Xing-Zhen Chen

Professor
PhD, University of Montreal
Office: 7-29A Medical Sciences Building
Laboratory: 7-29 and 7-30 Medical Sciences Building
Tel: 780-492-2294
Lab Tel: 780-492-2307
Email address: xzchen@ualberta.ca
Research Interests / Academic Activities
The transient receptor potential (TRP) superfamily of cation channels plays important roles in sensory physiology (such as vision and pain/mechano/chemo sensations), through responding to various stimuli, such as light, temperature, force and different chemicals.
Our laboratory mainly studies the function and regulation of TRPP2, -P3 and -V6 channels, of which dysfunction are associated with polycystic kidney disease, abnormal acid sensing, imbalance of calcium homeostasis and cancer, etc. In particular, we focus on
- Characterization of how they interact with and are regulated by interacting partner proteins
- Determination the amino acid residue(s) that constitutes the pore gate that controls the channel opening and closing, and
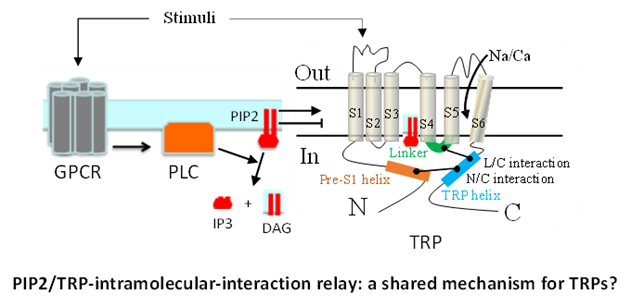
- Identification of intramolecular interactions that are critical for their channel function and mediate the regulation by upstream factors including phosphatidylinositol 4,5-bisphosphate (PIP2) and extracellular stimuli, and determination of whether this represents a shared mechanism for different TRPs.
Experimental methods and models: Molecular biology, protein-protein interaction, biotinylation, gene over-expression and knockdown, CRISPR/Cas9, immunostaining, electrophysiology (patch-clamp, two-microelectrode voltage-clamp, and lipid bilayer reconstitution), radiotracer transport measurements, pulse chase, cell proliferation and apoptosis assays. Experimental models include Xenopus oocytes, cultured mammalian cells, lipid bilayer, zebrafish and mice.
Select Publications
- Cai, R. and Chen, X.-Z. Roles of intramolecular interactions in the regulation of TRP channels. Reviews Physiol. Biochem. Pharmacol. doi: 10.1007/112_2022_74, 2022.
- Huang, Y., Li, S., Liu, Q., Wang, Z., Li, S., Liu, L., Zhao, W., Wang, K., Zhang, R., Wang, L., Wang, M., Ali, D.W., Michalak, M., Chen, X.-Z., Zhou, C., and Tang, J. The LCK-14-3-3ζ-TRPM8 axis regulates TRPM8 function/assembly and promotes pancreatic cancer malignancy. Cell Death Dis. 13, 524, 2022.
- Cai, R., Wang, L., Liu, X., Michalak, M., Tang, J., Peng, J.-B., and Chen, X.-Z. Auto-inhibitory intramolecular S5/S6 interaction in the TRPV6 channel regulates breast cancer cell migration and invasion. Commun. Biol. 4, 990, 2021.
- Cai, R., Tang, J., and Chen, X.-Z. Hydrophobic gates and proton binding in proton-activated chloride channel. iScience. 24, 103395, 2021.
- Zhou, C., Liang, Y., Zhou, L., Yan, Y., Zhang, R., Huang, Y., Wang, M., Tang, Y., Ali, D., Wang, Y., Michalak, M., Chen, X.-Z., and Tang, J. TSPAN1 promotes autophagy flux and mediates cooperation between WNT-CTNNB1 signaling and autophagy via the MIR454-FAM83A-TSPAN1 axis in pancreatic cancer. Autophagy. 17, 3175-3195, 2021.
- Cai, R., Liu, X., Zhang, R., Hofmann, L., Zheng, W., Amin, M.R., Wang, L., Hu, Q., Peng, J.-B., Michalak, M., Flockerzi, V., Ali, D., Chen, X.-Z., and Tang, J. Autoinhibition of TRPV6 channel and regulation by PI(4,5)P2. iScience. 23, 101444, 2020.
- Zhou, C., Yi, C., Yi, Y., Qin, W., Yan, Y., Dong, X., Zhang, X., Huang, Y., Zhang, R., Wei, J., Ali, A., Michalak, M., Chen, X.-Z., and Tang, J. LncRNA PVT1 promotes gemcitabine resistance of pancreatic cancer via activating Wnt/β-catenin and autophagy pathway through modulating the miR-619-5p/Pygo2 and miR-619-5p/ATG14 axes. Mol. Cancer. 19, 118, 2020.
- Wang, Z., Ng, C., Liu, X., Wang, Y., Li, B., Kashyap, P., Chaudhry, H.A., Castro, A., Kalontar, E.M., Ilyayev, L., Walker, R., Alexander, R.T., Qian, F., Chen, X.‑Z., and Yu, Y. The ion channel function of polycystin-1 in the polycystin-1/polycystin-2 complex. EMBO Rep. 20, e48336, 2019.
- Lee, J.J., Liu, X., O’Neill, D., Beggs, M.R., Flockerzi, V., Chen, X.-Z., Dimke, H., and Alexander, R.T. Activation of the calcium sensing receptor attenuates TRPV6-dependent intestinal calcium absorption. JCI Insight. 5, 128013, 2019.
- Zheng, W., Yang, X., Hu, R., Cai, R., Hofmann, L., Wang, Z., Hu, Q., Liu, X., Bulkley, D., Yu, Y., Tang, J., Flockerzi, V., Cao, Y., Cao, E., and Chen, X.-Z. Hydrophobic pore gates regulate ion permeation in polycystic kidney disease 2 and 2L1 channels. Nat. Commun. 9, 2302, 2018.
- Zheng, W., Cai, R., Hofmann, L., Nesin, V., Hu, Q., Long, W., Fahiti, M., Liu, X., Hussein, S., Kong, T., Li, J., Light, P.E., Tang, J., Flockerzi, V., Tsiokas, L., and Chen, X.-Z. Direct binding between pre-S1 and TRP-like domain in TRPP channels mediates gating and functional regulation by PIP2. Cell Rep. 22, 1560-1573, 2018.
- Zheng, W., Shen, F., Hu, R., Roy, B., Yang, J., Wang, Q., Zhang, F., King, J.C., Sergi, C., Liu, S.-M., Cordat, E., Tang, J., Cao, Y., Ali, D.W., and Chen, X.-Z. Far upstream element-binding protein 1 binds the untranslated region of PKD2 and suppresses its translation. J. Am. Soc. Nephrol. 27, 2645-2657, 2016.
Laboratory Members
Graduate students
Zoe Qinyi Xu
Lillian Wanyi Fang
Yuzi Wang
Amiee Yifang Wang
Xiaoling Deng
Rui Tian
Visiting scholar
Lin Xu