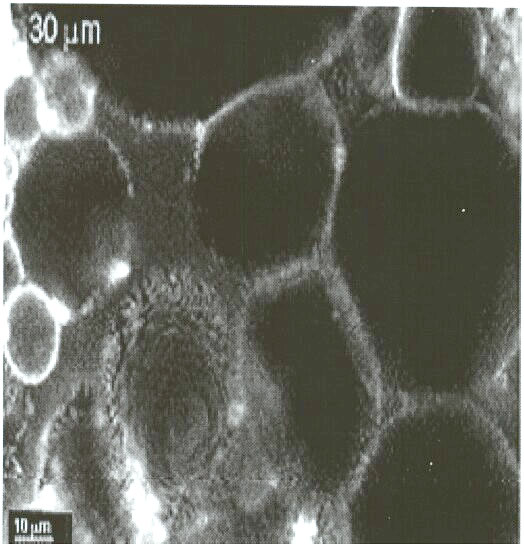
This confocal micrograph of the "emulsion gel" formed by mixing
Acinetobacter venetianus RAG-1 suspensions with n-hexadecane. Fluorescent
bacteria are seen in the thin films surrounding the larger (dark) hexadecane
drops. These bacterial behaviors are very important for bioremediation
and bioprocessing, and we are continuing work on these phenomena to better
understand and control the attachment of cells to the oil interface, and
the resulting uptake processes.
Transport Processes in Contaminated Soil
-
Soils at contaminated sitesoften contain a non-aqueous liquid phase
(NAPL) that changes the structure of the soil. Examples of NAPL are
crude oil, waste solvents and creosote.
-
Microbes can only degrade the contaminants that are accessible. The
fine pores in soil are too small for bacteria to enter, therefore, diffusion
of contaminants out of soil aggregates is an important process.
-
Diffusion of contaminants is controlled by the structure of the soil.
The following images give some examples of soil microstructure.
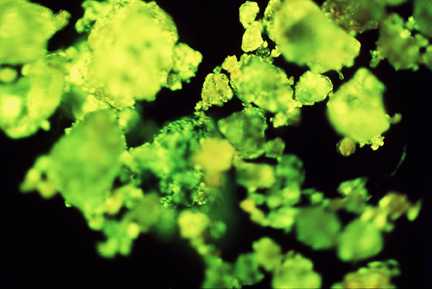
Creosote-contaminated clay- rich soil, showing fluorescence under ultra-violet
light due to contamination by polycyclic aromatic hydrocarbons (PAHs).
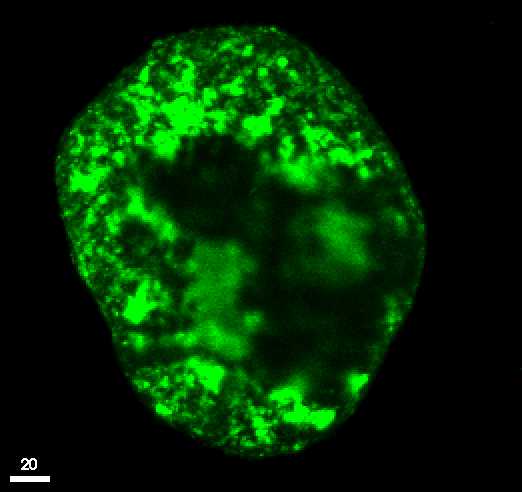
Scanning-laser microscopy image of an aggregate of the same soil. Bright
areas show high concentration of contaminants.
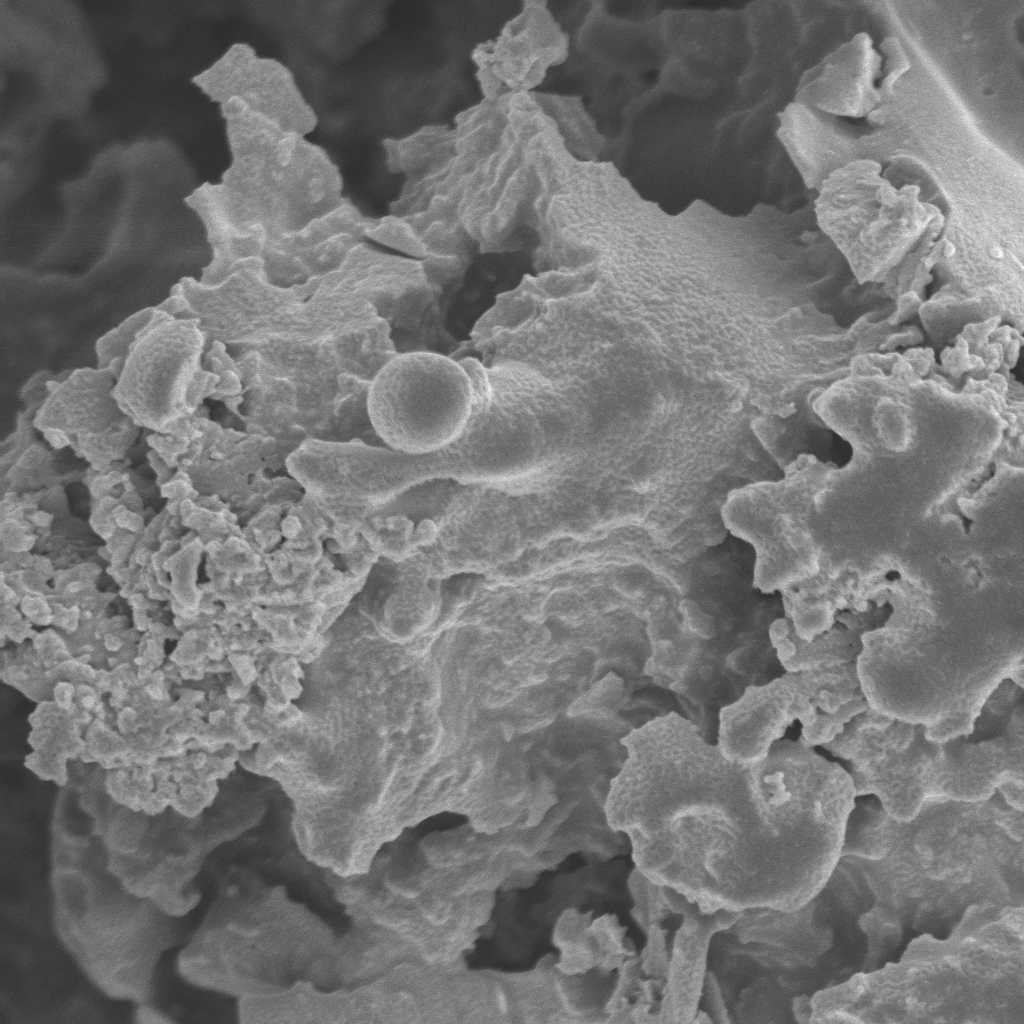
Scanning electron microscope view of the same contaminated soil. The
sample was frozen in liquid nitrogen and then fractured. The NAPL phase
in the soil is visible along the fractured surface.
The presence of this non-aqueous phase changes the nature of the soil
by filling the spaces between the soil granules. In aged soils from contaminated
sites, the NAPL is often a viscous oil or tar. The diffusion of
target compounds, such as PAHs, is much slower in this oil phase than
in water, so the release of the contaminants is very slow. This understanding
of the soil microstructure has been used to derive detailed mathematical
models for the release of contaminants from soil.
Bioreactor Design and Operation

Rotating drum bioreactors for treating municipal solid waste, producing
compost. Similar bioreactors have been used for treatment of contaminated
soil.

Engineered biopile facility for treating soil, with control of moisture,
oxygen supply and runoff.

Mixing tank for wastewater treatment. Similar vessels can be used for
treatment of contaminated soils and sludges.
- Transport processes, such as diffusion and heat transfer, and microbial
growth are crucial to these operations.
|













